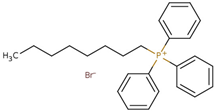
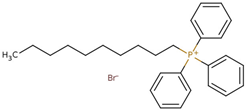
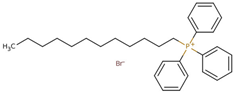
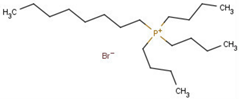
Abstract
A previously developed model to predict antibacterial activity of ionic liquids against a resistant A. baumannii strain was used to assess activity of phosphonium ionic liquids. Their antioxidant potential was additionally evaluated with newly developed models, which were based on public data. The accuracy of the models was rigorously evaluated using cross-validation as well as test set prediction. Six alkyl triphenylphosphonium and alkyl tributylphosphonium bromides with the C8, C10, and C12 alkyl chain length were synthesized and tested in vitro. Experimental studies confirmed their activity against A. baumannii as well as showed pronounced antioxidant properties. These results suggest that phosphonium ionic liquids could be promising lead structures against A. baumannii.
1. Introduction
Acinetobacter baumannii is an important nosocomial pathogen that is responsible for a wide range of human infections, resulting in high levels of morbidity and mortality. It is known that newly identified A. baumannii strains are resistant to most known antibiotics and able to survive for a prolonged period of time in a hospital setting, enhancing the ability to spread rapidly in the hospital which could result in the death of infected patients unless treated with nonresistant antibiotics [,,,]. The rapid global emergence of new antibiotic-resistant strains of A. baumannii demonstrates the high potential of this microorganism to respond quickly to environment changes. Therefore, creation of new effective antibacterial drugs is the most important task of modern drug development [].
Long-chain ionic liquids (ILs) comprised of 1,3-dialkylimidazolium, 1-alkylpyridinium, and N-alkyl guanidinium cations are considered extremely promising antimicrobial agents since they possess broad-spectrum activity against both Gram-positive and Gram-negative bacteria including multidrug-resistant (MDR) clinical isolates [,,,,], antifungal activity [,], as well as strong antibiofilm activity against a panel of pathogenic microorganisms. The length of the aliphatic chain of IL cations is known to play a crucial role towards its biological activity. Among the homologs investigated, cationic surfactants with an alkyl chain length of 12 and 14 carbon atoms have shown the highest efficiency as antimicrobial agents [,,,,]. It is worth noting that ILs comprised of nitrogen containing heterocyclic cations are the most commonly studied, whereas the phosphorus-containing ones have been studied to a lesser extent. However, phosphonium ILs occupy a special niche among promising biocides since they have some advantages over nitrogen-containing ILs such as higher thermal stability, as well as faster kinetics of their synthesis []. Moreover, long-chain tetraalkyl phosphonium salts were found to have broad-spectrum antimicrobial activity [,,,,], as well as pronounced antitumor activity []. In general, these compounds demonstrate higher antibacterial and antitumor activity, as well as less cytotoxicity as compared to their nitrogen-containing counterparts [,,]. An important feature of tetraalkyl phosphonium-based ILs is that their activity does not have a pronounced dependence on the length of alkyl radicals bound to cations [,,,]. For example, short-chain ILs comprised of tetrabutylphosphonium cations demonstrated high activity against sixty human tumor cell lines, which was similar to that of long-chain phosphonium salts []. Tributyl(2-hydroxyethyl)phosphonium docusate was found to exhibit antimicrobial and antibiofilm-forming activity to several antibiotic-resistant bacteria [,]. Another example is tetrakis(hydroxymethyl)phosphonium sulfate, a broad-spectrum biocide targeted at a wide range of bacteria, especially sulfate-reducing bacteria [].
Besides tetraalkyl phosphonium ILs, quaternary salts comprised of alkyl triphenylphosphonium cations are also of great interest since these compounds may have a specific biological activity. Thus, long-chain dodecyl(triphenyl)phosphonium ILs were found to induce expression of the plasma membrane of pleiotropic drug-resistant transporters in yeasts [,]. Mitochondria-targeted antioxidants (MTAs) are widely used in experiments for evaluating the impact of mitochondria on different pathological processes involving oxidative stress. These involve a wide range of compounds which have an antioxidant group linked to a mitochondria-targeted moiety, such as triphenylphosphonium cations []. It was found that SkQ1, a decyl(triphenyl)phosphonium cation conjugated to a quinone moiety, exhibited a strong antibacterial activity towards Gram-positive Bacillus subtilis, Mycobacterium sp., and Staphylococcus aureus and Gram-negative Photobacterium phosphoreum and Rhodobacter sphaeroides in sub micromolar and micromolar concentrations []. However, SkQ1 exhibited less antibiotic activity towards Escherichia coli due to the presence of the highly effective multidrug-resistant efflux pump AcrAB–TolC which promotes the expulsion of a wide range of molecules out of cells [].
Surprisingly, long-chain tetraalkyl phosphonium ILs as well as alkyl triphenylphosphonium ILs have not yet been studied for their activity against multidrug-resistant clinical isolate A. baumannii. Only in one recent study, newly synthesized 1,3-oxazolyl triphenylphosphonium salts were found to be active against A. baumannii []. However, the presence of a biologically active heterocyclic compound 1,3-oxazole in these compounds does not allow us to fully determine whether their activity was due to this moiety or to phosphonium cations. Therefore, we decided to explore the efficacy of phosphonium ILs comprised of inert alkyl and aryl radicals against A. baumannii. The ability of A. baumannii to form biofilms contributes to its survival in adverse environmental conditions including hospital environments and medical devices [,]. From this point of view, the evaluation of the antioxidant activity of phosphonium salts also seems relevant. Reactive oxygen species (ROS), such as hydrogen peroxide, superoxide, and hydroxyl radicals are known to serve as regulatory signals for many bacteria to maintain a healthy redox cycle and promote microbial attachment, consequently leading to the development of biofilms []. A disturbance in the redox cycle can lead to oxidative stress due to the accumulation of ROS. Oxidative stress in microorganisms was found to play an important role in the regulation of redox defense mechanisms, the production of the extracellular polymer substance (EPS) matrix, and biofilm heterogeneity []. Therefore, compounds that target oxidative stress regulators, such as antioxidants, could potentially be exploited as a novel strategy for biofilm control. To our knowledge, there are no systematic studies of the antioxidant activity of phosphonium salts. Only tetrakis(hydroxymethyl)phosphonium chloride (THPC) was reported as an efficient antioxidant due to its rapid oxygen-scavenging ability [,]. The properties of THPC are due to the presence of a weak P–CH2OH linkage which is readily cleaved in water solutions. The formed tris(hydroxymethyl)phosphine is a fairly strong reducing agent which can scavenge oxygen to produce tris(hydroxymethyl)phosphine oxide []. However, the question still remains as to the antioxidant activity of nonfunctionalized phosphonium salts comprised of inert alkyl or aryl radicals.
Thus, the aim of this research was to evaluate the efficacy of alkyl tributylphosphonium and alkyl triphenylphosphonium ILs with different alkyl chain lengths as antibacterial agents against A. baumannii as well as their antioxidant activity in in silico and in vitro studies.
