Key Factors in Effective Patient-Tailored Dosing of Fluoroquinolones in Urological Infections: Interindividual Pharmacokinetic and Pharmacodynamic Variability
Abstract
1. Introduction
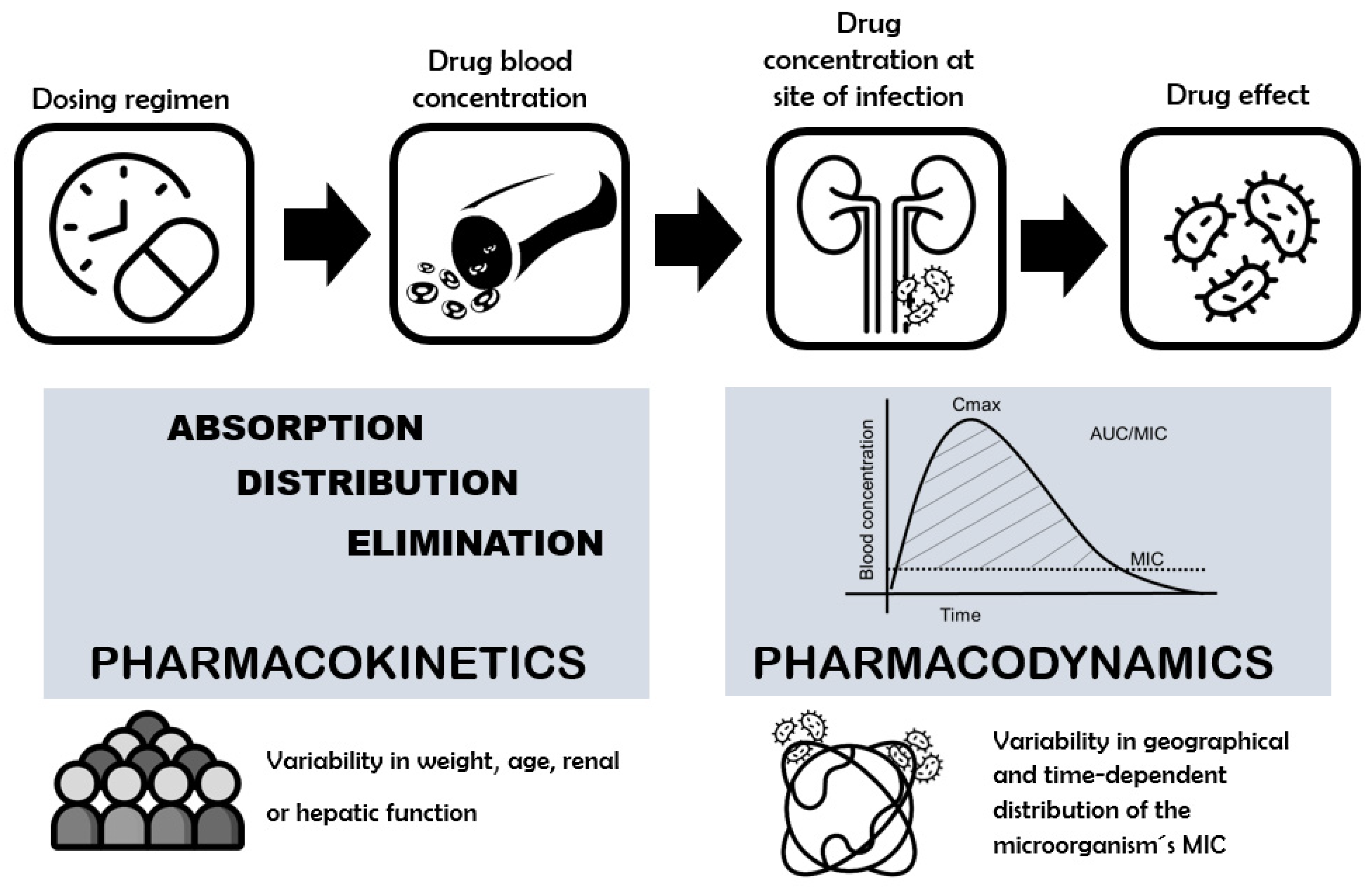
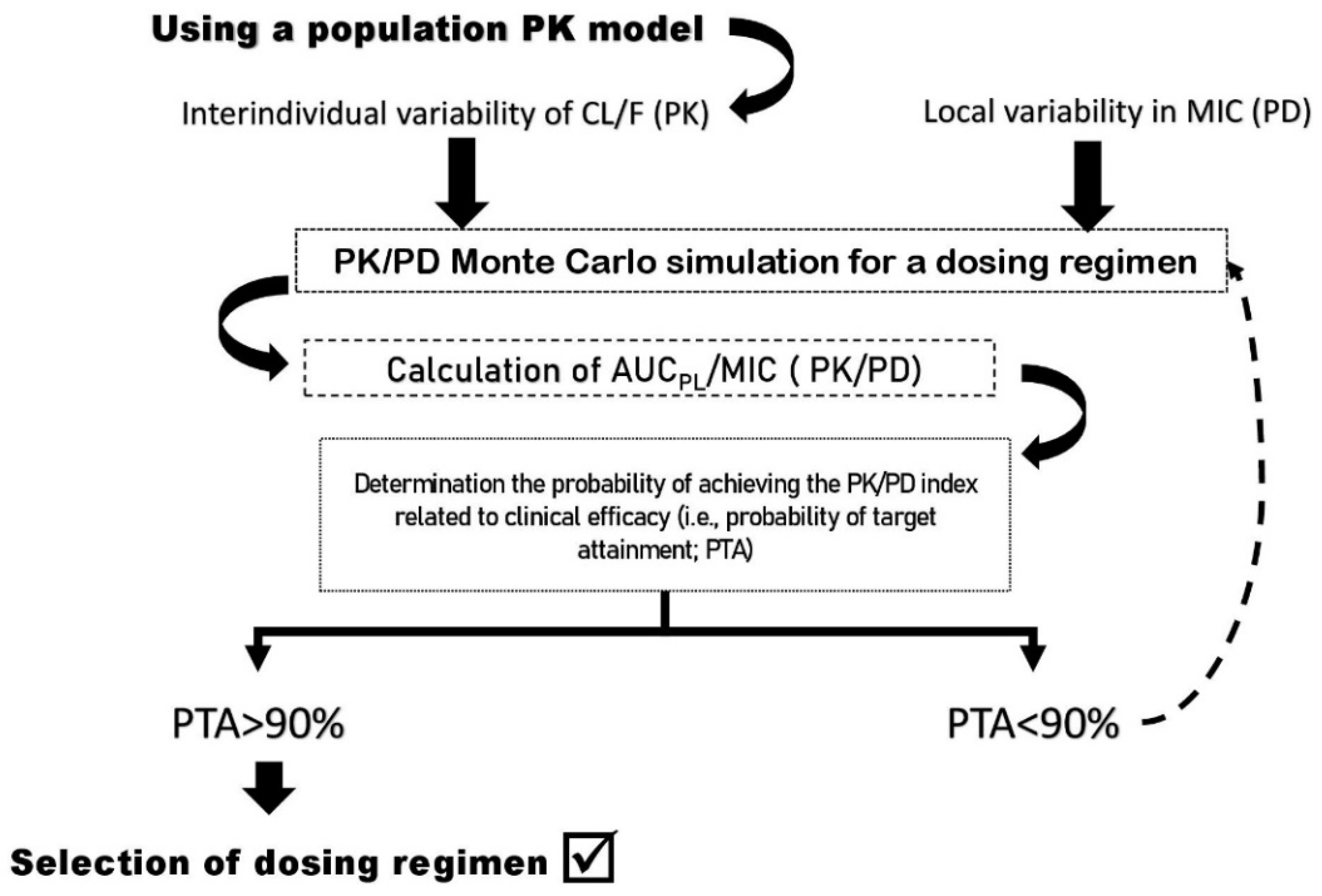
2. Concerns about the Clinical Efficacy of Fluoroquinolone Dosing: The Role of PK/PD Index as a Tool
3. Interindividual Pharmacokinetic Variability and Their Causes
3.1. Absorption Process: Role of Food
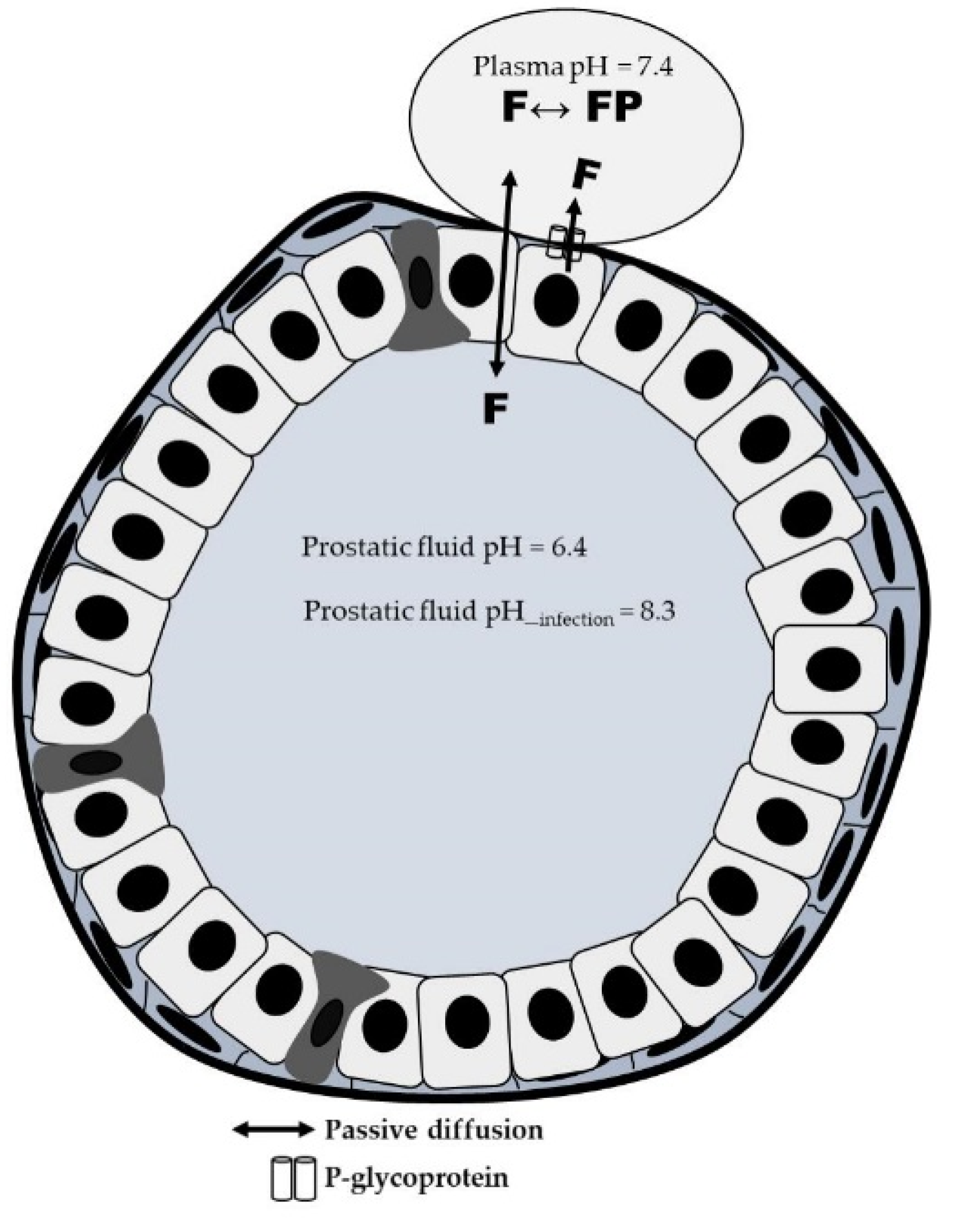
3.2. Distribution Process: Role of Patient´s Pathophysiological Characteristics
3.3. Elimination Process: Role of Renal and Hepatic Function
| Ciprofloxacin | ||||
|---|---|---|---|---|
| Study Characteristic | Vd (L) | Cl (L/h) | T½ (h) | Reference |
| Healthy, non-obese | ||||
| 200 mg Infusion IV 21–30 years | 199.1 (34.2) | 26.8 (5.7) | 4.2 (0.8) | Plaisance et al., 1987 [35] |
| 219.0 (35.8) | 44.6 (7.2) | 4.0 (0.3) | Allard et al., 1993 [60] | |
| 146.0 (27.4) | 25.2 (5.8) | 4. 4(0.9) | Drusano et al., 1986 [77] | |
| 750 mg Oral | ||||
| 21–29 years | 256.0 (80.0) 1 | 29.5 (5.9) 1 | 5.2 (0.7) | Plaisance et al., 1987 [35] |
| 46–68 years | 217.0 (45.0) 1 | 50.4 (14.4) 1 | 3.7 (0.4) | Drusano et al.,1986 [77] |
| Healthy, obese | ||||
| 400 mg Infusion IV 29 ± 7 years BMI = 36 ± 4 kg/m2 | 269.1 (51.6) | 53.8 (9.5) | 4.3 (0.6) | Allard et al., 1993 [60] |
| Patients with cirrhosis | ||||
| 750 mg Oral 52 ± 6 years | 218.1 (45.4) 1 | 45.9 (14.1) 1 | 3.7 (0.4) | Frost et al., 1989 [65] |
| Patients with renal disease | ||||
| 200 mg Infusion IV 22–62 years | ||||
| CLCR ≥ 100 mL/min | 191.7 (35.4) | 26.8 (5.7) | 4.3 (0.8) | Drusano et al., 1987 [66] |
| CLCR = 86–60 mL/min | 243.0 (97.1) | 26.3 (10.3) | 6.1 (1.6) | |
| CLCR = 11–57 mL/min | 183.2 (47.7) | 15.0 (3.8) | 7.7 (1.2) | |
| CLCR = 0 mL/min | 210.2 (70.8) | 15.4 (4.3) | 8.5 (3.3) | |
| 750 mg Oral 48–90 years | ||||
| CLCR ≥ 50 mL/min | 158.0 (46.5) 1 | 70.4 (48.9) 1 | 3.5 (1.2) | Gasser et al., 1987 [67] |
| CLCR < 50 mL/min | 113.8 (34.2) 1 | 29.4 (6.4) 1 | 6.3 (3.2) | |
| Elderly patients | ||||
| 200 mg Infusion IV 78 ± 11 years ClCR = 45 ± 16 mL/min | 100.8 (37.8) | 16.6 (6.8) | 5.8 (2.4) | Hirata et al., 1989 [63] |
| 200 mg Infusion IV 73 ± 11 years CLCR = 45 ± 16 mL/min | (61.0–118.0) | 18.4 (4.5) | ND | Cios et al., 2014 [61] |
| Acutely ill patients | ||||
| 200–400 mg Infusion IV 24–91 years ClCR = 63 ± 30 mL/min | 111.0 (33.0) | 17.0 (6.6) | ND | Forrest et al., 1993 [25] |
| 400–1200 mg Infusion IV 56–71 years GFR = 32–101 mL/min | 255.0 (51.0) | 25.4 (67.8) | ND | Abdulla et al., 2020 [68] |
| 400 mg Infusion IV 23–79 years ClCR =13–204 mL/min | 107.5 (21) | 18.6 (18.7) | ND | Li et al., 2019 [80] |
| 400–600 mg Infusion IV 24–89 years ClCR = 7–204 mL/min | ND | 15.2 (42.9) | ND | Roberts et al., 2019 [64] |
| 400 mg Infusion IV 55–77 years | 160 (51.2) | 10.7 (46.9) | ND | Roger et al., 2016 [79] |
