TEM,CTX-M,SHV Genes in ESBL-Producing Escherichia coli and Klebsiella pneumoniae Isolated from Clinical Samples in a County Clinical Emergency Hospital Romania-Predominance of CTX-M-15
Abstract
1. Introduction
2. Material and Methods
2.1. Bacterial Strains
2.2. Antimicrobial Susceptibility Testing
2.3. Molecular Testing
2.4. Statistical Analysis
3. Results
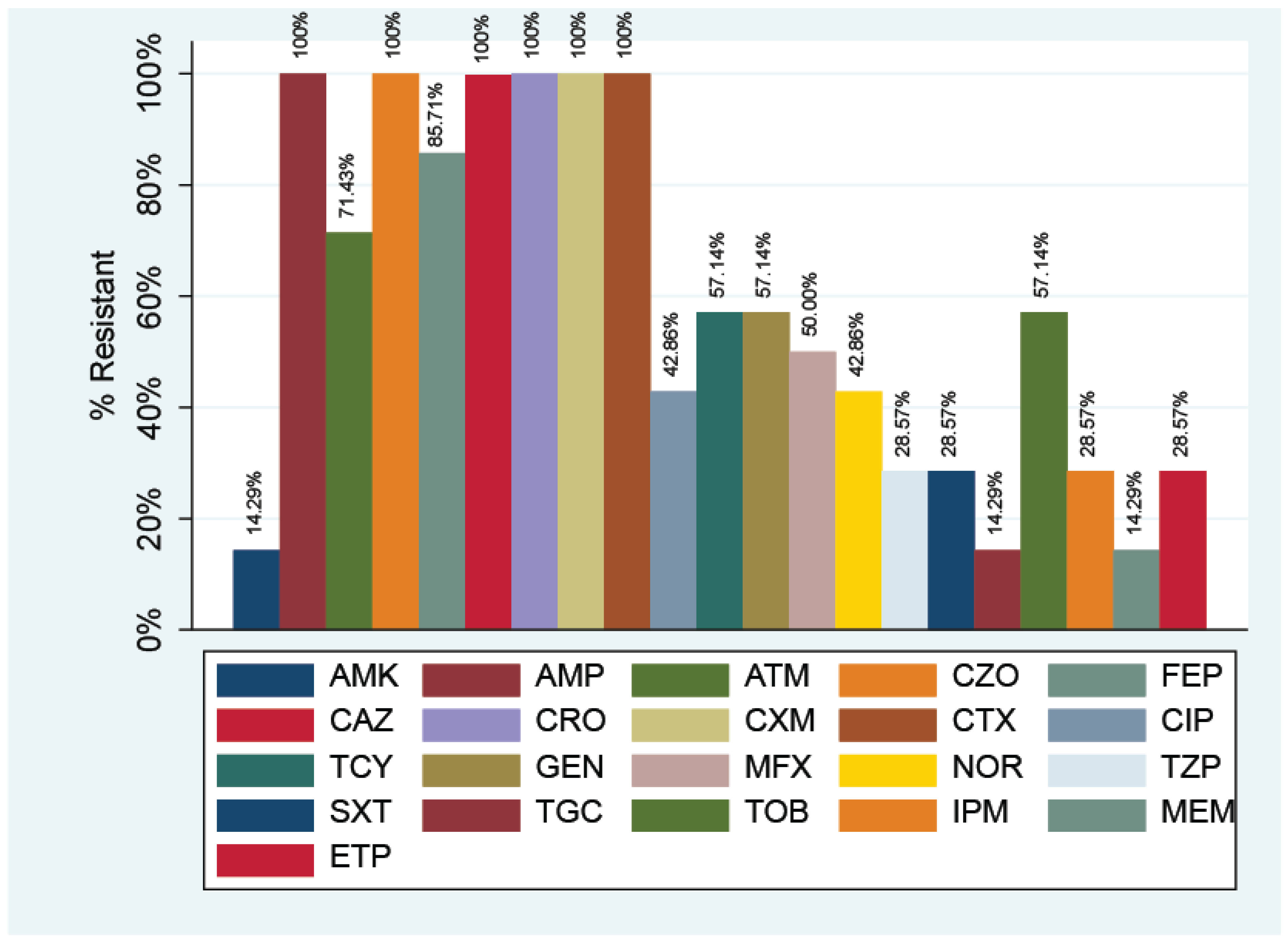
3.1. Antibiotic Resistance in E. coli
3.2. Genetic Characteristics of Multidrug-Resistant E. coli Strains
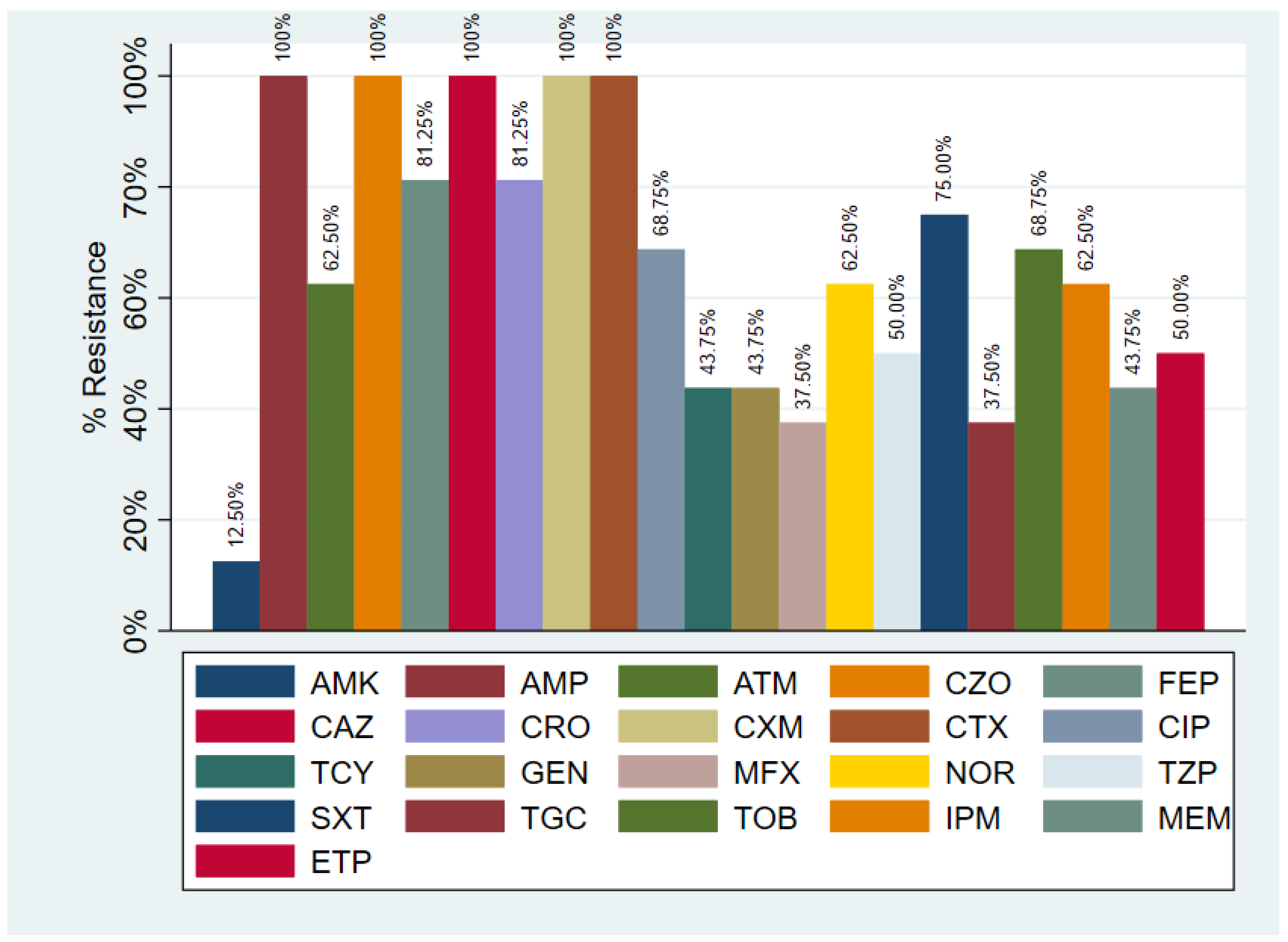
3.3. Antibiotic Resistance in K. pneumoniae
3.4. Genetic Characteristics of the K. pneumoniae Strains
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capelo-Martínez, J.L.; Igrejas, G. Antibiotic Drug Resistance; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 1–692. [Google Scholar]
- Carvalho, I.; Silva, N.; Carrola, J.; Silva, V.; Currie, C.; Igrejas, G.; Poeta, P. Antibiotic Resistance; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Chung, Y.S.; Hu, Y.S.; Shin, S.; Lim, S.K.; Yang, S.J.; Park, Y.H.; Park, K.T. Mechanisms of quinolone resistance in Escherichia coli isolated from companion animals, pet-owners, and non-pet-owners. J. Vet. Sci. 2017, 18, 449. [Google Scholar] [CrossRef] [PubMed]
- Caneiras, C.S.G.; Alises, S.M.; Lito, L.; Cristino, J.M.; Duarte, A. Molecular epidemiology of Klebsiella pneumoniae: Multiclonal dissemination of CTX-M-15 extended spectrum ß-lactamase. Eur. Respir. J. 2018, 52, PA3912. [Google Scholar]
- Caneiras, C.; Lito, L.; Melo-Cristino, J.; Duarte, A. Community- and hospital-acquired Klebsiella pneumoniae urinary tract infections in Portugal: Virulence and antibiotic resistance. Microorganisms 2019, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.; Chenouf, N.S.; Carvalho, J.A.; Castro, A.P.; Silva, V.; Capita, R.; Alonso-Calleja, C.; de Lurdes Nunes Enes Dapkevicius, M.; Igrejas, G.; Torres, C.; et al. Multidrug-resistant Klebsiella pneumoniae harboring extended spectrum β-lactamase encoding genes isolated from human septicemias. PLoS ONE 2021, 16, e0250525. [Google Scholar] [CrossRef]
- Knothe, H.; Shah, P.; Krcmery, V.; Antal, M.; Mitsuhashi, S. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection 1983, 11, 315–317. [Google Scholar] [CrossRef]
- Bradford, P.A. Extended-spectrum beta-lactamases in the 21st century: Characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 2001, 14, 933–951. [Google Scholar] [CrossRef]
- Bush, K. New beta-lactamases in gram-negative bacteria: Diversity and impact on the selection of antimicrobial therapy. Clin. Infect. Dis. 2001, 32, 1085–1089. [Google Scholar]
- Coudron, P.E.; Moland, E.S.; Sanders, C.C. Occurrence and detection of extended-spectrum beta-lactamases in members of the family Enterobacteriaceae at a veterans medical center: Seek and you may find. J. Clin. Microbiol. 1997, 35, 2593–2597. [Google Scholar] [CrossRef]
- Bush, K.; Fisher, J. Epidemiological expansion, structural studies, and clinical challenges of new β-lactamases from gram-negative bacteria. Annu. Rev. Microbiol. 2011, 65, 455–478. [Google Scholar] [CrossRef]
- Paterson, D.L.; Bonomo, R.A. Extended-spectrum beta-lactamases: A clinical update. Clin. Microbiol. Rev. 2005, 18, 657–686. [Google Scholar] [CrossRef]
- Canton, R.; Coque, T.M. The CTX-M beta-lactamase pandemic. Curr. Opin. Microbiol. 2006, 9, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Ghafourian, S.; Sadeghifard, N.; Soheili, S.; Sekawi, Z. Extended spectrum beta-lactamases: Definition, classification and epidemiology. Curr. Issues Mol. Biol. 2015, 17, 11–12. [Google Scholar] [PubMed]