2. Materials and Methods
2.1. QSAR Modeling of Antioxidant Activity
2.1.1. Dataset
The analysis was carried out using compounds with antioxidant activity obtained from the ChEMBL database and uploaded into OCHEM []. The structure of these compounds, their antioxidant activity, and literature sources are freely available on the OCHEM website.
The initial dataset of 1246 compounds consisted of a diverse chemical series with IC50 values of the molecules ranging from 0.0215 to 991 µM. The IC50 value determines the concentration of the sample required to inhibit 50% of the radicals. The IC50 values were converted into the −log(IC50) values and were used as the target variable to develop regression models. From the initial dataset, 20% of the compounds were randomly selected using OCHEM to form an external independent test set while the remaining molecules were used as the training set.
2.1.2. QSAR
QSAR models were created with three machine learning methods, including transformer convolutional neural network (CNN) [], transformer convolutional neural fingerprint (CNF) [], and random forest regression (RF) []. The RF models were developed using three descriptor sets including E-state indices [], ALogPS [,], and CDK 2.7.1 [], which were frequently top-performing descriptors, according to our previous studies. We used the optimized parameters setting of each machine learning method provided by the OCHEM platform.
Transformer convolutional neural network (transformer CNN). This method uses the internal latent representation of molecules based on their SMILES notation to extract information about the molecular structure []. The method predicts the target value based on the average individual prediction for a batch of augmented (noncanonical) SMILES belonging to the same molecule. The deviation within the predictions for augmented SMILES can be used to determine the uncertainty of predictions.
Transformer convolutional neural fingerprint (transformer CNF) is similar to transformer CNN, but instead of a convolutional neural network, it uses convolutional neural fingerprint for processing the latent representation of the neural network. Like transformer CNN, it uses the augmentation technique, which was originally proposed in computer vision and was recently introduced to QSAR studies [,].
Random forest (RF) consists of many individual trees devised to operate quickly over large datasets. RF is not heavily affected by correlated descriptors since it uses random samples to build each tree in the forest. The final prediction was the average of the individual trees [].
Descriptors
E-state indices. The calculation of electrotopological state indices is based on the chemical graph theory. E-state indices are 2D descriptors that combine both electronic and topological characteristics of the analyzed compounds [].
ALogPS. The program calculates two 2D descriptors, namely the 1-octanol–water partition coefficient [] and aqueous solubility [].
CDK 2.7.1. The Chemistry Development Kit (CDK) is a set of Java module libraries for processing chemical information. CDK 2.7.1 calculates 256 molecular descriptors such as geometrical, topological, constitutional, electronic, and hybrid descriptors [].
More details about the descriptors can be found elsewhere []. Pearson’s pairwise correlation method had been used for filtering each descriptor set before they were used as input for the machine learning methods. Additionally, unsupervised forward selection [] was applied to select a representative nonredundant set of descriptors.
A fivefold cross-validation method was used to evaluate the accuracy and robustness of the QSAR models. To avoid incorrect estimation of the models due to overfitting by variable selection, OCHEM repeats all model development steps within each validation fold. In addition, we also used the test set to further confirm the quality of the developed models [].
We used two criteria to assess the goodness of fit: the coefficient of determination q2 and the mean absolute error (MAE) []. OCHEM also supports estimation of the applicability domain [] of the developed machine learning models and the accuracy of predictions. Detailed descriptions of the machine learning methods, selected descriptors, statistical coefficients, and detailed validation procedures are provided in the OCHEM manual [].
2.2. Biology
2.2.1. Antibacterial Activity Evaluation
The MDR A. baumannii isolate was received from the Museum of Microbial Culture Collection of the Shupyk National Healthcare University of Ukraine collected at a Ukrainian hospital.
The antibacterial properties of PILs with high predicted activity were estimated against MDR (ampicillin-, oxacillin-, and ceftriaxone-resistant clinical isolate) A. baumannii by means of the disc diffusion method in a Mueller–Hinton agar []. The inoculum concentration of 1 × 105 colony-forming units (CFU) per mL was established using a 0.5 McFarland turbidity standard and a subsequent dilution of 0.02 mL of the tested compounds was applied on standard paper disks (6 mm) which were placed on the agar plate. The compound content on a disk was 0.25, 0.5, and 1.0 µM.
The antibacterial activity of the tested compounds was identified by measuring the zone diameter of growth inhibition, which indicated the degree of susceptibility or resistance of the A. baumannii isolate to the test compounds. The compounds which formed zones of bacterial growth inhibition > 15 mm could be considered active.
Furthermore, the antibacterial activity of the ILs was assessed with a 96-well plate microdilution assay, a technique adapted from the methodology described by Eloff et al. []. The bacterial inoculum was obtained by incubating microorganisms in a Mueller–Hinton broth for 24 h, adjusting it to a final concentration of 1 × 105 CFU per ml. The ILs were solubilized in sterile water, and the final concentrations for each IL were as follows: 0.4 µM, 0.78 µM, 1.56 µM, 3.12 µM, 6.25 µM, 12.5 µM, 25.0 µM, 50.0 µM, 100.0 µM, and 200.0 µM. Each plate included bacterial growth (positive control) and culture medium (negative control) for 24 h at 37 °C. The minimum concentrations without visible growth (using a binocular microscope) were defined as the concentrations which completely inhibited bacterial growth. Ofloxacin was used as a positive control in the MIC assay (0.015 µmol).
2.2.2. Antioxidant Activity Evaluation
The evaluation of antioxidant properties of the PILs (10 µM) was studied in vitro using the inhibition value of the rate of ascorbate-dependent radical lipid peroxidation and represented by the malonic dialdehyde (MDA) content using the reaction with thiobarbituric acid []. The amount of MDA was determined photometrically at 532 nm []. The intensity of lipid peroxidation processes was determined by the difference between the MDA content in the experimental and control samples (solvent) and presented as the percentage of control.
3. Results
3.1. Regression Model to Predict the Antioxidant Activity
The initial dataset of 1246 compounds with antioxidant activity collected from literature was split by chance into the training (997) and test (249) sets as described in the Materials and Methods.
In the preliminary stage of the analysis, all molecules were processed by the OCHEM software []; 2D coordinates of atoms were recalculated, counter ions and salts were removed from molecular structures, and molecular structures were neutralized and mesomerized.
The regression models were built using several methods, as described in the Materials and Methods section. The best-performing methods (see Table 1) included those based on representation learning (transformer CNN [] and transformer CNF []) as well as traditional descriptors (random forest, RF []). For the latter method, we selected the E-state [], ALogPS [], and CDK 2.7.1 [] descriptors, which contributed to the top-performing model for the RF.
The coefficient of determination q2 values of the developed models were 0.73–0.77 and 0.72–0.77 for the training and test sets, respectively. Thus, the models demonstrated high accuracy and robustness for prediction of the antioxidant activity. Other statistical parameters of the models were summarized in Table 1 as well as in the Supplementary Materials (Figure S2). A consensus model, which was an average of three individual models, was used to provide a quantitative evaluation of potential antioxidant agents as described in the next section. Moreover, the standard deviation of these individual models was used to estimate the applicability domain of the consensus model [].