| 200–400 mg Infusion IV 30–87 years GFR = 23–208 mL/min | ND | 20.3 (51.2) | ND | Gieling et al., 2020 [69] |
| Levofloxacin | ||||
|---|---|---|---|---|
| Study Characteristic | Vd (L) | Cl (L/h) | T½ (h) | Reference |
| Healthy young volunteers | ||||
| 500 mg Oral 22–36 years ClCR = 90–117 mL/min | 90.6 (11.9) 1 | 9.5 (1.7) 1 | 7.0 (0.8) | Chien et al., 1997 [36] |
| Healthy elderly volunteers | ||||
| 500 mg Oral 66–75 years ClCR = 47–80 mL/min | 70.8 (8.4) 1 | 7. 3 (1.9) 1 | 7.6 (2.0) | Chien et al., 1997 [36] |
| Patients with respiratory, urinary, and other infections | ||||
| 250–500 mg Infusion IV 47 ± 18 years ClCR = 86 ± 31 mL/min | ND | 9.3 (4.3) | ND | Preston et al., 1998 [70] |
| Patients adults with pulmonary tuberculosis | ||||
| 1000 mg Oral 30–54 years ClCR= 51–125 mL/min | (33.5–114.5) 1 | 7.6 (1.5–19.2) 1 | ND | Peloquin et al., 2008 [82] |
| Patients with bone and joint infections | ||||
| 750 mg Oral 57 ± 20 years BW = 72 ± 16 kg ClCR= 120 ± 74 mL/min | 90.6 1 (0.06) | 6.10 (0.17) 1 | ND | Eloy et al., 2020 [81] |
| Obese patients | ||||
| 750 mg Infusion IV 18–55 years BMI (kg/m2) = 49.3 ± 20.7 ClCR = 140 ± 64 mL/min | 83.8 (21.6) | 9.8 (4.2) | 5.9 (3.5) | Cook et al., 2011 [71] |
| Acutely hospitalized older patients with several degrees of renal function | ||||
| 125–750 mg Oral 81 ± 28 years ClCR = 18–50 mL/min | ND | 2.53 (1.46) 1 | ND | Cojutti et al., 2017 [90] |
| Intensive Care Unit | ||||
| Acute renal failure 500 mg Infusion IV 33–62 years | 114.0 (74–155) | 3.1 (2.9–3.2) | 34.5 (21.2–47.7) | Czock et al., 2006 [96] |
| Acute renal failure 33–62 years | 82.8 (50.0) | 2.5 (0.9) | 21.8 (5.5) | Hansen et al., 2001 [95] |
| Criticall ill in continuous hemodiafiltration 500 mg Infusion IV 59 ± 6 years ClCR = 70 ± 67 mL/min | ND | 3.6 (0.4) | ND | Wada et al., 2015 [91] |
| Continuous veno-venous hemofiltration 250 mg Infusion IV 23–70 years | ND | 1.8–3.6 | ND | Malone et al., 2001 [93] |
| Continuous veno-venous hemofiltration 500 mg Infusion IV 68 ± 5 years | 105.7 (36.4) | 3.26 (1.4) | 28.0 (4.5) | Guenter et al., 2002 [94] |
| Moxifloxacin | ||||
|---|---|---|---|---|
| Study with | Vd (L) | Cl (L/h) | T½ (h) | Reference |
| Healthy volunteers | ||||
| 200 mg Oral 33 ± 5 years | 222.0 (1.2) 1 | 13.1 (0.1) 1 | 11.8 (1.2) | Stass et al., 1998 [73] |
| 400 mg Oral 18–46 years | 175.9 (19.4) 1 | 101.0 (2.1) 1 | ND | Grosjean et al., 2012 [74] |
| Morbidly obese patients BMI > 40 kg/m2 400 mg Infusion IV 41 ± 12 years | 165.0 (30.0) | 9.6 (2) | 12.2 (2.2) | Keess et al., 2011 [75] |
| Hospitalized severe liver insufficiency with pneumonia or spontaneous bacterial peritonitis 400 mg Infusion IV 40–78 years | 154.1 (118.5–216.1) | 8.8 (6.4–10.5) | 10.4 (8.5–16.0) | Barth et al., 2008 [99] |
| Outpatients with pneumonia receiving hemodialysis 400 mg Oral 47–78 years | ND | 6.5 (1.9) 1 | ND | Tokimatsu et al., 2017 [101] |