- Bonnet, R. Growing group of extended-spectrum beta-lactamases: The CTX-M enzymes. Antimicrob. Agents Chemother. 2004, 48, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, S.; Shrestha, P.; Adhikari, B. Antimicrobial use in food animals and human health: Time to implement ‘One Health’ approach. Antimicrob. Resist. Infect. Control 2020, 9, 1–5. [Google Scholar] [CrossRef]
- Raut, S.; Adhikari, B. Global leadership against antimicrobial resistance ought to include developing countries. Lancet. Infect. Dis. 2016, 16, 775. [Google Scholar] [CrossRef][Green Version]
- Pokharel, S.; Raut, S.; Adhikari, B. Tackling antimicrobial resistance in low-income and middle-income countries. BMJ Glob. Health 2019, 4, e002104. [Google Scholar] [CrossRef]
- Raya, G.B.; Dhoubhadel, B.G.; Shrestha, D.; Raya, S.; Laghu, U.; Shah, A.; Raya, B.B.; Kafle, R.; Parry, C.M.; Ariyoshi, K. Multidrug-resistant and extended-spectrum beta-lactamase-producing uropathogens in children in Bhaktapur, Nepal. Trop. Med. Health 2020, 6, 487. [Google Scholar] [CrossRef]
- M100Ed32|Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; CLSI: Malvern, PA, USA, 2022; Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 1 March 2022).
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Monstein, H.J.; Östholm-Balkhed, Å.; Nilsson, M.V.; Nilsson, M.; Dornbusch, K.; Nilsson, L.E. Multiplex PCR amplification assay for the detection of blaSHV, blaTEM and blaCTX-M genes in Enterobacteriaceae. APMIS 2007, 115, 1400–1408. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, Y.; Chan, E.W.C.; Zhou, H.; Chen, S. Dissemination of the mcr-1 colistin resistance gene. Lancet. Infect. Dis. 2016, 16, 291–292. [Google Scholar] [CrossRef]
- Adelowo, O.O.; Fagade, O.E. The tetracycline resistance gene tet39 is present in both Gram-negative and Gram-positive bacteria from a polluted river, Southwestern Nigeria. Lett. Appl. Microbiol. 2009, 48, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Denton, M. Enterobacteriaceae. Int. J. Antimicrob. Agents 2007, 29 (Suppl. S3), S9–S22. [Google Scholar] [CrossRef]
- Oberoi, L.; Singh, N.; Sharma, P.; Aggarwal, A. ESBL, MBL and AMPC β lactamases producing superbugs—Havoc in the intensive care units of Punjab India. J. Clin. Diagn. Res. 2013, 7, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Cantón, R.; Bryan, J. Global antimicrobial resistance: From surveillance to stewardship. Part 1: Surveillance and risk factors for resistance. Expert Rev. Anti. Infect. Ther. 2012, 10, 1269–1271. [Google Scholar] [CrossRef]
- Peirano, G.; Pitout, J.D.D. Extended-spectrum β-lactamase-producing Enterobacteriaceae: Update on molecular epidemiology and treatment options. Drugs 2019, 79, 1529–1541. [Google Scholar] [CrossRef]
- Jean, S.S.; Hsueh, P.R. High burden of antimicrobial resistance in Asia. Int. J. Antimicrob. Agents 2011, 37, 291–295. [Google Scholar] [CrossRef]
- Bonelli, R.R.; Moreira, B.M.; Picão, R.C. Antimicrobial resistance among Enterobacteriaceae in South America: History, current dissemination status and associated socioeconomic factors. Drug Resist. Updat. 2014, 17, 24–36. [Google Scholar] [CrossRef]
- Leopold, S.J.; van Leth, F.; Tarekegn, H.; Schultsz, C. Antimicrobial drug resistance among clinically relevant bacterial isolates in sub-Saharan Africa: A systematic review. J. Antimicrob. Chemother. 2014, 69, 2337–2353. [Google Scholar] [CrossRef]
- Sonda, T.; Kumburu, H.; van Zwetselaar, M.; Alifrangis, M.; Lund, O.; Kibiki, G.; Aarestrup, F.M. Meta-analysis of proportion estimates of Extended-Spectrum-Beta-Lactamase-producing Enterobacteriaceae in East Africa hospitals. Antimicrob. Resist. Infect. Control 2016, 5, 1–9. [Google Scholar] [CrossRef]