Table 1.
Statistical coefficients of the regression models to predict antioxidant activity.
Table 1.
Statistical coefficients of the regression models to predict antioxidant activity.
| N | Method | Training Set a | Test Set a | ||
|---|---|---|---|---|---|
| q2 | MAEc | q2 | MAE | ||
| 1 | Transformer CNN | 0.77 ± 0.02 | 0.3 ± 0.01 | 0.75 ± 0.05 | 0.28 ± 0.02 |
| 2 | Transformer CNF | 0.76 ± 0.02 | 0.3 ± 0.01 | 0.77 ± 0.04 | 0.28 ± 0.02 |
| 3 | RF | 0.73 ± 0.02 | 0.32 ± 0.01 | 0.72 ± 0.04 | 0.3 ± 0.02 |
| 4 | Consensusb | 0.77 ± 0.02 | 0.29 ± 0.01 | 0.77 ± 0.04 | 0.27 ± 0.02 |
a The training and test datasets included 997 and 249 molecules, respectively. b The consensus model was the average of the transformer CNN, transformer CNF, and RF models. c MAE is the mean absolute error and q2 is the coefficient of determination, respectively. The RF model was based on the E-state [], ALogPS [] and CDK 2.7.1 [] descriptors.
3.2. Selection of Compounds with the Help of In Silico Tools
A virtual set of ILs was generated as a combinatorial library based on the recommendations of an experienced synthetic organic chemist (who suggested which types of modifications of PILs could be synthesized). It included eleven compounds with different substitution patterns (see the Supplementary Materials). All the compounds were run through the previously published consensus classification model []. Six compounds predicted as active among those with the most confident predictions (>60%) were selected (Table S1). The compounds were analyzed using a set of structural alerts [] implemented in OCHEM to screen and exclude compounds with potential toxicity. All the selected compounds passed these filters. These compounds were then tested using a consensus antioxidant model developed in the previous section. All the compounds were predicted as moderately antioxidant active compounds [] (see Table S2). Therefore, all the six compounds were retained for synthesis and experimental testing (Table 2 and Table S1). The results of biological testing of the newly synthesized compounds confirmed the theoretical predictions of their activities (Table 2, Table 3 and Table 4) as described in the following sections.

Table 2.
Chemical structures of the synthesized compounds tested for their antibacterial activity.
3.3. Synthesis of PILs
Below, we provide a description of the synthetic protocols used to synthesize the analyzed compounds.
3.3.1. Initial Materials and Structure Confirmation
The following chemicals were used for the synthesis of ionic liquids: triphenylphosphine (95%), tributylphosphine (93.5%), 1-bromododecane (97%), 1-bromodecane (98%), 1-bromooctane (99%), acetonitrile (99.5%), ethyl acetate (99.7%), and hexane (95%) (Sigma-Aldrich, St. Louis, MO, USA). 1H NMR spectra were recorded in CDCl3 and DMSO-d6 on a Varian Gemini-2000 (400 MHz) spectrometer using TMS (tetramethylsilane) as the internal standard.
3.3.2. Synthesis of Ionic Liquids
Long-chain alkyl triphenylphosphonium (1–3) and alkyl tributylphosphonium (4,5) ILs were synthesized according to Scheme 1. To the stirred solution of triphenylphosphine or tributylphosphine (0.05 mol) in 50 mL of acetonitrile, we added bromoalkane (0.06 mol), and the mixture was heated to reflux for 24 h. Acetonitrile was distilled, the product was washed with an ethyl acetate–hexane mixture (1:3 v/v, 3 × 100 mL). The residual solvents were removed in vacuum (10 mbar) at 60 °C.
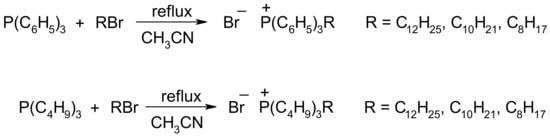
Scheme 1.
Synthesis of phosphonium ILs (see also Supplementary Table S3).
- Octyl(triphenyl)phosphonium bromide (PPh3C8–Br), CASRN 42036-78-2.Colorless solid, m.p.: 61–63 °C.1H NMR (400 MHz, DMSO-d6): σ = 0.81 (t, 3H, CH3), 1.19 (m, 8H, CH2), 1.48 (m, 4H, PCH2CH2CH2CH2), 3.56 (m, 2H, PCH2), 7.76–7.84 (m, 15H, Ar–H).
- Decyl(triphenyl)phosphonium bromide (PPh3C10–Br), CASRN 32339-43-8.White solid, m.p.: 86–88 °C.1H NMR (400 MHz, DMSO-d6): σ = 0.83 (t, 3H, CH3), 1.2 (m, 12H, CH2), 1.46 (m, 4H, PCH2CH2CH2CH2), 3.6 (m, 2H, PCH2), 7.8–7.9 (m, 15H, Ar–H).
- Dodecyl(triphenyl)phosphonium bromide (PPh3C12–Br), CASRN 15510-55-1.White solid, m.p.: 89–92 °C.1H NMR (400 MHz, DMSO-d6): σ = 0.83 (t, 3H, CH3), 1.2 (m, 16H, CH2), 1.46 (m, 4H, PCH2CH2CH2CH2), 3.6 (m, 2H, PCH2), 7.8–7.9 (m, 15H, Ar–H).
- Octyl(tributyl)phosphonium bromide (PBu3C8–Br), CARSN 57702-65-5.Liquid.1H NMR (400 MHz, CDCl3): σ = 0.86 (t, 3H, CH3), 0.96 (t, 9H, CH2CH2CH2CH3), 1.24 (m, 8H, CH2), 1.51 (m, 16H, PCH2CH2CH2CH2), 2.44 (m, 8H, PCH2).
- Decyl(tributyl)phosphonium bromide (Pbu3C10–Br), CASRN 99045-50-8.Viscous liquid.1H NMR (400 MHz, CDCl3): σ = 0.85 (t, 3H, CH3), 0.96 (t, 9H, CH2CH2CH2CH3), 1.24 (m, 12H, CH2), 1.51 (m, 16H, PCH2CH2CH2CH2), 2.43 (m, 8H, PCH2).
- Dodecyl(tributyl)phosphonium bromide (Pbu3C12–Br), CASRN 15294-63-0.White solid, m.p.: 30–32 °C.1H NMR (400 MHz, CDCl3): σ = 0.86 (t, 3H, CH3), 0.96 (t, 9H, CH2CH2CH2CH3), 1.24 (m, 16H, CH2), 1.52 (m, 16H, PCH2CH2CH2CH2), 2.44 (m, 8H, PCH2).
3.4. In Vitro Evaluation of the Activity of the PILs
3.4.1. Antibacterial Activity
The biological study results of the PILs with predicted activity against MDR A. baumannii are presented in Table 3 and Figure 1.