| Critical ill patients receiving continuous hemodiafiltration 400 mg IV infusion 25–76 years | 266 (154–514) | 15.7 (8.1–49.39) | 12.3 (3.7–34.0) | Czock et al., 2006 [96] |
| Intensive care unit with COPD 2 400 mg Infusion IV 70 ± 10 years | 115.0 (40.0) | 8.85 (2.6) | 9.7 (3.7) | Sionidou et al., 2019 [100] |
4. Antibacterial Activity of FQs: Interregional Variability in Pharmacodynamic Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization. Critically Important Antimicrobials for Human Medicine, 6th revision; World Health Organization: Geneva, Switzerland, 2018; Available online: https://apps.who.int/iris/bitstream/handle/10665/312266/9789241515528-eng.pdf?ua=1 (accessed on 19 March 2022).
- Gray, C.; Loshak, H. Fluoroquinolones for the treatment of intra-abdominal infections. In CADTH Rapid Response Report: Summary with Critical Appraisal; Canadian Agency for Drugs and Technologies in Health: Kanata, ON, Canada, 2019; Available online: https://www.cadth.ca/sites/default/files/pdf/htis/2019/RC1094%20FQ%20for%20Intra-Ab%20Infection%20Final.pdf (accessed on 19 March 2022).
- Morales, D.R.; Slattery, J.; Pinheiro, L.; Kurz, X.; Hedenmalm, K. Indications for Systemic Fluoroquinolone Therapy in Europe and Prevalence of Primary-Care Prescribing in France, Germany and the UK: Descriptive Population-Based Study. Clin. Drug Investig. 2018, 38, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Bonkat, G.; Bartoletti, R.; Bruyére, F.; Cai, T.; Geerlings, S.E.; Köves, B.; Schubert, S.; Pilatz, A.; Veeratterapillay, R.; Wagenlehner, F. European Association of Urology: Guidelines on Urological Infections; European Association of Urology: Arnhem, The Netherlands, 2020; Available online: https://uroweb.org/guideline/urological-infections/ (accessed on 19 March 2022).
- Stamatiou, K.; Pierris, N. Mounting resistance of uropathogens to antimicrobial agents: A retrospective study in patients with chronic bacterial prostatitis relapse. Investig. Clin. Urol. 2017, 58, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Zowawi, H.M.; Harris, P.N.; Roberts, M.J.; Tambyah, P.A.; Schembri, M.A.; Pezzani, M.D.; Williamson, D.A.; Paterson, D.L. The emerging threat of multidrug-resistant Gram-negative bacteria in urology. Nat. Rev. Urol. 2015, 12, 570–584. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Fluoroquinolone and Quinolone Antibiotics: PRAC Recommends Restrictions on Use New Restrictions Follow Review of Disabling and Potentially Long-Lasting Side Effects. Available online: https://www.ema.europa.eu/en/documents/press-release/fluoroquinolone-quinolone-antibiotics-prac-recommends-restrictions-use_en.pdf (accessed on 19 March 2022).
- FDA Drug Safety Communication: FDA Advises Restricting Fluoroquinolone Antibiotic Use for Certain Uncomplicated Infections 2016. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-advises-restricting-fluoroquinolone-antibiotic-use-certain (accessed on 19 March 2022).