- Chong, Y.; Shimoda, S.; Shimono, N. Current epidemiology, genetic evolution and clinical impact of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect. Genet. Evol. 2018, 61, 185–188. [Google Scholar] [CrossRef]
- Rus, M.; Licker, M.; Musuroi, C.; Seclaman, E.; Muntean, D.; Cirlea, N.; Tamas, A.; Vulpie, S.; Horhat, F.G.; Baditoiu, L. Distribution of NDM1 carbapenemase-producing proteeae strains on high-risk hospital wards. Infect. Drug Resist. 2020, 13, 4751–4761. [Google Scholar] [CrossRef] [PubMed]
- Axente, C.; Licker, M.; Moldovan, R.; Hogea, E.; Muntean, D.; Horhat, F.; Bedreag, O.; Sandesc, D.; Papurica, M.; Dugaesescu, D.; et al. Antimicrobial consumption, costs and resistance patterns: A two year prospective study in a Romanian intensive care unit. BMC Infect. Dis. 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cristea, O.M.; Zlatian, O.M.; Dinescu, S.N.; Bălăşoiu, A.T.; Avramescu, C.; Bălăşoiu, M.; Niculescu, M.; Calina, D. A Comparative Study on Antibiotic Resistance of Klebsiella Strains from Surgical and Intensive Care Wards. Curr. Health Sci. J. 2016, 42, 169–179. [Google Scholar] [PubMed]
- Ghenea, A.E.; Cioboată, R.; Drocaş, A.I.; Țieranu, E.N.; Vasile, C.M.; Moroşanu, A.; Țieranu, C.G.; Salan, A.-I.; Popescu, M.; Turculeanu, A.; et al. Prevalence and antimicrobial resistance of Klebsiella strains isolated from a county hospital in Romania. Antibiotics 2021, 10, 868. [Google Scholar] [CrossRef]
- Bradford, P.A.; Cherubin, C.E.; Idemyor, V.; Rasmussen, B.A.; Bush, K. Multiply resistant Klebsiella pneumoniae strains from two Chicago hospitals: Identification of the extended-spectrum TEM-12 and TEM-10 ceftazidime-hydrolyzing beta-lactamases in a single isolate. Antimicrob. Agents Chemother. 1994, 38, 761–766. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Champs, C.; Sirot, D.; Chanal, C.; Poupart, M.C.; Dumas, M.P.; Sirot, J. Concomitant dissemination of three extended-spectrum beta-lactamases among different Enterobacteriaceae isolated in a French hospital. J. Antimicrob. Chemother. 1991, 27, 441–457. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kwon, Y.; Pai, H.; Kim, J.W.; Cho, D.T. Survey of Klebsiella pneumoniae strains producing extended-spectrum beta-lactamases: Prevalence of SHV-12 and SHV-2a in Korea. J. Clin. Microbiol. 1998, 36, 1446–1449. [Google Scholar] [CrossRef]
- Pałucha, A.; Mikiewicz, B.; Hryniewicz, W.; Gniadkowski, M. Concurrent outbreaks of extended-spectrum beta-lactamase-producing organisms of the family Enterobacteriaceae in a Warsaw hospital. J. Antimicrob. Chemother. 1999, 44, 489–499. [Google Scholar] [CrossRef][Green Version]
- Rosenthal, V.D.; Duszynska, W.; Ider, B.E.; Gurskis, V.; Al-Ruzzieh, M.A.; Myatra, S.N.; Gupta, D.; Belkebir, S.; Upadhyay, N.; Zand, F.; et al. International nosocomial infection control consortium (INICC) report, data summary of 45 countries for 2013–2018, adult and pediatric units, device-associated module. Am. J. Infect. Control 2021, 49, 1267–1274. [Google Scholar] [CrossRef]
- Rodloff, A.C.; Goldstein, E.J.C.; Torres, A. Two decades of imipenem therapy. J. Antimicrob. Chemother. 2006, 58, 916–929. [Google Scholar] [CrossRef]
- Wu, M.J.; Feng, Y.S.; Sung, W.P.; Surampalli, R.Y. Quantification and analysis of airborne bacterial characteristics in a nursing care institution. J. Air Waste Manag. Assoc. 2011, 61, 732–739. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Almsick, V.; Schuler, F.; Mellmann, A.; Schwierzeck, V. The use of long-read sequencing technologies in infection control: Horizontal transfer of a blaCTX-M-27 containing lncFII plasmid in a patient screening sample. Microorganisms 2022, 10, 491. [Google Scholar] [CrossRef] [PubMed]