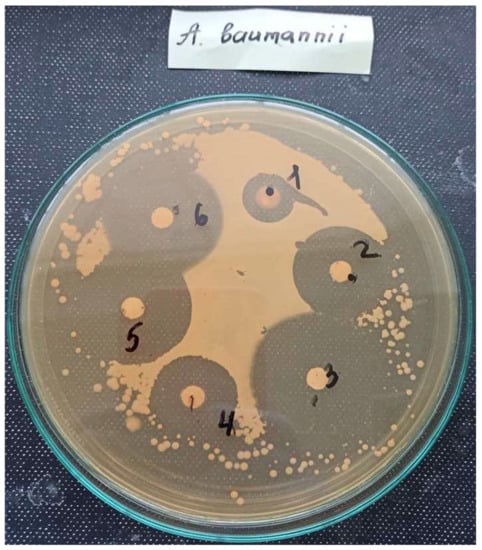
Figure 1.
Inhibition zone diameters of the six studied PILs (content on a disk, 1.25 µmoles) of an MDR clinical isolate of A. baumannii on agar plates.

Table 3.
Inhibition zone diameters (mm) formed by the PILs against A. baumannii (n = 3).
Table 3.
Inhibition zone diameters (mm) formed by the PILs against A. baumannii (n = 3).
| N | Compound | Compound Content on a Disk (µmoles) | |||
|---|---|---|---|---|---|
| 0.01 | 0.05 | 0.25 | 1.25 | ||
| 1 | PPh3C8–Br | 8.1 ± 0.3 | 12.3 ± 0.6 | 15.5 ± 0.3 | 17.3 ± 0.6 |
| 2 | PPh3C10–Br | 20.4 ± 0.9 | 25.4 ± 0.3 | 28.6 ± 0.9 | 31.5 ± 0.6 |
| 3 | PPh3C12–Br | 26.2 ± 0.6 | 30.1 ± 0.6 | 34.4 ± 0.9 | 41.8 ± 0.9 |
| 4 | Pbu3C8–Br | 14.5 ± 0.3 | 18.8 ± 0.3 | 22.3 ± 0.6 | 26.1 ± 0.3 |
| 5 | Pbu3C10–Br | 20.7 ± 0.3 | 25.4 ± 0.6 | 31.3 ± 0.3 | 34.2 ± 0.3 |
| 6 | Pbu3C12–Br | 26.1 ± 0.6 | 30.3 ± 0.3 | 33.2 ± 0.6 | 36.6 ± 0.6 |
| 7 | Ampicillin, oxacillin, ceftriaxone | 6 ± 0.3 | 6 ± 0.3 | 6 ± 0.3 | 6 ± 0.3 |
The results regarding the PILs’ antibacterial activity against MDR A. baumannii (Table 3 and Figure 1) demonstrated anti-A. baumannii activity of all the six investigated PILs in the 0.01–1.25 µmoles content on a disk. PILs dodecyl(triphenyl)phosphonium bromide (3), decyl(triphenyl)phosphonium bromide (2), dodecyl(tributyl)phosphonium bromide (6), and decyl(tributyl)phosphonium (5) bromide with the C12 and C10 alkyl chain length were found to be more active with inhibition zone diameters in the range of 31.5–41.8 mm with the content on a disk of 1.25 µmoles. PILs octyl(triphenyl)phosphonium bromide (1) and octyl(tributyl)phosphonium bromide (4) with the C8 alkyl chain length demonstrated a lower activity ranging from 17.3 to 26.1 mm (inhibition zone diameters) (content on a disk of 1.25 µmoles). A similar tendency was observed with decreasing µmoles on a disk to 0.01.
The MIC values were determined for PILs 2, 4–6 as 12.5 µM. Furthermore, PIL 3 showed a high activity, with the MIC value of 6.25 µM, and PIL 1 had the MIC value of 25.0 µM. The obtained in vitro results confirmed the in silico prediction of antibacterial activity of the studied compounds.
3.4.2. Antioxidant Activity
It is known that oxidative stress induced by microbial pathogens not only leads to disruption of the key metabolic processes in the body, but also regulates the replication of microbial pathogens and promotes the formation of bacterial resistance []. Antibiotics are able to regulate the production of reactive oxygen species and the bactericidal action of antimicrobial agents under aerobic conditions []. Thus, compounds with high antioxidant activity can be important to decrease oxidative stress and help the human body to fight bacteria.
The structural features and established anti-A. baumannii properties of the studied PILs formed the basis for the study of their potential antioxidant activity (AOA). The results of the AOA estimation of the test compounds by free radical lipid peroxidation are presented in Table 4.
The known synthetic antioxidant ionol (butylhydroxytoluene) was used as a reference. This compound is widely used in the production of food products (food additive E321), personal care products, cosmetics, and in some pharmaceuticals [].

Table 4.
Antioxidant activity of PILs (n = 3).
Table 4.
Antioxidant activity of PILs (n = 3).
| N | Compound | Inhibition Fate of MDA, % |
|---|---|---|
| 1 | PPh3C8–Br | 31.5 ± 2.0 |
| 2 | PPh3C10–Br | 34.2 ± 1.2 |
| 3 | PPh3C12–Br | 33.3 ± 1.5 |
| 4 | PPh3C8–Br | 31.5 ± 2.0 |
| 5 | PPh3C10–Br | 34.2 ± 1.2 |
| 6 | PBu3C12–Br | 33.1 ± 2.2 |
| Ionol a | 31.4 ± 2.5 | |
| 1% DMSO b | 14 ± 0.5 |
The concentration of all the compounds was 10 mg/mL. Note: a reference antioxidant, b reference negative control. The inhibition rates indicated were reported after correction for the activity of the reference negative control (i.e., we observed 45.4% for ionol and reported a corrected value of 31.4%).
Surprisingly, both alkyl triphenylphosphonium and alkyl tributylphosphonium ILs demonstrated a similar ability to inhibit lipid peroxidation processes at the ionol level ranging between 30.2% and 34.2% (Table 4). It is worth noting that the mechanism of antioxidant activity of these compounds is not described. In the case of alkyl triphenylphosphonium salts, nucleophilic radical addition of hydroxyl radicals to phenyl rings could be assumed, likely to cationic heterocyclic compounds []. However, aliphatic alkyl tributylphosphonium ILs also showed high antioxidant activity, similar to the triphenylphosphonium-based ones. Thus, a possible mechanism of hydroxyl radicals scavenging by phosphonium ILs may involve a break of an a-C–H bond to form an a-phosphonium intermediate which is then intercepted by another radical to create a new a-C–OH bond (Scheme 2).
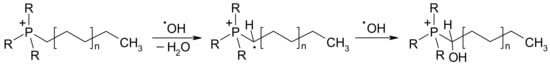
Scheme 2.
Possible mechanism of hydroxyl radical inhibition by phosphonium ILs.