- Bonkat, G.; Pilatz, A.; Wagenlehner, F. Time to Adapt Our Practice? The European Commission Has Restricted the Use of Fluoroquinolones since March 2019. Eur. Urol. 2019, 76, 273–275. [Google Scholar] [CrossRef]
- Almalki, Z.S.; Yue, X.; Xia, Y.; Wigle, P.R.; Guo, J.J. Utilization, Spending, and Price Trends for Quinolones in the US Medicaid Programs: 25 Years’ Experience 1991–2015. PharmacoEconomics Open 2017, 1, 123–131. [Google Scholar] [CrossRef]
- Sumi, C.D.; Heffernan, A.J.; Lipman, J.; Roberts, J.A.; Sime, F.B. What Antibiotic Exposures Are Required to Suppress the Emergence of Resistance for Gram-Negative Bacteria? A Systematic Review. Clin. Pharmacokinet. 2019, 58, 1407–1443. [Google Scholar] [CrossRef]
- Martinez, M.N.; Papich, M.G.; Drusano, G.L. Dosing regimen matters: The importance of early intervention and rapid attainment of the pharmacokinetic/pharmacodynamic target. Antimicrob. Agents Chemother. 2012, 56, 2795–2805. [Google Scholar] [CrossRef]
- Heffernan, A.J.; Sime, F.B.; Lipman, J.; Roberts, J.A. Individualising Therapy to Minimize Bacterial Multidrug Resistance. Drugs 2018, 78, 621–641. [Google Scholar] [CrossRef]
- Asin-Prieto, E.; Rodriguez-Gascon, A.; Isla, A. Applications of the pharmacokinetic/pharmacodynamic (PK/PD) analysis of antimicrobial agents. J. Infect. Chemother. 2015, 21, 319–329. [Google Scholar] [CrossRef]
- Ambrose, P.G.; Bhavnani, S.M.; Rubino, C.M.; Louie, A.; Gumbo, T.; Forrest, A.; Drusano, G.L. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: It’s not just for mice anymore. Clin. Infect. Dis. 2007, 44, 79–86. [Google Scholar] [CrossRef]
- Onufrak, N.J.; Forrest, A.; Gonzalez, D. Pharmacokinetic and Pharmacodynamic Principles of Anti-infective Dosing. Clin. Ther. 2016, 38, 1930–1947. [Google Scholar] [CrossRef] [PubMed]
- Bonkat, G.; Wagenlehner, F. In the Line of Fire: Should Urologists Stop Prescribing Fluoroquinolones as Default? Eur. Urol. 2019, 75, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Mehvar, R. Estimation of pharmacokinetic parameters based on the patient-adjusted population data. Am. J. Pharm. Educ. 2006, 70, 96. [Google Scholar] [CrossRef] [PubMed]
- de Velde, F.; Mouton, J.W.; de Winter, B.C.M.; van Gelder, T.; Koch, B.C.P. Clinical applications of population pharmacokinetic models of antibiotics: Challenges and perspectives. Pharmacol. Res. 2018, 134, 280–288. [Google Scholar] [CrossRef]
- Mouton, J.W.; Dudley, M.N.; Cars, O.; Derendorf, H.; Drusano, G.L. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: An update. J. Antimicrob. Chemother. 2005, 55, 601–607. [Google Scholar] [CrossRef]
- Sy, S.K.; Zhuang, L.; Derendorf, H. Pharmacokinetics and pharmacodynamics in antibiotic dose optimization. Expert Opin. Drug Metab. Toxicol. 2016, 12, 93–114. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on the Use of Pharmacokinetics and Pharmacodynamics in the Development of Antimicrobial Medicinal Products. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-use-pharmacokinetics-pharmacodynamics-development-antimicrobial-medicinal-products_en.pdf (accessed on 15 March 2022).
- Bhavnani, S.M.; Forrest, A.; Hammel, J.P.; Drusano, G.L.; Rubino, C.M.; Ambrose, P.G. Pharmacokinetics-pharmacodynamics of quinolones against Streptococcus pneumoniae in patients with community-acquired pneumonia. Diagn. Microbiol. Infect. Dis. 2008, 62, 99–101. [Google Scholar] [CrossRef]
- Drusano, G.L.; Preston, S.L.; Fowler, C.; Corrado, M.; Weisinger, B.; Kahn, J. Relationship between fluoroquinolone area under the curve: Minimum inhibitory concentration ratio and the probability of eradication of the infecting pathogen, in patients with nosocomial pneumonia. J. Infect. Dis. 2004, 189, 1590–1597. [Google Scholar] [CrossRef]
- Forrest, A.; Nix, D.E.; Ballow, C.H.; Goss, T.F.; Birmingham, M.C.; Schentag, J.J. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 1993, 37, 1073–1081. [Google Scholar] [CrossRef]
- Ambrose, P.G. Quinolone In Vitro Susceptibility Test Interpretive Criteria Evaluations. The National Antimicrobial Susceptibility Testing Committee for the United States. USCAST 001, 15 October 2018. Available online: https://app.box.com/s/e14zs4u4tpxs02ppjb97czmckvbm99sg (accessed on 19 March 2022).
- Rattanaumpawan, P.; Nachamkin, I.; Bilker, W.B.; Roy, J.A.; Metlay, J.P.; Zaoutis, T.E.; Lautenbach, E.; CDC Prevention Epicenters Program. High fluoroquinolone MIC is associated with fluoroquinolone treatment failure in urinary tract infections caused by fluoroquinolone susceptible Escherichia coli. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 25. [Google Scholar] [CrossRef]
- Peloquin, C.A.; Cumbo, T.J.; Nix, D.E.; Sands, M.F.; Schentag, J.J. Evaluation of intravenous ciprofloxacin in patients with nosocomial lower respiratory tract infections. Impact of plasma concentrations, organism, minimum inhibitory concentration, and clinical condition on bacterial eradication. Arch. Intern. Med. 1989, 149, 2269–2273. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F.; Esposito, S.; Leone, S.; Lucini, V.; Pannacci, M.; Ma, L.; Drusano, G.L. Feedback dose alteration significantly affects probability of pathogen eradication in nosocomial pneumonia. Eur. Respir. J. 2009, 34, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Le, W.J.; Li, S.; Cao, Y.P.; Su, X.H. Meta-analysis of the efficacy of moxifloxacin in treating Mycoplasma genitalium infection. Int. J. STD AIDS 2017, 28, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Noreddin, A.M.; Hoban, D.J.; Zhanel, G.G. Comparison of gatifloxacin and levofloxacin administered at various dosing regimens to hospitalised patients with community-acquired pneumonia: Pharmacodynamic target attainment study using North American surveillance data for Streptococcus pneumoniae. Int. J. Antimicrob. Agents 2005, 26, 120–125. [Google Scholar] [CrossRef]
- Haeseker, M.; Stolk, L.; Nieman, F.; Hoebe, C.; Neef, C.; Bruggeman, C.; Verbon, A. The ciprofloxacin target AUC: MIC ratio is not reached in hospitalized patients with the recommended dosing regimens. Br. J. Clin. Pharmacol. 2013, 75, 180–185. [Google Scholar] [CrossRef]
- Trang, M.; Dudley, M.N.; Bhavnani, S.M. Use of Monte Carlo simulation and considerations for PK-PD targets to support antibacterial dose selection. Curr. Opin. Pharmacol. 2017, 36, 107–113. [Google Scholar] [CrossRef]
- Rizk, M.L.; Bhavnani, S.M.; Drusano, G.; Dane, A.; Eakin, A.E.; Guina, T.; Jang, S.H.; Tomayko, J.F.; Wang, J.; Zhuang, L.; et al. Considerations for Dose Selection and Clinical Pharmacokinetics/Pharmacodynamics for the Development of Antibacterial Agents. Antimicrob. Agents Chemother. 2019, 63, e02309-18. [Google Scholar] [CrossRef]
- Plaisance, K.I.; Drusano, G.L.; Forrest, A.; Bustamante, C.I.; Standiford, H.C. Effect of dose size on bioavailability of ciprofloxacin. Antimicrob. Agents Chemother. 1987, 31, 956–958. [Google Scholar] [CrossRef]