- Vubil, D.; Figueiredo, R.; Reis, T.; Canha, C.; Boaventura, L.; Da Silva, G.J. Outbreak of KPC-3-producing ST15 and ST348 Klebsiella pneumoniae in a Portuguese hospital. Epidemiol. Infect. 2017, 145, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Schaufler, K.; Semmler, T.; Wieler, L.H.; Wöhrmann, M.; Baddam, R.; Ahmed, N.; Müller, K.; Kola, A.; Fruth, A.; Ewers, C.; et al. Clonal spread and interspecies transmission of clinically relevant ESBL-producing Escherichia coli of ST410—Another successful pandemic clone? FEMS Microbiol. Ecol. 2016, 92, fiv155. [Google Scholar] [CrossRef]
- Louka, C.; Ravensbergen, S.J.; Ott, A.; Zhou, X.; Garcia-Cobos, S.; Friedrich, A.W.; Pournaras, S.; Rosema, S.; Rossen, J.W.; Stienstra, Y.; et al. Predominance of CTX-M-15-producing ST131 strains among ESBL-producing Escherichia coli isolated from asylum seekers in the Netherlands. J. Antimicrob. Chemother. 2021, 76, 70–76. [Google Scholar] [CrossRef]
- Licker, M.; Baditoiu, L.; Muntean, D.; Dragomirescu, L.; Horhat, F.; Piluţ, C.; Hogea, E.; Deutsch, P.; Adam, A.; Moldovan, R. Surveillance of multi-drug resistant pathogens in two Romanian university hospitals. Int. J. Infect. Control 2012, 8, 4. [Google Scholar] [CrossRef][Green Version]
- Poulou, A.; Grivakou, E.; Vrioni, G.; Koumaki, V.; Pittaras, T.; Pournaras, S.; Tsakris, A. Modified CLSI Extended-Spectrum β-Lactamase (ESBL) Confirmatory Test for Phenotypic Detection of ESBLs among Enterobacteriaceae Producing Various β-Lactamases. J. Clin. Microbiol. 2014, 52, 1483. [Google Scholar] [CrossRef]
- Hanberger, H.; Edlund, C.; Furebring, M.; Giske, C.G.; Melhus, Å.; Nilsson, L.E.; Petersson, J.; Sjölin, J.; Ternhag, A.; Werner, M.; et al. Rational use of aminoglycosides—Review and recommendations by the Swedish reference group for antibiotics (SRGA). Scand. J. Infect. Dis. 2013, 45, 161–175. [Google Scholar] [CrossRef]
- Leibovici, L.; Vidal, L.; Paul, M. Aminoglycoside drugs in clinical practice: An evidence-based approach. J. Antimicrob. Chemother. 2009, 63, 246–251. [Google Scholar] [CrossRef]
- Cho, S.Y.; Choi, S.M.; Park, S.H.; Lee, D.G.; Choi, J.H.; Yoo, J.H. Amikacin therapy for urinary tract infections caused by extended-spectrum β-lactamase-producing Escherichia coli. Korean J. Intern. Med. 2016, 31, 156. [Google Scholar] [CrossRef]
- Yang, Y.; Bhachech, N.; Bradford, P.A.; Jett, B.D.; Sahm, D.F.; Bush, K. Ceftazidime-resistant Klebsiella pneumoniae and Escherichia coli isolates producing TEM-10 and TEM-43 beta-lactamases from St. Louis, Missouri. Antimicrob. Agents Chemother. 1998, 42, 1671–1676. [Google Scholar] [CrossRef][Green Version]
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-spectrum β-lactamases: An update on their characteristics, epidemiology and detection. JAC-Antimicrob. Resist. 2021, 3, dlab092. [Google Scholar] [CrossRef] [PubMed]
- Rocha, F.R.; Pinto, V.P.T.; Barbosa, F.C.B. The spread of CTX-M-type extended-spectrum β-lactamases in Brazil: A systematic review. Microb. Drug Resist. 2016, 22, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, N.; Leitão, J.; Manageiro, V.; Ferreira, E.; Caniça, M. Spread of extended-spectrum β-lactamase CTX-M-producing Escherichia coli clinical isolates in community and nosocomial environments in Portugal. Antimicrob. Agents Chemother. 2007, 51, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