4. Discussion
The previously created QSAR model [] (see also Figure S1) was used to identify six anti-A. baumannii agents. Selected alkyl triphenylphosphonium and alkyl tributylphosphonium bromides with the C8, C10, and C12 alkyl chain length demonstrated a high activity against the MDR clinical isolate of A. baumannii as confirmed by the inhibition zone diameters of these compounds that were in the range of 33–40 mm (Table 3). These results support previous studies, which demonstrated great prospects of ILs as therapeutic agents of complex action in biomedical applications [,,]. Previous studies showed that ILs with an alkyl chain length of 12 and 14 carbon atoms had the highest efficiency as antimicrobial and antitumor agents [,,]. The compounds investigated in this study had the alkyl chain length of 8 and 12, and those with the C10 and C12 alkyl chain length were found to be more active against A. baumannii (Table 3), thus confirming the previously reported trend.
We also developed in silico models to estimate the antioxidant activity of these compounds using different machine-learning techniques of the OCHEM platform []. The regression models demonstrated good stability, robustness, and predictive power, as verified by cross-validation and prediction of the independent test set. The model predicted the antioxidant activity of the analyzed compounds which was further confirmed by experimental studies. Indeed, we found that all the six PILs had pronounced antioxidant properties ranging from 30% to 34%, which was similar to that of the reference antioxidant ionol.
Effective antioxidant therapy is important to optimize the treatment of infectious diseases. The possibility of using certain antioxidants as antimicrobial and antiviral agents which can block the replication of microbial pathogens is widely considered an important strategy [,]. A correlation between the degree of reduction of the persistent potential of resistant microorganisms under the influence of certain xenobiotics and the level of their antioxidant activity was previously established: compounds with high antioxidant activity most effectively blocked the persistent properties of microbial pathogens []. The use of therapeutic strategies aimed at the development of antimicrobial agents with antioxidant properties is considered one of the promising areas for imbalance-regulating redox processes caused by multidrug-resistant pathogens [,,]. Biologically active compounds with a dual direction of action can lead to the successful progress of this task. Therefore, the presented in vitro and in silico analysis of the antioxidant properties of new highly active PILs make them promising pharmacologically active agents against A. baumannii. New theoretical and experimental studies to elucidate their mechanism of action will be important for a rational design of this class of compounds.
5. Conclusions
We investigated the antibacterial activity of new ionic liquids against A. baumannii. To study the antioxidant activity, predictive in silico models based on different machine-learning techniques were built using the OCHEM platform. The developed regression models demonstrated good stability, robustness, and predictive power, as verified by cross-validation and prediction of the test set.
Six PILs were synthesized and their anti-A. baumannii activity and antioxidant properties were evaluated in vitro. The studied alkyl triphenylphosphonium and alkyl tributylphosphonium bromides with the C8, C10, and C12 alkyl chain length demonstrated activity against the MDR clinical isolate of A. baumannii in the range of 17.3–41.8 mm (inhibition zone diameters) and 6.25–25.0 µM (MIC). The PILs with the C10 and C12 alkyl chain length were found to be more active, with inhibition zone diameters in the range of 31.5–41.8 mm (with MIC values in the range of 6.25–12.5 µM). The alkyl chains shorter than eight or longer than 12 carbon atoms resulted in the loss of activity of the PILs.
All the tested PILs had similar antioxidant properties at the reference antioxidant ionol level ranging from 30% to 34%. These results suggest that the investigated compounds are interesting and promising lead structures against A. baumannii. Additional experimental studies to elucidate their mechanism of action will be important for a further rational design of these compounds.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/antibiotics11040491/s1. Figure S1. Consensus classification machine-learning model [] built by the OCHEM platform using several machine learning algorithms [,]. The training and test sets included 210 and 53 molecules, respectively. The cross-validation results were reported for the training set. Figure S2. QSAR models developed using OCHEM (http://ochem.eu) in this study. (a–c) Statistical coefficients calculated for the regression models by a different MLT. (d) Consensus model calculated by averaging the previous three models. Table S1. Anti- A. baumannii activity calculated by using the consensus classification model for 11 virtual compounds. Table S2. Antioxidant activity predicted by using the consensus regression model for six tested ILs. Table S3. Comparison of the melting point values of the ILs with those previously reported in the literature [,,,,,].
Author Contributions
Conceptualization, L.O.M. and I.V.T.; Methodology, D.M.H., V.V.K., K.Y.D., V.S.B. and I.V.S.; Software, I.V.T. and V.V.K.; Validation, V.V.K.; Formal analysis, I.V.S.; Investigation, S.P.R., D.M.H., L.O.M. and K.Y.D.; Resources, I.V.T.; Data curation, V.V.K. and D.M.H.; Writing—original draft preparation, L.O.M., D.M.H., V.V.K. and S.P.R.; Writing—review and editing, I.V.T. and L.O.M.; Visualization, D.M.H.; Supervision, L.O.M. and I.V.T.; Project administration, L.O.M. and I.V.T.; Funding Acquisition, I.V.T. and L.O.M. All authors have read and agreed to the published version of the manuscript.
Funding
The project was partially funded by the German Federal Ministry for Education and Research (BMBF) under grant agreement No. 01DK20018. The content of this publication is the full responsibility of the authors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data and models are available at http://ochem.eu/article/139940 (accessed on 4 April 2022).
Conflicts of Interest
I.V.T. is the CEO of BIGCHEM GmbH, which licenses the OCHEM software. Other authors declare no conflict of interest.