- Chien, S.C.; Rogge, M.C.; Gisclon, L.G.; Curtin, C.; Wong, F.; Natarajan, J.; Williams, R.R.; Fowler, C.L.; Cheung, W.K.; Chow, A.T. Pharmacokinetic profile of levofloxacin following once-daily 500-milligram oral or intravenous doses. Antimicrob. Agents Chemother. 1997, 41, 2256–2260. [Google Scholar] [CrossRef]
- Stass, H.; Kubitza, D. Pharmacokinetics and elimination of moxifloxacin after oral and intravenous administration in man. J. Antimicrob. Chemother. 1999, 43 (Suppl. B), 83–90. [Google Scholar] [CrossRef]
- Nix, D.E.; Watson, W.A.; Lener, M.E.; Frost, R.W.; Krol, G.; Goldstein, H.; Lettieri, J.; Schentag, J.J. Effects of aluminum and magnesium antacids and ranitidine on the absorption of ciprofloxacin. Clin. Pharmacol. Ther. 1989, 46, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Pitman, S.K.; Hoang, U.T.P.; Wi, C.H.; Alsheikh, M.; Hiner, D.A.; Percival, K.M. Revisiting Oral Fluoroquinolone and Multivalent Cation Drug-Drug Interactions: Are They Still Relevant? Antibiotics 2019, 8, 108. [Google Scholar] [CrossRef]
- Lee, L.J.; Hafkin, B.; Lee, I.D.; Hoh, J.; Dix, R. Effects of food and sucralfate on a single oral dose of 500 milligrams of levofloxacin in healthy subjects. Antimicrob. Agents Chemother. 1997, 41, 2196–2200. [Google Scholar] [CrossRef]
- Olivera, M.E.; Manzo, R.H.; Junginger, H.E.; Midha, K.K.; Shah, V.P.; Stavchansky, S.; Dressman, J.B.; Barends, D.M. Biowaiver monographs for immediate release solid oral dosage forms: Ciprofloxacin hydrochloride. J. Pharm. Sci. 2011, 100, 22–33. [Google Scholar] [CrossRef]
- Radwan, A.; Zaid, A.N.; Jaradat, N.; Odeh, Y. Food effect: The combined effect of media pH and viscosity on the gastrointestinal absorption of ciprofloxacin tablet. Eur. J. Pharm. Sci. 2017, 101, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Calvo, R.; Lukas, J.C.; Rodriguez, M.; Leal, N.; Suarez, E. The role of unbound drug in pharmacokinetics/pharmacodynamics and in therapy. Curr. Pharm. Des. 2006, 12, 977–987. [Google Scholar] [CrossRef]
- Gonzalez, D.; Schmidt, S.; Derendorf, H. Importance of relating efficacy measures to unbound drug concentrations for anti-infective agents. Clin. Microbiol. Rev. 2013, 26, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Aminimanizani, A.; Beringer, P.; Jelliffe, R. Comparative pharmacokinetics and pharmacodynamics of the newer fluoroquinolone antibacterials. Clin. Pharmacokinet. 2001, 40, 169–187. [Google Scholar] [CrossRef]
- Fish, D.N.; Chow, A.T. The clinical pharmacokinetics of levofloxacin. Clin. Pharmacokinet. 1997, 32, 101–119. [Google Scholar] [CrossRef]
- Bergogne-Bérézin, E. Clinical role of protein binding of quinolones. Clin. Pharmacokinet. 2002, 41, 741–750. [Google Scholar] [CrossRef]
- Drusano, G.L.; Preston, S.L.; Van Guilder, M.; North, D.; Gombert, M.; Oefelein, M.; Boccumini, L.; Weisinger, B.; Corrado, M.; Kahn, J. A population pharmacokinetic analysis of the penetration of the prostate by levofloxacin. Antimicrob. Agents Chemother. 2000, 44, 2046–2051. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bulitta, J.B.; Kinzig, M.; Naber, C.K.; Wagenlehner, F.M.; Sauber, C.; Landersdorfer, C.B.; Sörgel, F.; Naber, K.G. Population pharmacokinetics and penetration into prostatic, seminal, and vaginal fluid for ciprofloxacin, levofloxacin, and their combination. Chemotherapy 2011, 57, 402–416. [Google Scholar] [CrossRef] [PubMed]
- Kiang, T.K.; Hafeli, U.O.; Ensom, M.H. A comprehensive review on the pharmacokinetics of antibiotics in interstitial fluid spaces in humans: Implications on dosing and clinical pharmacokinetic monitoring. Clin. Pharmacokinet. 2014, 53, 695–730. [Google Scholar] [CrossRef] [PubMed]
- Gergs, U.; Ihlefeld, D.; Clauss, T.; Weiss, M.; Pönicke, K.; Hofmann, G.O.; Neumann, J. Population Pharmacokinetics of Levofloxacin in Plasma and Bone of Patients Undergoing Hip or Knee Surgery. Clin. Pharmacol. Drug Dev. 2018, 7, 692–698. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.; Weidner, W.; Sörgel, F.; Naber, K.G. The role of antibiotics in chronic bacterial prostatitis. Int. J. Antimicrob. Agents 2005, 26, 1–7. [Google Scholar] [CrossRef]
- Zimmermann, E.S.; Laureano, J.V.; Dos Santos, C.N.; Schmidt, S.; Lagishetty, C.V.; de Castro, W.V.; Dalla Costa, T. Simultaneous Semimechanistic Population Analyses of Levofloxacin in Plasma, Lung, and Prostate To Describe the Influence of Efflux Transporters on Drug Distribution following Intravenous and Intratracheal Administration. Antimicrob. Agents Chemother. 2015, 60, 946–954. [Google Scholar] [CrossRef]
- Zimmermann, E.S.; de Miranda Silva, C.; Neris, C.; Torres, B.G.D.S.; Schmidt, S.; Dalla Costa, T. Population pharmacokinetic modeling to establish the role of P-glycoprotein on ciprofloxacin distribution to lung and prostate following intravenous and intratracheal administration to Wistar rats. Eur. J. Pharm. Sci. 2019, 127, 319–329. [Google Scholar] [CrossRef]
- Sadiq, M.W.; Nielsen, E.I.; Khachman, D.; Conil, J.M.; Georges, B.; Houin, G.; Laffont, C.M.; Karlsson, M.O.; Friberg, L.E. A whole-body physiologically based pharmacokinetic (WB-PBPK) model of ciprofloxacin: A step towards predicting bacterial killing at sites of infection. J. Pharmacokinet. Pharmacodyn. 2017, 44, 69–79. [Google Scholar] [CrossRef]
- Rasool, M.F.; Ali, S.; Khalid, S.; Khalid, R.; Majeed, A.; Imran, I.; Saeed, H.; Usman, M.; Ali, M.; Alali, A.S.; et al. Development and evaluation of physiologically based pharmacokinetic drug-disease models for predicting captopril pharmacokinetics in chronic diseases. Sci. Rep. 2021, 11, 8589. [Google Scholar] [CrossRef]
- Rasool, M.F.; Khalid, S.; Majeed, A.; Saeed, H.; Imran, I.; Mohany, M.; Al-Rejaie, S.S.; Alqahtani, F. Development and Evaluation of Physiologically Based Pharmacokinetic Drug-Disease Models for Predicting Rifampicin Exposure in Tuberculosis and Cirrhosis Populations. Pharmaceutics 2019, 11, 578. [Google Scholar] [CrossRef]
- Gerhart, J.G.; Carreño, F.O.; Edginton, A.N.; Sinha, J.; Perrin, E.M.; Kumar, K.R.; Rikhi, A.; Hornik, C.P.; Harris, V.; Ganguly, S.; et al. Best Pharmaceuticals for Children Act—Pediatric Trials Network Steering Committee Development and Evaluation of a Virtual Population of Children with Obesity for Physiologically Based Pharmacokinetic Modeling. Clin. Pharmacokinet. 2022, 61, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Derendorf, H.; Schmidt, S. Rowland and Tozer’s Clinical Pharmacokinetics and Pharmacodynamics: Concepts and Applications, 5th ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2019; ISBN1 1496385896. ISBN2 9781496385895. [Google Scholar]
- Allard, S.; Kinzig, M.; Boivin, G.; Sorgel, F.; LeBel, M. Intravenous ciprofloxacin disposition in obesity. Clin. Pharmacol. Ther. 1993, 54, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Cios, A.; Wyska, E.; Szymura-Oleksiak, J.; Grodzicki, T. Population pharmacokinetic analysis of ciprofloxacin in the elderly patients with lower respiratory tract infections. Exp. Gerontol. 2014, 57, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Forrest, A.; Ballow, C.H.; Nix, D.E.; Birmingham, M.C.; Schentag, J.J. Development of a population pharmacokinetic model and optimal sampling strategies for intravenous ciprofloxacin. Antimicrob. Agents Chemother. 1993, 37, 1065–1072. [Google Scholar] [CrossRef]
