Dosing Colistimethate Every 8 h Results in Higher Plasma Concentrations of Active Colistin Than Every 12-Hourly Dosing without Increase in Nephrotoxicity: A Phase 1 Pharmacokinetics Trial in Healthy Adult Volunteers
Abstract
:1. Introduction
2. Results
2.1. Demographic and Other Baseline Characteristics
2.2. Pharmacokinetic Parameters of Colistin A, Colistin B, CMS A, and CMS B
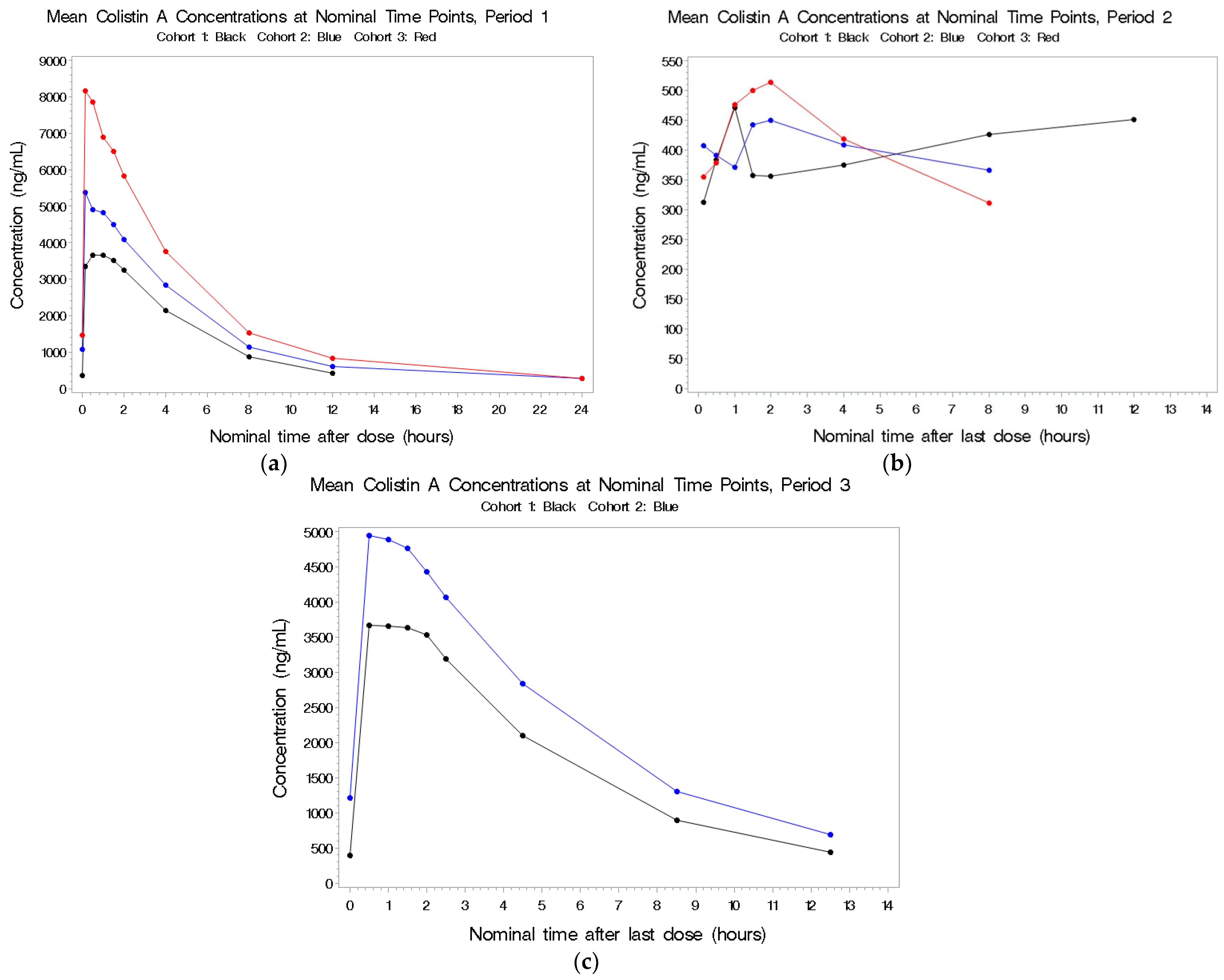
2.2.1. Colistin A
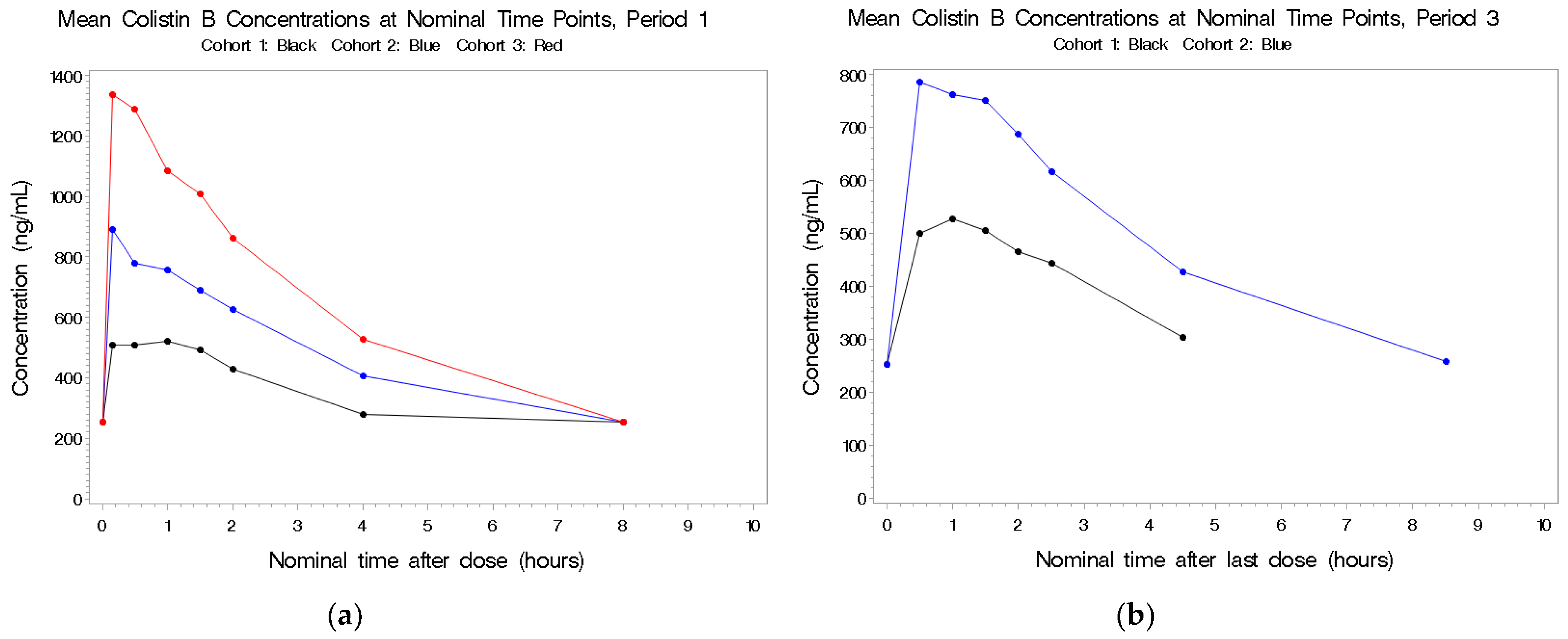
2.2.2. Colistin B
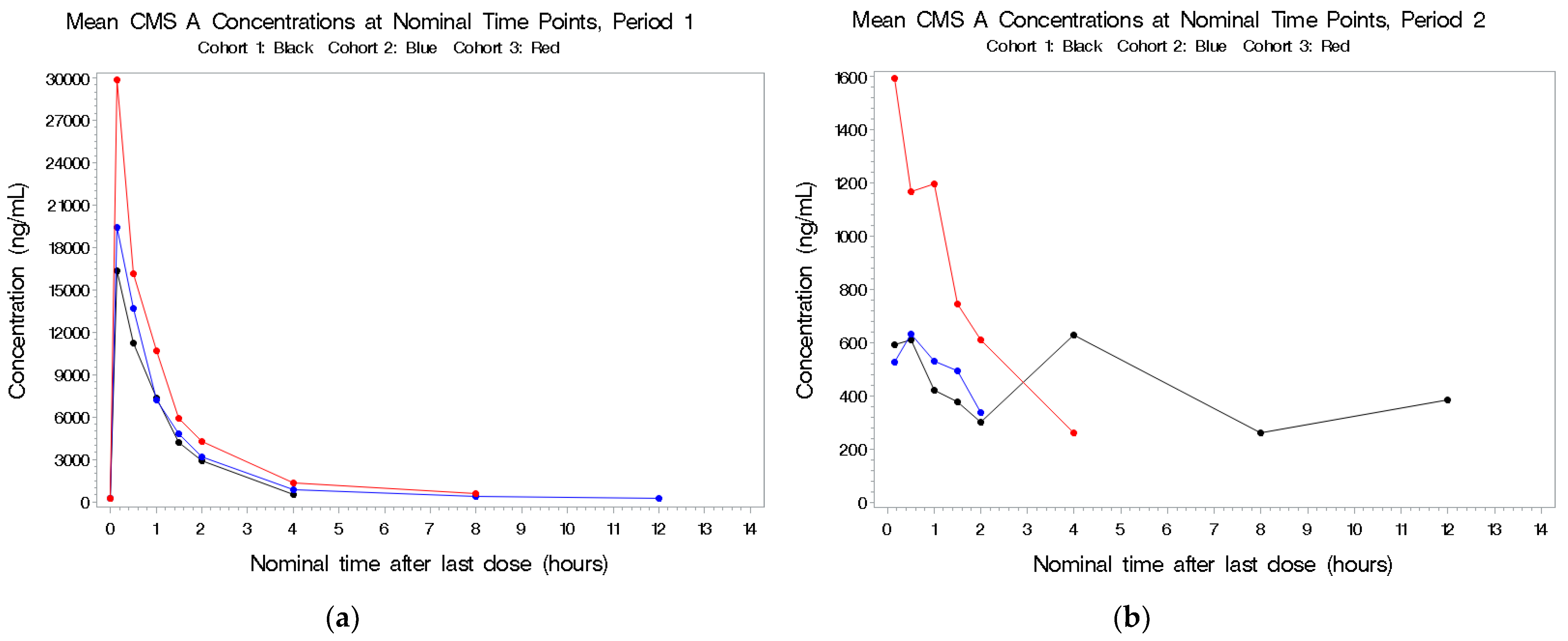
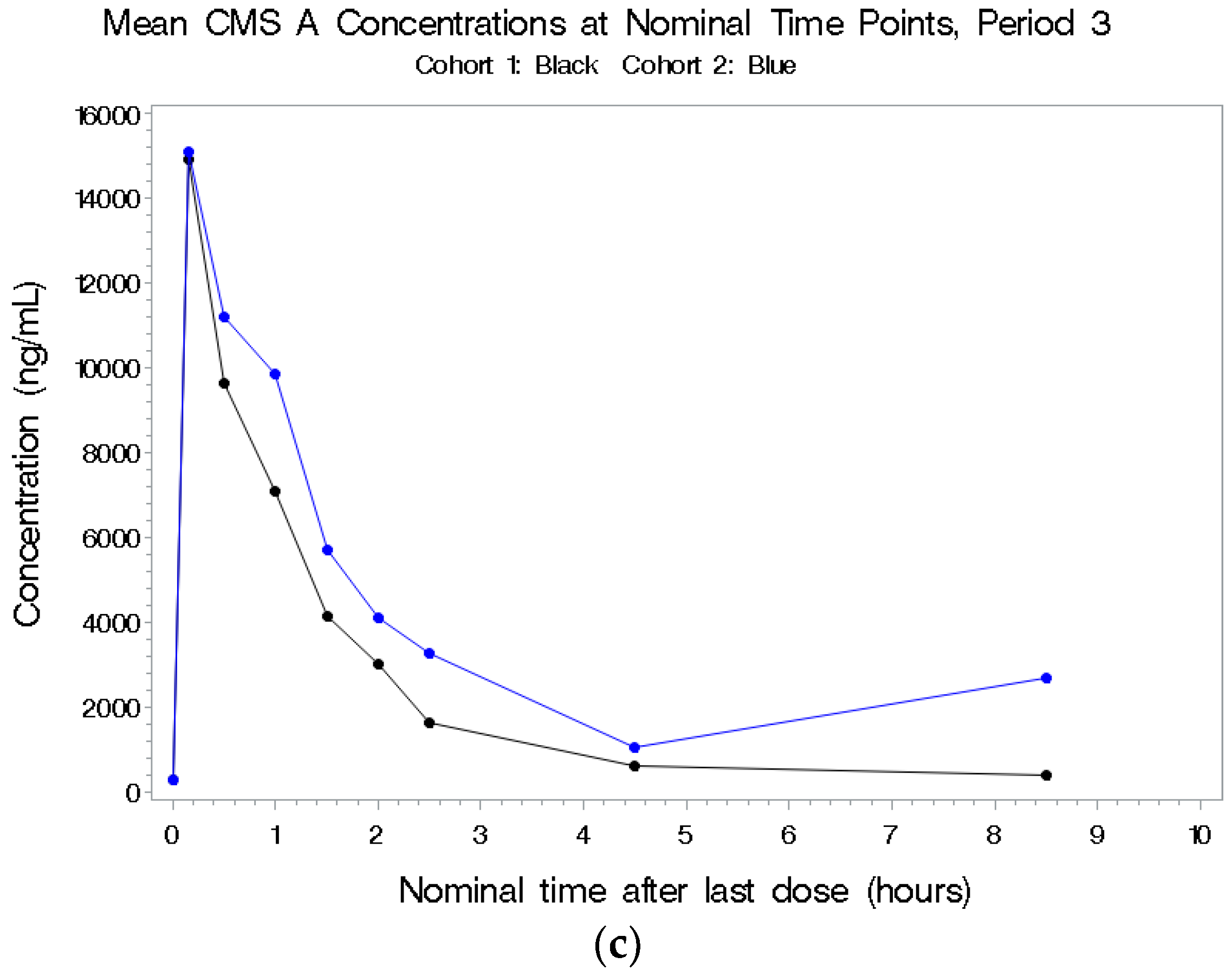
2.2.3. CMS A