- Kirtikliene, T.; Mierauskaitė, A.; Razmienė, I.; Kuisiene, N. Genetic characterization of multidrug-resistant E. coli isolates from bloodstream infections in Lithuania. Microorganisms 2022, 10, 449. [Google Scholar] [CrossRef] [PubMed]
- Melegh, S.; Schneider, G.; Horváth, M.; Jakab, F.; Emödy, L.; Tigyi, Z. Identification and characterization of CTX-M-15 producing Klebsiella pneumoniae clone ST101 in a Hungarian university teaching hospital. Acta Microbiol. Immunol. Hung. 2015, 62, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Schmiedel, J.; Falgenhauer, L.; Domann, E.; Bauerfeind, R.; Prenger-Berninghoff, E.; Imirzalioglu, C.; Chakraborty, T. Multiresistant extended-spectrum β-lactamase-producing Enterobacteriaceae from humans, companion animals and horses in central Hesse, Germany. BMC Microbiol. 2014, 14, 1–13. [Google Scholar] [CrossRef]
- Silva, M.M.; Fernandes, M.R.; Sellera, F.P.; Cerdeira, L.; Medeiros, L.K.G.; Garino, F.; Azevedo, S.S.; Lincopan, N. Multidrug-resistant CTX-M-15-producing Klebsiella pneumoniae ST231 associated with infection and persistent colonization of dog. Diagn. Microbiol. Infect Dis. 2018, 92, 259–261. [Google Scholar] [CrossRef]
- Carvalho, I.; Cunha, R.; Martins, C.; Martínez-Álvarez, S.; Chenouf, N.S.; Pimenta, P.; Pereira, A.R.; Ramos, S.; Sadi, M.; Martins, Â.; et al. Antimicrobial resistance genes and diversity of clones among FAECAL ESBL-producing Escherichia coli isolated from healthy and sick dogs living in Portugal. Antibiotics 2021, 10, 1013. [Google Scholar] [CrossRef]
- Maciuca, I.E.; Williams, N.J.; Tuchilus, C.; Dorneanu, O.; Guguianu, E.; Carp-Carare, C.; Rimbu, C.; Timofte, D. High prevalence of Escherichia coli-producing CTX-M-15 extended-spectrum beta-lactamases in poultry and human clinical isolates in Romania. Microb. Drug Resist. 2015, 21, 651–662. [Google Scholar] [CrossRef]
- Rodríguez-Navarro, J.; Miró, E.; Brown-Jaque, M.; Hurtado, J.C.; Moreno, A.; Muniesa, M.; González-López, J.J.; Vila, J.; Espinal, P.; Navarro, F. Comparison of commensal and clinical isolates for diversity of plasmids in Escherichia coli and Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2020, 64, e02064-19. [Google Scholar] [CrossRef] [PubMed]
- Belas, A.; Marques, C.; Aboim, C.; Pomba, C. Emergence of Escherichia coli ST131 H30/H30-Rx subclones in companion animals. J. Antimicrob. Chemother. 2019, 74, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Chanoine, M.H.; Blanco, J.; Leflon-Guibout, V.; Demarty, R.; Alonso, M.P.; Caniça, M.M.; Park, Y.J.; Lavigne, J.P.; Pitout, J.; Johnson, J.R. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. J. Antimicrob. Chemother. 2008, 61, 273–281. [Google Scholar] [CrossRef]
- Younes, A.; Hamouda, A.; Dave, J.; Amyes, S.G.B. Prevalence of transferable blaCTX-M-15 from hospital- and community-acquired Klebsiella pneumoniae isolates in Scotland. J. Antimicrob. Chemother. 2011, 66, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Xercavins, M.; Jiménez, E.; Padilla, E.; Riera, M.; Freixas, N.; Boix-Palop, L.; Pérez, J.; Calbo, E. High clonal diversity of ESBL-producing Klebsiella pneumoniae isolates from clinical samples in a non-outbreak situation. A cohort study. Antimicrob. Resist. Infect. Control 2020, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Haenni, M.; Ponsin, C.; Métayer, V.; Médaille, C.; Madec, J.Y. Veterinary hospital-acquired infections in pets with a ciprofloxacin-resistant CTX-M-15-producing Klebsiella pneumoniae ST15 clone. J. Antimicrob. Chemother. 2012, 67, 770–771. [Google Scholar] [CrossRef] [PubMed]