Abbreviations
| AOA | Antioxidant activity |
| CDK | Chemistry Development Kit |
| CFU | Colony-forming unit |
| CNF | Convolutional neural fingerprint |
| CNN | Convolutional neural network |
| ILs | Ionic liquids |
| MAE | Mean absolute error |
| MDR | Multidrug-resistant |
| OCHEM | Online chemical database and modeling environment |
| PILs | Phosphonium ionic liquids |
| QSAR | Quantitative structure–activity relationship |
| RF | Random forest |
References
- Chen, L.-K.; Kuo, S.-C.; Chang, K.-C.; Cheng, C.-C.; Yu, P.-Y.; Chang, C.-H.; Chen, T.-Y.; Tseng, C.-C. Clinical Antibiotic-Resistant Acinetobacter Baumannii Strains with Higher Susceptibility to Environmental Phages than Antibiotic-Sensitive Strains. Sci. Rep. 2017, 7, 6319. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-López, R.; Solano-Gálvez, S.G.; Juárez Vignon-Whaley, J.J.; Abello Vaamonde, J.A.; Padró Alonzo, L.A.; Rivera Reséndiz, A.; Muleiro Álvarez, M.; Vega López, E.N.; Franyuti-Kelly, G.; Álvarez-Hernández, D.A.; et al. Acinetobacter Baumannii Resistance: A Real Challenge for Clinicians. Antibiotics 2020, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Namiganda, V.; Mina, Y.; Meklat, A.; Touati, D.; Bouras, N.; Barakate, M.; Sabaou, N. Antibiotic Resistance Pattern of Acinetobacter Baumannii Strains Isolated from Different Clinical Specimens and Their Sensibility Against Bioactive Molecules Produced by Actinobacteria. Arab. J. Sci. Eng. 2019, 44, 6267–6275. [Google Scholar] [CrossRef]
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and Pathophysiological Overview of Acinetobacter Infections: A Century of Challenges. Clin. Microbiol. Rev. 2017, 30, 409–447. [Google Scholar] [CrossRef] [Green Version]
- Nazarov, P.A.; Sorochkina, A.I.; Karakozova, M.V. New Functional Criterion for Evaluation of Homologous MDR Pumps. Front. Microbiol. 2020, 11, 2844. [Google Scholar] [CrossRef]
- Łuczak, J.; Jungnickel, C.; Łącka, I.; Stolte, S.; Hupka, J. Antimicrobial and Surface Activity of 1-Alkyl-3-Methylimidazolium Derivatives. Green Chem. 2010, 12, 593–601. [Google Scholar] [CrossRef]
- Cornellas, A.; Perez, L.; Comelles, F.; Ribosa, I.; Manresa, A.; Garcia, M.T. Self-Aggregation and Antimicrobial Activity of Imidazolium and Pyridinium Based Ionic Liquids in Aqueous Solution. J. Colloid Interface Sci. 2011, 355, 164–171. [Google Scholar] [CrossRef]
- Hodyna, D.; Kovalishyn, V.; Rogalsky, S.; Blagodatnyi, V.; Petko, K.; Metelytsia, L. Antibacterial Activity of Imidazolium-Based Ionic Liquids Investigated by QSAR Modeling and Experimental Studies. Chem. Biol. Drug Des. 2016, 88, 422–433. [Google Scholar] [CrossRef]
- Florio, W.; Becherini, S.; D’Andrea, F.; Lupetti, A.; Chiappe, C.; Guazzelli, L. Comparative Evaluation of Antimicrobial Activity of Different Types of Ionic Liquids. Mater. Sci. Eng. C 2019, 104, 109907. [Google Scholar] [CrossRef]
- Semenyuta, I.V.; Trush, M.M.; Kovalishyn, V.V.; Rogalsky, S.P.; Hodyna, D.M.; Karpov, P.; Xia, Z.; Tetko, I.V.; Metelytsia, L.O. Structure-Activity Relationship Modeling and Experimental Validation of the Imidazolium and Pyridinium Based Ionic Liquids as Potential Antibacterials of MDR Acinetobacter Baumannii and Staphylococcus Aureus. Int. J. Mol. Sci. 2021, 22, 563. [Google Scholar] [CrossRef]
- Schrekker, H.S.; Donato, R.K.; Fuentefria, A.M.; Bergamo, V.; Oliveira, L.F.; Machado, M.M. Imidazolium Salts as Antifungal Agents: Activity against Emerging Yeast Pathogens, without Human Leukocyte Toxicity. Med. Chem. Commun. 2013, 4, 1457–1460. [Google Scholar] [CrossRef]
- Bergamo, V.Z.; Donato, R.K.; Dalla Lana, D.F.; Donato, K.J.Z.; Ortega, G.G.; Schrekker, H.S.; Fuentefria, A.M. Imidazolium Salts as Antifungal Agents: Strong Antibiofilm Activity against Multidrug-Resistant Candida Tropicalis Isolates. Lett. Appl. Microbiol. 2015, 60, 66–71. [Google Scholar] [CrossRef]
- Reddy, G.K.K.; Nancharaiah, Y.V.; Venugopalan, V.P. Long Alkyl-Chain Imidazolium Ionic Liquids: Antibiofilm Activity against Phototrophic Biofilms. Colloids Surf. B Biointerfaces 2017, 155, 487–496. [Google Scholar] [CrossRef]
- Atefi, F.; Garcia, M.T.; Singer, R.D.; Scammells, P.J. Phosphonium Ionic Liquids: Design, Synthesis and Evaluation of Biodegradability. Green Chem. 2009, 11, 1595–1604. [Google Scholar] [CrossRef] [Green Version]
- Kanazawa, A.; Ikeda, T.; Endo, T. Synthesis and Antimicrobial Activity of Dimethyl- and Trimethyl-Substituted Phosphonium Salts with Alkyl Chains of Various Lengths. Antimicrob. Agents Chemother. 1994, 38, 945–952. [Google Scholar] [CrossRef] [Green Version]
- Cieniecka-Rosłonkiewicz, A.; Pernak, J.; Kubis-Feder, J.; Ramani, A.; Robertson, A.J.; Seddon, K.R. Synthesis, Anti-Microbial Activities and Anti-Electrostatic Properties of Phosphonium-Based Ionic Liquids. Green Chem. 2005, 7, 855–862. [Google Scholar] [CrossRef]
- O’Toole, G.A.; Wathier, M.; Zegans, M.E.; Shanks, R.M.Q.; Kowalski, R.; Grinstaff, M.W. Diphosphonium Ionic Liquids as Broad-Spectrum Antimicrobial Agents. Cornea 2012, 31, 810–816. [Google Scholar] [CrossRef] [Green Version]
- Wylie, M.P.; Bell, S.E.J.; Nockemann, P.; Bell, R.; McCoy, C.P. Phosphonium Ionic Liquid-Infused Poly(Vinyl Chloride) Surfaces Possessing Potent Antifouling Properties. ACS Omega 2020, 5, 7771–7781. [Google Scholar] [CrossRef]
- Ermolaev, V.V.; Arkhipova, D.M.; Miluykov, V.A.; Lyubina, A.P.; Amerhanova, S.K.; Kulik, N.V.; Voloshina, A.D.; Ananikov, V.P. Sterically Hindered Quaternary Phosphonium Salts (QPSs): Antimicrobial Activity and Hemolytic and Cytotoxic Properties. Int. J. Mol. Sci. 2022, 23, 86. [Google Scholar] [CrossRef]
- Kumar, V.; Malhotra, S.V. Antitumor Activity of Ionic Liquids on Human Tumor Cell Lines. In Ionic Liquid Applications: Pharmaceuticals, Therapeutics, and Biotechnology; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2010; Volume 1038, pp. 91–102. ISBN 9780841225473. [Google Scholar]
- Kumar, V.; Malhotra, S.V. Study on the Potential Anti-Cancer Activity of Phosphonium and Ammonium-Based Ionic Liquids. Bioorg. Med. Chem. Lett. 2009, 19, 4643–4646. [Google Scholar] [CrossRef]