- Hirata, C.A.; Guay, D.R.; Awni, W.M.; Stein, D.J.; Peterson, P.K. Steady-state pharmacokinetics of intravenous and oral ciprofloxacin in elderly patients. Antimicrob. Agents Chemother. 1989, 33, 1927–1931. [Google Scholar] [CrossRef]
- Roberts, J.A.; Alobaid, A.S.; Wallis, S.C.; Perner, A.; Lipman, J.; Sjövall, F. Defining optimal dosing of ciprofloxacin in patients with septic shock. J. Antimicrob. Chemother. 2019, 74, 1662–1669. [Google Scholar] [CrossRef]
- Frost, R.W.; Lettieri, J.T.; Krol, G.; Shamblen, E.C.; Lasseter, K.C. The effect of cirrhosis on the steady-state pharmacokinetics of oral ciprofloxacin. Clin. Pharmacol. Ther. 1989, 45, 608–616. [Google Scholar] [CrossRef]
- Drusano, G.L.; Weir, M.; Forrest, A.; Plaisance, K.; Emm, T.; Standiford, H.C. Pharmacokinetics of intravenously administered ciprofloxacin in patients with various degrees of renal function. Antimicrob. Agents Chemother. 1987, 31, 860–864. [Google Scholar] [CrossRef]
- Gasser, T.C.; Ebert, S.C.; Graversen, P.H.; Madsen, P.O. Ciprofloxacin pharmacokinetics in patients with normal and impaired renal function. Antimicrob. Agents Chemother. 1987, 31, 709–712. [Google Scholar] [CrossRef]
- Abdulla, A.; Rogouti, O.; Hunfeld, N.G.M.; Endeman, H.; Dijkstra, A.; van Gelder, T.; Muller, A.E.; de Winter, B.C.M.; Koch, B.C.P. Population pharmacokinetics and target attainment of ciprofloxacin in critically ill patients. Eur. J. Clin. Pharmacol. 2020, 76, 957–967. [Google Scholar] [CrossRef]
- Gieling, E.M.; Wallenburg, E.; Frenzel, T.; de Lange, D.W.; Schouten, J.A.; Ten Oever, J.; Kolwijck, E.; Burger, D.M.; Pickkers, P.; Ter Heine, R.; et al. Higher Dosage of Ciprofloxacin Necessary in Critically Ill Patients: A New Dosing Algorithm Based on Renal Function and Pathogen Susceptibility. Clin. Pharmacol. Ther. 2020, 108, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Preston, S.L.; Drusano, G.L.; Berman, A.L.; Fowler, C.L.; Chow, A.T.; Dornseif, B.; Reichl, V.; Natarajan, J.; Corrado, M. Pharmacodynamics of levofloxacin: A new paradigm for early clinical trials. JAMA 1998, 279, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.M.; Martin, C.; Adams, V.R.; Morehead, R.S. Pharmacokinetics of intravenous levofloxacin administered at 750 milligrams in obese adults. Antimicrob. Agents Chemother. 2011, 55, 3240–3243. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.P.; Cojutti, P.; Pea, F. Levofloxacin dosing regimen in severely morbidly obese patients (BMI >/=40 kg/m(2)) should be guided by creatinine clearance estimates based on ideal body weight and optimized by therapeutic drug monitoring. Clin. Pharmacokinet. 2014, 53, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Stass, H.; Dalhoff, A.; Kubitza, D.; Schuhly, U. Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a new 8-methoxy quinolone, administered to healthy subjects. Antimicrob. Agents Chemother. 1998, 42, 2060–2065. [Google Scholar] [CrossRef]
- Grosjean, P.; Urien, S. Reevaluation of moxifloxacin pharmacokinetics and their direct effect on the QT interval. J. Clin. Pharmacol. 2012, 52, 329–338. [Google Scholar] [CrossRef]
- Kees, M.G.; Weber, S.; Kees, F.; Horbach, T. Pharmacokinetics of moxifloxacin in plasma and tissue of morbidly obese patients. J. Antimicrob. Chemother. 2011, 66, 2330–2335. [Google Scholar] [CrossRef]
- de Vroom, S.L.; van Hest, R.M.; van Daalen, F.V.; Kuil, S.D.; Mathôt, R.A.A.; Geerlings, S.E.; Jager, N.G.L. Pharmacokinetic/pharmacodynamic target attainment of ciprofloxacin in adult patients on general wards with adequate and impaired renal function. Int. J. Antimicrob. Agents 2020, 56, 106166. [Google Scholar] [CrossRef]
- Drusano, G.L.; Plaisance, K.I.; Forrest, A.; Standiford, H.C. Dose ranging study and constant infusion evaluation of ciprofloxacin. Antimicrob. Agents Chemother. 1986, 30, 440–443. [Google Scholar] [CrossRef]
- Roberts, J.A.; Cotta, M.O.; Cojutti, P.; Lugano, M.; Della Rocca, G.; Pea, F. Does Critical Illness Change Levofloxacin Pharmacokinetics? Antimicrob. Agents Chemother. 2015, 60, 1459–1463. [Google Scholar] [CrossRef]
- Roger, C.; Wallis, S.C.; Louart, B.; Lefrant, J.Y.; Lipman, J.; Muller, L.; Roberts, J.A. Comparison of equal doses of continuous venovenous haemofiltration and haemodiafiltration on ciprofloxacin population pharmacokinetics in critically ill patients. J. Antimicrob. Chemother. 2016, 71, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zoller, M.; Fuhr, U.; Huseyn-Zada, M.; Maier, B.; Vogeser, M.; Zander, J.; Taubert, M. Ciprofloxacin in critically ill subjects: Considering hepatic function, age and sex to choose the optimal dose. J. Antimicrob. Chemother. 2019, 74, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Eloy, G.; Lebeaux, D.; Launay, M.; Fernandez-Gerlinger, M.P.; Billaud, E.; Douez, E.; Mainardi, J.L.; Bouyer, B.; Jullien, V. Influence of Renal Function and Age on the Pharmacokinetics of Levofloxacin in Patients with Bone and Joint Infections. Antibiotics 2020, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Peloquin, C.A.; Hadad, D.J.; Molino, L.P.; Palaci, M.; Boom, W.H.; Dietze, R.; Johnson, J.L. Population pharmacokinetics of levofloxacin, gatifloxacin, and moxifloxacin in adults with pulmonary tuberculosis. Antimicrob. Agents Chemother. 2008, 52, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, J.F.; Liu, Y.B.; Xiao, Z.K.; Huang, J.A.; Si, B.; Sun, S.H.; Xia, Q.M.; Wu, X.J.; Cao, G.Y.; et al. Population pharmacokinetics of oral levofloxacin 500 mg once-daily dosage in community-acquired lower respiratory tract infections: Results of a prospective multicenter study in China. J. Infect. Chemother. 2009, 15, 293–300. [Google Scholar] [CrossRef]
- Kiem, S.; Ryu, S.M.; Lee, Y.M.; Schentag, J.J.; Kim, Y.W.; Kim, H.K.; Jang, H.J.; Joo, Y.D.; Jin, K.; Shin, J.G.; et al. Population pharmacokinetics of levofloxacin in Korean patients. J. Chemother. 2016, 28, 308–313. [Google Scholar] [CrossRef]
- Chien, S.C.; Chow, A.T.; Natarajan, J.; Williams, R.R.; Wong, F.A.; Rogge, M.C.; Nayak, R.K. Absence of age and gender effects on the pharmacokinetics of a single 500-milligram oral dose of levofloxacin in healthy subjects. Antimicrob. Agents Chemother. 1997, 41, 1562–1565. [Google Scholar] [CrossRef][Green Version]
- Kervezee, L.; Stevens, J.; Birkhoff, W.; Kamerling, I.M.; de Boer, T.; Dröge, M.; Meijer, J.H.; Burggraaf, J. Identifying 24 h variation in the pharmacokinetics of levofloxacin: A population pharmacokinetic approach. Br. J. Clin. Pharmacol. 2016, 81, 256–268. [Google Scholar] [CrossRef]
- Nomura, K.; Fujimoto, Y.; Morimoto, Y.; Kanbayashi, Y.; Matsumoto, Y.; Taniwaki, M. Population pharmacokinetics of levofloxacin as prophylaxis for febrile neutropenia. Intern. Med. 2008, 47, 375–378. [Google Scholar] [CrossRef][Green Version]