- Donati, V.; Feltrin, F.; Hendriksen, R.S.; Svendsen, C.A.; Cordaro, G.; Garciá-Fernández, A.; Lorenzetti, S.; Lorenzetti, R.; Battisti, A.; Franco, A. Extended-spectrum-beta-lactamases, AmpC beta-lactamases and plasmid mediated quinolone resistance in klebsiella spp. from companion animals in Italy. PLoS ONE 2014, 9, e90564. [Google Scholar]
- Usein, C.R.; Papagheorghe, R.; Oprea, M.; Condei, M.; Strãuţ, M. Molecular characterization of bacteremic Escherichia coli isolates in Romania. Folia Microbiol. 2016, 61, 221–226. [Google Scholar] [CrossRef]
- Miftode, E.; Dorneanu, O.; Badescu, A.; Ghibu, L.; Leca, D.; Vremera, T.; Mereuţă, A. Emergence of a new group CTX-M enzyme in Romania and risk factors for extended spectrum beta-lactamase producing E. coli infections. Rev. Med.-Chir. A Soc. Med. Si Nat. Din Iasi 2012, 116, 477–480. [Google Scholar]
- Popa, L.I.; Gheorghe, I.; Barbu, I.C.; Surleac, M.; Paraschiv, S.; Măruţescu, L.; Popa, M.; Pîrcălăbioru, G.G.; Talapan, D.; Niţă, M.; et al. Multidrug Resistant Klebsiella pneumoniae ST101 clone survival chain from inpatients to hospital effluent after chlorine treatment. Front. Microbiol. 2021, 11, 3403. [Google Scholar] [CrossRef]
- Timofte, D.; Maciuca, I.E.; Williams, N.J.; Wattret, A.; Schmidt, V. Veterinary hospital dissemination of CTX-M-15 extended-spectrum beta-lactamase-producing Escherichia coli ST410 in the United Kingdom. Microb. Drug Resist. 2016, 22, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Shnaiderman-Torban, A.; Navon-Venezia, S.; Dahan, R.; Dor, Z.; Taulescu, M.; Paitan, Y.; Edery, N.; Steinman, A. CTX-M-15 producing Escherichia coli sequence type 361 and sequence type 38 causing bacteremia and umbilical infection in a neonate foal. J. Equine Vet. Sci. 2020, 85, 102881. [Google Scholar] [CrossRef] [PubMed]
- Lamoureaux, T.L.; Frase, H.; Antunes, N.T.; Vakulenko, S.B. Antibiotic resistance and substrate profiles of the class a carbapenemase KPC-6. Antimicrob. Agents Chemother. 2012, 56, 6006–6008. [Google Scholar] [CrossRef] [PubMed]
- Wysocka, M.; Zamudio, R.; Oggioni, M.R.; Gołębiewska, J.; Bronk, M.; Krawczyk, B. Genetic background and antibiotic resistance profiles of K. pneumoniae NDM-1 strains isolated from Uti, Abu, and the Gi tract, from one hospital in Poland, in Relation to strains nationally and worldwide. Genes 2021, 12, 1285. [Google Scholar] [CrossRef]
- Alcalá, L.; Alonso, C.A.; Simón, C.; González-Esteban, C.; Orós, J.; Rezusta, A.; Ortega, C.; Torres, C. Wild Birds, Frequent carriers of extended-spectrum β-lactamase (ESBL) producing Escherichia coli of CTX-M and SHV-12 types. Microb. Ecol. 2016, 72, 861–869. [Google Scholar] [CrossRef]
- Veldman, K.; van Tulden, P.; Kant, A.; Testerink, J.; Mevius, D. Characteristics of cefotaxime-resistant Escherichia coli from wild birds in The Netherlands. Appl. Environ. Microbiol. 2013, 79, 7556–7561. [Google Scholar] [CrossRef]
- Liakopoulos, A.; Mevius, D.; Ceccarelli, D. A review of SHV extended-spectrum β-lactamases: Neglected yet ubiquitous. Front. Microbiol. 2016, 7, 1374. [Google Scholar] [CrossRef]
- Perilli, M.; Dell’Amico, E.; Segatore, B.; De Massis, M.R.; Bianchi, C.; Luzzaro, F.; Rossolini, G.M.; Toniolo, A.; Nicoletti, G.; Amicosante, G. Molecular characterization of extended-spectrum β-lactamases produced by nosocomial isolates of Enterobacteriaceae from an Italian nationwide survey. J. Clin. Microbiol. 2002, 40, 611–614. [Google Scholar] [CrossRef][Green Version]