- Choi, S.Y.; Rodríguez, H.; Gunaratne, H.Q.N.; Puga, A.V.; Gilpin, D.; McGrath, S.; Vyle, J.S.; Tunney, M.M.; Rogers, R.D.; McNally, T. Dual Functional Ionic Liquids as Antimicrobials and Plasticisers for Medical Grade PVCs. RSC Adv. 2014, 4, 8567–8581. [Google Scholar] [CrossRef]
- Okoro, C.C. The Biocidal Efficacy of Tetrakis-Hydroxymethyl Phosphonium Sulfate (THPS) Based Biocides on Oil Pipeline PigRuns Liquid Biofilms. Pet. Sci. Technol. 2015, 33, 1366–1372. [Google Scholar] [CrossRef]
- Knorre, D.A.; Markova, O.V.; Smirnova, E.A.; Karavaeva, I.E.; Sokolov, S.S.; Severin, F.F. Dodecyltriphenylphosphonium Inhibits Multiple Drug Resistance in the Yeast Saccharomyces Cerevisiae. Biochem. Biophys. Res. Commun. 2014, 450, 1481–1484. [Google Scholar] [CrossRef]
- Galkina, K.V.; Besedina, E.G.; Zinovkin, R.A.; Severin, F.F.; Knorre, D.A. Penetrating Cations Induce Pleiotropic Drug Resistance in Yeast. Sci. Rep. 2018, 8, 8131. [Google Scholar] [CrossRef]
- Nazarov, P.A.; Osterman, I.A.; Tokarchuk, A.V.; Karakozova, M.V.; Korshunova, G.A.; Lyamzaev, K.G.; Skulachev, M.V.; Kotova, E.A.; Skulachev, V.P.; Antonenko, Y.N. Mitochondria-Targeted Antioxidants as Highly Effective Antibiotics. Sci. Rep. 2017, 7, 1394. [Google Scholar] [CrossRef]
- Trush, M.M.; Kovalishyn, V.; Hodyna, D.; Golovchenko, O.V.; Chumachenko, S.; Tetko, I.V.; Brovarets, V.S.; Metelytsia, L. In Silico and in Vitro Studies of a Number PILs as New Antibacterials against MDR Clinical Isolate Acinetobacter Baumannii. Chem. Biol. Drug Des. 2020, 95, 624–630. [Google Scholar] [CrossRef]
- Eze, E.C.; Chenia, H.Y.; Zowalaty, M.E.E. Acinetobacter Baumannii Biofilms: Effects of Physicochemical Factors, Virulence, Antibiotic Resistance Determinants, Gene Regulation, and Future Antimicrobial Treatments. Infect. Drug Resist. 2018, 11, 2277–2299. [Google Scholar] [CrossRef] [Green Version]
- Qi, L.; Li, H.; Zhang, C.; Liang, B.; Li, J.; Wang, L.; Du, X.; Liu, X.; Qiu, S.; Song, H. Relationship between Antibiotic Resistance, Biofilm Formation, and Biofilm-Specific Resistance in Acinetobacter Baumannii. Front. Microbiol. 2016, 7, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, K.S.; Mawang, C.I.; Daniel-Jambun, D.; Lim, Y.Y.; Lee, S.M. Current Anti-Biofilm Strategies and Potential of Antioxidants in Biofilm Control. Expert Rev. Anti-Infect. Ther. 2018, 16, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Gambino, M.; Cappitelli, F. Mini-Review: Biofilm Responses to Oxidative Stress. Biofouling 2016, 32, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Deene, Y.D.; Hurley, C.; Venning, A.; Vergote, K.; Mather, M.; Healy, B.J.; Baldock, C. A Basic Study of Some Normoxic Polymer Gel Dosimeters. Phys. Med. Biol. 2002, 47, 3441–3463. [Google Scholar] [CrossRef]
- Jirasek, A.; Hilts, M.; Shaw, C.; Baxter, P. Investigation of Tetrakis Hydroxymethyl Phosphonium Chloride as an Antioxidant for Use in X-Ray Computed Tomography Polyacrylamide Gel Dosimetry. Phys. Med. Biol. 2006, 51, 1891–1906. [Google Scholar] [CrossRef]
- Sushko, I.; Novotarskyi, S.; Körner, R.; Pandey, A.K.; Rupp, M.; Teetz, W.; Brandmaier, S.; Abdelaziz, A.; Prokopenko, V.V.; Tanchuk, V.Y.; et al. Online Chemical Modeling Environment (OCHEM): Web Platform for Data Storage, Model Development and Publishing of Chemical Information. J. Comput. Aided Mol. Des. 2011, 25, 533–554. [Google Scholar] [CrossRef] [Green Version]
- Karpov, P.; Godin, G.; Tetko, I.V. Transformer-CNN: Swiss Knife for QSAR Modeling and Interpretation. J. Cheminform. 2020, 12, 17. [Google Scholar] [CrossRef] [Green Version]
- Tetko, I.V.; Karpov, P.; Bruno, E.; Kimber, T.B.; Godin, G. Augmentation Is What You Need! In Proceedings of the Artificial Neural Networks and Machine Learning—ICANN 2019: Workshop and Special Sessions; Tetko, I.V., Kůrková, V., Karpov, P., Theis, F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 831–835. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Hall, L.H.; Kier, L.B. Electrotopological State Indices for Atom Types: A Novel Combination of Electronic, Topological, and Valence State Information. J. Chem. Inf. Comput. Sci. 1995, 35, 1039–1045. [Google Scholar] [CrossRef]
- Tetko, I.V.; Tanchuk, V.Y. Application of Associative Neural Networks for Prediction of Lipophilicity in ALOGPS 2.1 Program. J. Chem. Inf. Comput. Sci. 2002, 42, 1136–1145. [Google Scholar] [CrossRef]
- Tetko, I.V.; Tanchuk, V.Y.; Kasheva, T.N.; Villa, A.E. Estimation of Aqueous Solubility of Chemical Compounds Using E-State Indices. J. Chem. Inf. Comput. Sci. 2001, 41, 1488–1493. [Google Scholar] [CrossRef]
- Willighagen, E.L.; Mayfield, J.W.; Alvarsson, J.; Berg, A.; Carlsson, L.; Jeliazkova, N.; Kuhn, S.; Pluskal, T.; Rojas-Chertó, M.; Spjuth, O.; et al. The Chemistry Development Kit (CDK) v2.0: Atom Typing, Depiction, Molecular Formulas, and Substructure Searching. J. Cheminform. 2017, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Bjerrum, E.J. SMILES Enumeration as Data Augmentation for Neural Network Modeling of Molecules. arXiv 2017, arXiv:1703.07076. [Google Scholar]
- Whitley, D.C.; Ford, M.G.; Livingstone, D.J. Unsupervised Forward Selection: A Method for Eliminating Redundant Variables. J. Chem. Inf. Comput. Sci. 2000, 40, 1160–1168. [Google Scholar] [CrossRef]
- Tetko, I.V.; Sushko, I.; Pandey, A.K.; Zhu, H.; Tropsha, A.; Papa, E.; Oberg, T.; Todeschini, R.; Fourches, D.; Varnek, A. Critical Assessment of QSAR Models of Environmental Toxicity against Tetrahymena Pyriformis: Focusing on Applicability Domain and Overfitting by Variable Selection. J. Chem. Inf. Model. 2008, 48, 1733–1746. [Google Scholar] [CrossRef] [Green Version]
- Sushko, I.; Novotarskyi, S.; Körner, R.; Pandey, A.K.; Kovalishyn, V.V.; Prokopenko, V.V.; Tetko, I.V. Applicability Domain for in Silico Models to Achieve Accuracy of Experimental Measurements. J. Chemom. 2010, 24, 202–208. [Google Scholar] [CrossRef]
- OCHEM Introduction-OCHEM User’s Manual-OCHEM Docs. Available online: http://docs.ochem.eu//display/MAN.html (accessed on 27 November 2021).