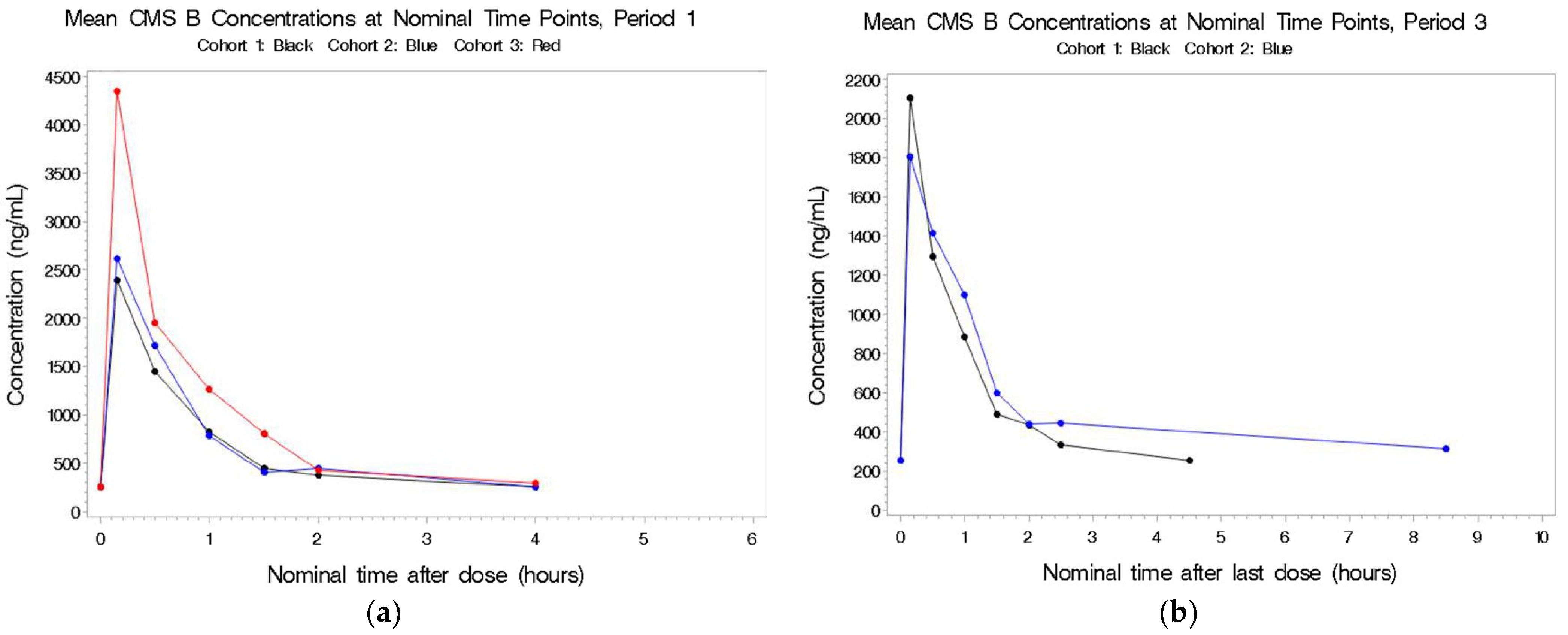
2.2.4. CMS B
2.3. Comparison of Colistin Analyte Concentrations in Plasma and BAL
2.3.1. Colistin A
2.3.2. Colistin B
2.3.3. CMS A
2.3.4. CMS B
2.4. Subject Safety and Tolerability
2.5. Nephrotoxicity and Kidney Biomarkers as Indicators of Future Nephrotoxicity
2.6. Neurotoxicity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Trial Design, Study Population, and Ethics Approval
4.3. Study Objectives
4.4. Methodology
4.5. Pharmacokinetic Assessments
4.5.1. Plasma Samples
4.5.2. Bronchoalveolar Lavage Samples
4.6. Kidney Biomarkers
4.7. Pharmacokinetic Analysis