- Poyart, C.; Mugnier, P.; Quesne, G.; Berche, P.; Trieu-Cuot, P. A novel extended-spectrum TEM-type beta-lactamase (TEM-52) associated with decreased susceptibility to moxalactam in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 1998, 42, 108–113. [Google Scholar] [CrossRef][Green Version]
- Pai, H.; Lyu, S.; Lee, J.H.; Kim, J.; Kwon, Y.; Kim, J.W.; Choe, K.W. Survey of extended-spectrum beta-lactamases in clinical isolates of Escherichia coli and Klebsiella pneumoniae: Prevalence of TEM-52 in Korea. J. Clin. Microbiol. 1999, 37, 1758–1763. [Google Scholar] [CrossRef]
| No. | Origin | Resistance Phenotype | Beta-Lactamases | tet Genes |
|---|---|---|---|---|
| 1 | Purulent secretion | AMP ATM CZO FEP CAZ CRO CXM CTX | CTX-M-15 TEM-1 | ND |
| 2 | Purulent secretion | AMP ATM CZO FEP CAZ CRO CXM CTX GEN TCY | CTX-M-15 TEM-1 | ND |
| 3 | Purulent secretion | AMP ATM CZO FEP CAZ CRO CXM CTX CIP MFX NOR GEN TOB SXT TGC TCY | CTX-M-15 TEM-1 | tet(A) |
| 4 | Purulent secretion | AMK AMP TZP ATM CZO FEP CAZ CRO CXM CTX CIP MFX NOR GEN TOB SXT IPM MEM ETP | CTX-M-15 TEM-1 | ND |
| 5 | Tracheal aspirate | AMP CZO CAZ CRO CXM CTX MFX GEN TOB | CTX-M-15 | ND |
| 6 | Tracheal aspirate | AMP ATM CZO FEP CAZ CRO CXM CTX GEN TOB TCY ETP | TEM-1 | tet(A) |
| 7 | Catheter | AMP CZO FEP CAZ CRO CXM CTX CIP MFX NOR TOB TCY IPM | CTX-M-15 TEM-1 | tet(A) |
| 8 | Purulent secretion | AMP ATM CZO FEP CAZ CRO CXM CTX | CTX-M-15 TEM-1 | ND |
| 9 | Purulent secretion | AMP ATM CZO FEP CAZ CRO CXM CTX GEN TCY | CTX-M-15 TEM-1 | tet(B) |
| 10 | Purulent secretion | AMK AMP TZP ATM CZO FEP CAZ CRO CXM CTX CIP MFX NOR GEN TOB SXT TGC TCY | CTX-M-15 TEM-1 | tet(A) |
| 11 | Purulent secretion | AMP TZP ATM CZO FEP CAZ CRO CXM CTX CIP MFX NOR GEN TOB SXT IPM MEM ETP | CTX-M-15 SHV-1 | ND |
| 12 | Tracheal aspirate | AMP CZO CAZ CRO CXM CTX NOR | CTX-M-15 | ND |
| 13 | Tracheal aspirate | AMP TZP ATM CZO FEP CAZ CRO CXM CTX TCY ETP | CTX-M-15 TEM-1 | tet(A) |
| 14 | Catheter | AMP CZO FEP CAZ CRO CXM CTX CIP MFX NOR TOB TCY IPM | CTX-M-15 TEM-1 | tet(A) |
| No. | Origin | Resistance Phenotype | Beta-Lactamases | tet Genes |
|---|---|---|---|---|
| 1 | Sputum | AMK AMP TZP ATM CZO FEP CAZ CRO CXM CTX CIP NOR GEN TOB SXT TGC TCY IPM MEM ETP | CTX-M-15 SHV-1 TEM-1 | tet(A) |
| 2 | Sputum | AMP CZO FEP CAZ CRO CXM CTX CIP MFX NOR GEN TOB SXT TCY IPM MEM | CTX-M-15 SHV-1 | tet(A) |
| 3 | Peritoneal fluid | AMP ATM CZO FEP CAZ CRO CXM CTX CIP MFX NOR SXT IPM | CTX-M-15 SHV-1 TEM-1 | ND |
| 4 | Peritoneal fluid | AMP TZP ATM CZO FEP CAZ CRO CXM CTX CIP MFX NOR SXT TGC IPM MEM ETP | CTX-M-15 SHV-1 TEM-1 | ND |
| 5 | Purulent secretion | AMP CZO CAZ CXM CTX NOR | SHV-1 | ND |
| 6 | Purulent secretion | AMK AMP ATM CZO FEP CAZ CRO CXM CTX CIP NOR GEN TOB SXT TGC MEM ETP | CTX-M-15 SHV-1 | ND |
| 7 | Purulent secretion | AMP CZO CAZ CXM CTX CIP TOB TCY | SHV-1 | tet(A) |
| 8 | Purulent secretion | AMP CZO CAZ CXM CTX NOR TCY | SHV-1 | tet(A) |
| 9 | Purulent secretion | AMP TZP ATM CZO FEP CAZ CRO CXM CTX SXT TCY | SHV-1 | tet(A) |
| 10 | Purulent secretion | AMP TZP ATM CZO FEP CAZ CRO CXM CTX CIP TOB SXT TGC IPM MEM ETP | CTX-M-15 SHV-1 | ND |
| 11 | Purulent secretion | AMP CZO FEP CAZ CRO CXM CTX CIP TOB SXT IPM | CTX-M-15 SHV-1 | ND |
| 12 | Purulent secretion | AMP TZP CZO FEP CAZ CRO CXM CTX TOB | CTX-M-15 SHV-1 | ND |
| 13 | Purulent secretion | AMP ATM CZO FEP CAZ CRO CXM CTX GEN TOB SXT TGC IPM ETP | CTX-M-15 SHV-1 TEM-1 | ND |
| 14 | Tracheal aspirate | AMP TZP ATM CZO FEP CAZ CRO CXM CTX CIP MFX NOR GEN TOB SXT IPM ETP | CTX-M-15 SHV-1 TEM-1 | ND |
| 15 | Tracheal aspirate | AMP TZP ATM CZO FEP CAZ CRO CXM CTX CIP MFX NOR GEN TOB SXT TGC TCY IPM MEM ETP | CTX-M-15 SHV-1 TEM-1 | ND |