- van den Elsen, S.H.J.; Sturkenboom, M.G.G.; Van’t Boveneind-Vrubleuskaya, N.; Skrahina, A.; van der Werf, T.S.; Heysell, S.K.; Mpagama, S.; Migliori, G.B.; Peloquin, C.A.; Touw, D.J.; et al. Population Pharmacokinetic Model and Limited Sampling Strategies for Personalized Dosing of Levofloxacin in Tuberculosis Patients. Antimicrob. Agents Chemother. 2018, 62, e01092-18. [Google Scholar] [CrossRef]
- Cao, G.; Zhang, J.; Wu, X.; Yu, J.; Chen, Y.; Ye, X.; Zhu, D.; Zhang, Y.; Guo, B.; Shi, Y. Pharmacokinetics and pharmacodynamics of levofloxacin injection in healthy Chinese volunteers and dosing regimen optimization. J. Clin. Pharm. Ther. 2013, 38, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Cojutti, P.G.; Ramos-Martin, V.; Schiavon, I.; Rossi, P.; Baraldo, M.; Hope, W.; Pea, F. Population Pharmacokinetics and Pharmacodynamics of Levofloxacin in Acutely Hospitalized Older Patients with Various Degrees of Renal Function. Antimicrob. Agents Chemother. 2017, 61, e02134-16. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Kobayashi, M.; Ono, Y.; Mizugaki, A.; Katabami, K.; Maekawa, K.; Miyamoto, D.; Yanagida, Y.; Hayakawa, M.; Sawamura, A.; et al. Pharmacokinetics and the optimal regimen for levofloxacin in critically ill patients receiving continuous hemodiafiltration. J. Intensive Care. 2015, 3, 22. [Google Scholar] [CrossRef]
- Canouï, E.; Kerneis, S.; Morand, P.; Enser, M.; Gauzit, R.; Eyrolle, L.; Leclerc, P.; Contejean, A.; Zheng, Y.; Anract, P.; et al. Oral levofloxacin: Population pharmacokinetics model and pharmacodynamics study in bone and joint infections. J. Antimicrob. Chemother. 2022, 77, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Malone, R.S.; Fish, D.N.; Abraham, E.; Teitelbaum, I. Pharmacokinetics of levofloxacin and ciprofloxacin during continuous renal replacement therapy in critically ill patients. Antimicrob. Agents Chemother. 2001, 45, 2949–2954. [Google Scholar] [CrossRef]
- Guenter, S.G.; Iven, H.; Boos, C.; Bruch, H.P.; Muhl, E. Pharmacokinetics of levofloxacin during continuous venovenous hemodiafiltration and continuous venovenous hemofiltration in critically ill patients. Pharmacotherapy 2002, 22, 175–183. [Google Scholar] [CrossRef]
- Hansen, E.; Bucher, M.; Jakob, W.; Lemberger, P.; Kees, F. Pharmacokinetics of levofloxacin during continuous veno-venous hemofiltration. Intensive Care Med. 2001, 27, 371–375. [Google Scholar] [CrossRef]
- Czock, D.; Husig-Linde, C.; Langhoff, A.; Schopke, T.; Hafer, C.; de Groot, K.; Swoboda, S.; Kuse, E.; Haller, H.; Fliser, D.; et al. Pharmacokinetics of moxifloxacin and levofloxacin in intensive care unit patients who have acute renal failure and undergo extended daily dialysis. Clin. J. Am. Soc. Nephrol. 2006, 1, 1263–1268. [Google Scholar] [CrossRef]
- Sullivan, J.T.; Woodruff, M.; Lettieri, J.; Agarwal, V.; Krol, G.J.; Leese, P.T.; Watson, S.; Heller, A.H. Pharmacokinetics of a once-daily oral dose of moxifloxacin (Bay 12-8039), a new enantiomerically pure 8-methoxy quinolone. Antimicrob. Agents Chemother. 1999, 43, 2793–2797. [Google Scholar] [CrossRef][Green Version]
- Nightingale, C.H. Moxifloxacin, a new antibiotic designed to treat community-acquired respiratory tract infections: A review of microbiologic and pharmacokinetic-pharmacodynamic characteristics. Pharmacotherapy 2000, 20, 245–256. [Google Scholar] [CrossRef]
- Barth, J.; Jager, D.; Mundkowski, R.; Drewelow, B.; Welte, T.; Burkhardt, O. Single- and multiple-dose pharmacokinetics of intravenous moxifloxacin in patients with severe hepatic impairment. J. Antimicrob. Chemother. 2008, 62, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Sionidou, M.; Manika, K.; Pitsiou, G.; Kontou, P.; Chatzika, K.; Zarogoulidis, P.; Kioumis, I. Moxifloxacin in Chronic Obstructive Pulmonary Disease: Pharmacokinetics and Penetration into Bronchial Secretions in Ward and Intensive Care Unit Patients. Antimicrob. Agents Chemother. 2019, 63, e01974-18. [Google Scholar] [CrossRef]
- Tokimatsu, I.; Shigemura, K.; Kotaki, T.; Yoshikawa, H.; Yamamichi, F.; Tomo, T.; Arakawa, S.; Fujisawa, M.; Kadota, J.I. A Prospective Study of the Efficacy, Safety and Pharmacokinetics of Enteral Moxifloxacin in the Treatment of Hemodialysis Patients with Pneumonia. Intern. Med. 2017, 56, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Hasunuma, T.; Tohkin, M.; Kaniwa, N.; Jang, I.J.; Yimin, C.; Kaneko, M.; Saito, Y.; Takeuchi, M.; Watanabe, H.; Yamazoe, Y.; et al. Absence of ethnic differences in the pharmacokinetics of moxifloxacin, simvastatin, and meloxicam among three East Asian populations and Caucasians. Br. J. Clin. Pharmacol. 2016, 81, 1078–1090. [Google Scholar] [CrossRef] [PubMed]
- Bulik, C.C.; Bader, J.C.; Zhang, L.; Van Wart, S.A.; Rubino, C.M.; Bhavnani, S.M.; Sweeney, K.L.; Ambrose, P.G. PK-PD Compass: Bringing infectious diseases pharmacometrics to the patient’s bedside. J. Pharmacokinet. Pharmacodyn. 2017, 44, 161–177. [Google Scholar] [CrossRef]
- Täubel, J.; Prasad, K.; Rosano, G.; Ferber, G.; Wibberley, H.; Cole, S.T.; Van Langenhoven, L.; Fernandes, S.; Djumanov, D.; Sugiyama, A. Effects of the Fluoroquinolones Moxifloxacin and Levofloxacin on the QT Subintervals: Sex Differences in Ventricular Repolarization. J. Clin. Pharmacol. 2020, 60, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Bidell, M.R.; Lodise, T.P. Fluoroquinolone-Associated Tendinopathy: Does Levofloxacin Pose the Greatest Risk? Pharmacotherapy 2016, 36, 679–693. [Google Scholar] [CrossRef]
- Cicali, B.; Lingineni, K.; Cristofoletti, R.; Wendl, T.; Hoechel, J.; Wiesinger, H.; Chaturvedula, A.; Vozmediano, V.; Schmidt, S. Quantitative Assessment of Levonorgestrel Binding Partner Interplay and Drug-Drug Interactions Using Physiologically Based Pharmacokinetic Modeling. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 48–58. [Google Scholar] [CrossRef]
- Kim, C.; Lo Re, V.; Rodriguez, M.; Lukas, J.C.; Leal, N.; Campo, C.; García-Bea, A.; Suarez, E.; Schmidt, S.; Vozmediano, V. Application of a dual mechanistic approach to support bilastine dose selection for older adults. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 1006–1017. [Google Scholar] [CrossRef]
- Li, J.; Roberts, J.; Working Group of Anti-infective Pharmacology of the International Society of Antimicrobial Chemotherapy (ISAC). Antibiotic pharmacokinetics/pharmacodynamics: Where are we heading? Int. J. Antimicrob. Agents 2021, 58, 106369. [Google Scholar] [CrossRef]
- Magri, V.; Boltri, M.; Cai, T.; Colombo, R.; Cuzzocrea, S.; De Visschere, P.; Giuberti, R.; Granatieri, C.M.; Latino, M.A.; Larganà, G.; et al. Multidisciplinary approach to prostatitis. Arch. Ital. Urol. Androl. 2019, 90, 227–248. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing. Antimicrobial Wild Type Distribution of Microorganism. Available online: https://mic.eucast.org/Eucast2/ (accessed on 15 March 2022).