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Eloff, J.N. Sensitive and Quick Microplate Method to Determine the Minimal Inhibitory Concentration of Plant Extracts for Bacteria. Planta med. 1998, 64, 711–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esterbauer, H.; Cheeseman, K.H. Determination of Aldehydic Lipid Peroxidation Products: Malonaldehyde and 4-Hydroxynonenal. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1990; Volume 186, pp. 407–421. ISBN 00766879. [Google Scholar]
- Papastergiadis, A.; Mubiru, E.; Van Langenhove, H.; De Meulenaer, B. Malondialdehyde Measurement in Oxidized Foods: Evaluation of the Spectrophotometric Thiobarbituric Acid Reactive Substances (TBARS) Test in Various Foods. J. Agric. Food Chem. 2012, 60, 9589–9594. [Google Scholar] [CrossRef] [PubMed]
- Sushko, I.; Salmina, E.; Potemkin, V.A.; Poda, G.; Tetko, I.V. ToxAlerts: A Web Server of Structural Alerts for Toxic Chemicals and Compounds with Potential Adverse Reactions. J. Chem. Inf. Model. 2012, 52, 2310–2316. [Google Scholar] [CrossRef]
- Phongpaichit, S.; Nikom, J.; Rungjindamai, N.; Sakayaroj, J.; Hutadilok-Towatana, N.; Rukachaisirikul, V.; Kirtikara, K. Biological Activities of Extracts from Endophytic Fungi Isolated from Garcinia Plants. FEMS Immunol. Med. Microbiol. 2007, 51, 517–525. [Google Scholar] [CrossRef] [Green Version]
- Chua, S.L.; Ding, Y.; Liu, Y.; Cai, Z.; Zhou, J.; Swarup, S.; Drautz-Moses, D.I.; Schuster, S.C.; Kjelleberg, S.; Givskov, M.; et al. Reactive Oxygen Species Drive Evolution of Pro-Biofilm Variants in Pathogens by Modulating Cyclic-Di-GMP Levels. Open Biol. 2016, 6, 160162. [Google Scholar] [CrossRef] [Green Version]
- Grant, S.S.; Hung, D.T. Persistent Bacterial Infections, Antibiotic Tolerance, and the Oxidative Stress Response. Virulence 2013, 4, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Yehye, W.A.; Rahman, N.A.; Ariffin, A.; Abd Hamid, S.B.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M. Understanding the Chemistry behind the Antioxidant Activities of Butylated Hydroxytoluene (BHT): A Review. Eur. J. Med. Chem. 2015, 101, 295–312. [Google Scholar] [CrossRef]
- Tauber, J.; Imbri, D.; Opatz, T. Radical Addition to Iminium Ions and Cationic Heterocycles. Molecules 2014, 19, 6190. [Google Scholar] [CrossRef]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Curreri, A.M.; Mitragotri, S.; Tanner, E.E.L. Recent Advances in Ionic Liquids in Biomedicine. Adv. Sci. 2021, 8, 2004819. [Google Scholar] [CrossRef]
- Nikfarjam, N.; Ghomi, M.; Agarwal, T.; Hassanpour, M.; Sharifi, E.; Khorsandi, D.; Ali Khan, M.; Rossi, F.; Rossetti, A.; Nazarzadeh Zare, E.; et al. Antimicrobial Ionic Liquid-Based Materials for Biomedical Applications. Adv. Funct. Mater. 2021, 31, 2104148. [Google Scholar] [CrossRef]
- Farghaly, T.A.; Abdalla, M.M. Synthesis, Tautomerism, and Antimicrobial, Anti-HCV, Anti-SSPE, Antioxidant, and Antitumor Activities of Arylazobenzosuberones. Bioorg. Med. Chem. 2009, 17, 8012–8019. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Heleno, S.A.; Reis, F.S.; Stojkovic, D.; Queiroz, M.J.R.P.; Vasconcelos, M.H.; Sokovic, M. Chemical Features of Ganoderma Polysaccharides with Antioxidant, Antitumor and Antimicrobial Activities. Spec. Issue Ganoderma Phytochem. 2015, 114, 38–55. [Google Scholar] [CrossRef] [Green Version]
- Kartashova, O.L.; Utkina, T.M.; Zhestkov, A.V.; Kurkin, V.A.; Zolotarev, P.N. Effect of phytosubstances with antioxidant activity on persistence properties of microorganisms. Antibiot. Chemoter. 2009, 54, 16–18. [Google Scholar]
- Angiolella, L.; Sacchetti, G.; Efferth, T. Antimicrobial and Antioxidant Activities of Natural Compounds. Evid.-Based Complement. Alternat. Med. 2018, 2018, 1945179. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Nur, H.; Ismail, A.F.; Sharif, S.; RamaKrishna, S.; Berto, F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants 2020, 9, 1309. [Google Scholar] [CrossRef]
- Özgeriş, B. Design, Synthesis, Characterization, and Biological Evaluation of Nicotinoyl Thioureas as Antimicrobial and Antioxidant Agents. J. Antibiot. 2021, 74, 233–243. [Google Scholar] [CrossRef]
- Tetko, I.V. Associative Neural Network. Methods Mol. Biol. Clifton NJ 2008, 458, 185–202. [Google Scholar] [CrossRef] [Green Version]
- Frank, E.; Hall, M.; Trigg, L.; Holmes, G.; Witten, I.H. Data Mining in Bioinformatics Using Weka. Bioinformatics 2004, 20, 2479–2481. [Google Scholar] [CrossRef] [Green Version]
- N-Octyl Triphenylphosphonium Bromide. Available online: https://www.chemsrc.com/en/cas/42036-78-2_799327.html (accessed on 18 February 2022).
- Kuz’menok, N.M.; Mikhalyonok, S.G.; Arol, A.S.; Shevchuk, M.O.; Bezborodov, V.S.; Krakhalev, M.N.; Sutormin, V.S.; Prishchepa, O.O.; Zharkova, G.M.; Zyryanov, V.Y. Synthesis of Organotriphenylphosphonium Halides, Quaternary Ammonium Salts and Study of Their Application as Surfactants Soluble in Liquid Crystals. Zhidkie Krist. Ikh Prakt. Ispolzovanie 2020, 20, 6–18. [Google Scholar] [CrossRef]
- Decyl-TPP. Available online: https://www.chemsrc.com/en/cas/32339-43-8_255481.html (accessed on 18 February 2022).
- Ivashchenko, S.P. Lipids. XLIV. A New Synthesis of 1-Alken-1-Yl Alkyl Ethers Using the Wittig Reaction. Zh. Org. Khimii. 1966, 2, 2181–2183. [Google Scholar]
- Kurt, M. Antiseptic Detergent Compositions. Avaialble online: https://patents.google.com/patent/US3281365A (accessed on 1 April 2022).
- Tributyl(Dodecyl)Phosphonium Bromide. Available online: https://www.chemsrc.com/en/cas/15294-63-0_1102483.html (accessed on 19 February 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).