| 16 | Catheter | AMK AMP TZP ATM CZO FEP CAZ CRO CXM CTX CIP MFX NOR GEN TOB SXT TCY IPM MEM ETP | CTX-M-15 SHV-1 TEM-1 | ND |
| 17 | Sputum | AMK AMP TZP ATM CZO FEP CAZ CRO CXM CTX CIP NOR GEN TOB SXT TGC TCY IPM MEM ETP | CTX-M-15 SHV-1 TEM-1 | ND |
| 18 | Sputum | AMP CZO FEP CAZ CRO CXM CTX CIP MFX NOR GEN TOB SXT TCY IPM MEM | CTX-M-15 SHV-1 | tet(A) |
| 19 | Peritoneal fluid | AMP ATM CZO FEP CAZ CRO CXM CTX CIP MFX NOR SXT IPM | CTX-M-15 SHV-1 TEM-1 | ND |
| 20 | Peritoneal fluid | AMP TZP ATM CZO FEP CAZ CRO CXM CTX CIP MFX NOR SXT TGC IPM MEM ETP | CTX-M-15 SHV-1 TEM-1 | ND |
| 21 | Wound secretion | AMP CZO CAZ CXM CTX NOR | SHV-1 | ND |
| 22 | Wound secretion | AMP ATM CZO FEP CAZ CRO CXM CTX CIP NOR GEN TOB SXT TGC MEM ETP | CTX-M-15 SHV-1 | ND |
| 23 | Wound secretion | AMP CZO CAZ CXM CTX CIP TOB TCY | SHV-1 | tet(A) |
| 24 | Wound secretion | AMP CZO CAZ CXM CTX NOR TCY | SHV-1 | tet(A) |
| 25 | Wound secretion | AMP TZP ATM CZO FEP CAZ CRO CXM CTX SXT TCY | SHV-1 | tet(A) |
| 26 | Wound secretion | AMP TZP ATM CZO FEP CAZ CRO CXM CTX CIP TOB SXT TGC IPM MEM ETP | CTX-M-15 SHV-1 | ND |
| 27 | Wound secretion | AMP CZO FEP CAZ CRO CXM CTX CIP TOB SXT IPM | CTX-M-15 SHV-1 | ND |
| 28 | Wound secretion | AMP TZP CZO FEP CAZ CRO CXM CTX TOB | SHV-1 | ND |
| 29 | Wound secretion | AMP ATM CZO FEP CAZ CRO CXM CTX GEN TOB SXT TGC IPM ETP | CTX-M-15 SHV-1 TEM-1 | ND |
| 30 | Tracheal aspirate | AMP TZP ATM CZO FEP CAZ CRO CXM CTX CIP MFX NOR GEN TOB SXT IPM ETP | CTX-M-15 SHV-1 TEM-1 | ND |
| 31 | Tracheal aspirate | AMP TZP ATM CZO FEP CAZ CRO CXM CTX CIP MFX NOR GEN TOB SXT TGC TCY IPM MEM ETP | CTX-M-15 SHV-1 TEM-1 | tet(A) |
| 32 | Cateter | AMP TZP ATM CZO FEP CAZ CRO CXM CTX CIP MFX NOR GEN TOB SXT TCY IPM MEM ETP | CTX-M-15 SHV-1 TEM-1 | tet(A) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghenea, A.E.; Zlatian, O.M.; Cristea, O.M.; Ungureanu, A.; Mititelu, R.R.; Balasoiu, A.T.; Vasile, C.M.; Salan, A.-I.; Iliuta, D.; Popescu, M.; et al. TEM,CTX-M,SHV Genes in ESBL-Producing Escherichia coli and Klebsiella pneumoniae Isolated from Clinical Samples in a County Clinical Emergency Hospital Romania-Predominance of CTX-M-15. Antibiotics 2022, 11, 503. https://doi.org/10.3390/antibiotics11040503
Ghenea AE, Zlatian OM, Cristea OM, Ungureanu A, Mititelu RR, Balasoiu AT, Vasile CM, Salan A-I, Iliuta D, Popescu M, et al. TEM,CTX-M,SHV Genes in ESBL-Producing Escherichia coli and Klebsiella pneumoniae Isolated from Clinical Samples in a County Clinical Emergency Hospital Romania-Predominance of CTX-M-15. Antibiotics. 2022; 11(4):503. https://doi.org/10.3390/antibiotics11040503
Chicago/Turabian StyleGhenea, Alice Elena, Ovidiu Mircea Zlatian, Oana Mariana Cristea, Anca Ungureanu, Radu Razvan Mititelu, Andrei Theodor Balasoiu, Corina Maria Vasile, Alex-Ioan Salan, Daniel Iliuta, Mihaela Popescu, and et al. 2022. "TEM,CTX-M,SHV Genes in ESBL-Producing Escherichia coli and Klebsiella pneumoniae Isolated from Clinical Samples in a County Clinical Emergency Hospital Romania-Predominance of CTX-M-15" Antibiotics 11, no. 4: 503. https://doi.org/10.3390/antibiotics11040503
APA StyleGhenea, A. E., Zlatian, O. M., Cristea, O. M., Ungureanu, A., Mititelu, R. R., Balasoiu, A. T., Vasile, C. M., Salan, A.-I., Iliuta, D., Popescu, M., Udriștoiu, A.-L., & Balasoiu, M. (2022). TEM,CTX-M,SHV Genes in ESBL-Producing Escherichia coli and Klebsiella pneumoniae Isolated from Clinical Samples in a County Clinical Emergency Hospital Romania-Predominance of CTX-M-15. Antibiotics, 11(4), 503. https://doi.org/10.3390/antibiotics11040503