- Clinical Laboratory Standards Institute. Available online: https://clsi.org/ (accessed on 15 March 2022).
- Dalhoff, A. Global fluoroquinolone resistance epidemiology and implictions for clinical use. Interdiscip. Perspect. Infect. Dis. 2012, 2012, 976273. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmeister, A.S.; Jones, R.N. The Importance of Antimicrobial Resistance Monitoring Worldwide and the Origins of SENTRY Antimicrobial Surveillance Program. Open Forum. Infect. Dis. 2019, 6, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- National Healthcare Safety Network (NHSN). CDC Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Healthcare Quality Promotion (DHQP). Available online: https://www.cdc.gov/nhsn/index.html (accessed on 19 March 2022).
- European Antimicrobial Resistance Surveillance Network (EARS-Net). European Centre for Disease Prevention and Control. Available online: https://www.ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/ears-net (accessed on 19 March 2021).
- Shortridge, D.; Gales, A.C.; Streit, J.M.; Huband, M.D.; Tsakris, A.; Jones, R.N. Geographic and Temporal Patterns of Antimicrobial Resistance in Pseudomonas aeruginosa Over 20 Years From the SENTRY Antimicrobial Surveillance Program, 1997–2016. Open Forum Infect. Dis. 2019, 6, S63–S68. [Google Scholar] [CrossRef] [PubMed]
- Stewardson, A.J.; Vervoort, J.; Adriaenssens, N.; Coenen, S.; Godycki-Cwirko, M.; Kowalczyk, A.; Huttner, B.D.; Lammens, C.; Malhotra-Kumar, S.; Goossens, H.; et al. Study Group Effect of outpatient antibiotics for urinary tract infections on antimicrobial resistance among commensal Enterobacteriaceae: A multinational prospective cohort study. Clin. Microbiol. Infect. 2018, 24, 972–979. [Google Scholar] [CrossRef]
- Critchley, I.A.; Cotroneo, N.; Pucci, M.J.; Mendes, R. The burden of antimicrobial resistance among urinary tract isolates of Escherichia coli in the United States in 2017. PLoS ONE 2019, 14, e0220265. [Google Scholar] [CrossRef]
- Wagenlehner, F. Urogenital infections. World J. Urol. 2020, 38, 1–2. [Google Scholar] [CrossRef]
- Sader, H.S.; Farrell, D.J.; Flamm, R.K.; Jones, R.N. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalized in intensive care units in United States and European hospitals (2009–2011). Diagn. Microbiol. Infect. Dis. 2014, 78, 443–448. [Google Scholar] [CrossRef]
- de Kraker, M.E.; Jarlier, V.; Monen, J.C.; Heuer, O.E.; van de Sande, N.; Grundmann, H. The changing epidemiology of bacteraemias in Europe: Trends from the European Antimicrobial Resistance Surveillance System. Clin. Microbiol. Infect. 2013, 19, 860–868. [Google Scholar] [CrossRef]
- Valero, A.; Isla, A.; Rodríguez-Gascón, A.; Calvo, B.; Canut, A.; Solinís, M.Á. Pharmacokinetic/pharmacodynamic analysis as a tool for surveillance of the activity of antimicrobials against Pseudomonas aeruginosa strains isolated in critically ill patients. Enferm. Infecc. Microbiol. Clin. 2019, 37, 380–386. [Google Scholar] [CrossRef]
- Valero, A.; Rodríguez-Gascón, A.; Isla, A.; Barrasa, H.; Del Barrio-Tofiño, E.; Oliver, A.; Canut, A.; Solinís, M.Á. Pseudomonas aeruginosa Susceptibility in Spain: Antimicrobial Activity and Resistance Suppression Evaluation by PK/PD Analysis. Pharmaceutics 2021, 13, 1899. [Google Scholar] [CrossRef] [PubMed]
- Kandil, H.; Cramp, E.; Vaghela, T. Trends in Antibiotic Resistance in Urologic Practice. Eur. Urol. Focus 2016, 2, 363–373. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estradé, O.; Vozmediano, V.; Carral, N.; Isla, A.; González, M.; Poole, R.; Suarez, E. Key Factors in Effective Patient-Tailored Dosing of Fluoroquinolones in Urological Infections: Interindividual Pharmacokinetic and Pharmacodynamic Variability. Antibiotics 2022, 11, 641. https://doi.org/10.3390/antibiotics11050641
Estradé O, Vozmediano V, Carral N, Isla A, González M, Poole R, Suarez E. Key Factors in Effective Patient-Tailored Dosing of Fluoroquinolones in Urological Infections: Interindividual Pharmacokinetic and Pharmacodynamic Variability. Antibiotics. 2022; 11(5):641. https://doi.org/10.3390/antibiotics11050641
Chicago/Turabian StyleEstradé, Oskar, Valvanera Vozmediano, Nerea Carral, Arantxa Isla, Margarita González, Rachel Poole, and Elena Suarez. 2022. "Key Factors in Effective Patient-Tailored Dosing of Fluoroquinolones in Urological Infections: Interindividual Pharmacokinetic and Pharmacodynamic Variability" Antibiotics 11, no. 5: 641. https://doi.org/10.3390/antibiotics11050641
APA StyleEstradé, O., Vozmediano, V., Carral, N., Isla, A., González, M., Poole, R., & Suarez, E. (2022). Key Factors in Effective Patient-Tailored Dosing of Fluoroquinolones in Urological Infections: Interindividual Pharmacokinetic and Pharmacodynamic Variability. Antibiotics, 11(5), 641. https://doi.org/10.3390/antibiotics11050641