4.8. Bioanalytical Methods
4.9. Safety Assessments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bergen, P.J.; Li, J.; Rayner, C.R.; Nation, R.L. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2006, 50, 1953–1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalopoulos, A.S.; Falagas, M.E. Colistin: Recent data on pharmacodynamics properties and clinical efficacy in critically ill patients. Ann. Intensive Care 2011, 1, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavascki, A.P.; Nation, R.J. Nephrotoxicity of polymyxins: Is there any difference between colistimethate and polymyxin B? Antimicrob. Agents Chemother. 2017, 61, e02319-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couet, W.; Grégoire, N.; Gobin, P.; Saulnier, P.J.; Frasca, D.; Marchand, S.; Mimoz, O. Pharmacokinetics of Colistin and Colistimethate Sodium After a Single 80-mg Intravenous Dose of CMS in Young Healthy Volunteers. Clin. Pharmacol. Ther. 2011, 89, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, J.; Yagisawa, M. The history of antibiotics: The Japanese story. J. Infect. Chemother. 2002, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Kuti, J.L.; Kim, A.; Cloutier, D.J.; Nicolau, D.P. Evaluation of Plazomicin, Tigecycline, and Meropenem Pharmacodynamic Exposure against Carbapenem-Resistant Enterobacteriaceae in Patients with Bloodstream Infection or Hospital-Acquired/Ventilator-Associated Pneumonia from the CARE Study (ACHN-490-007). Infect. Dis. Ther. 2019, 8, 383–396. [Google Scholar] [CrossRef] [Green Version]
- Abdelsalam, M.F.A.; Abdalla, M.S.; El-Abhar, H.S.E. Prospective, comparative clinical study between high-dose colistin monotherapy and colistin-meropenem combination therapy for treatment of hospital-acquired pneumonia and ventilator-associated pneumonia caused by multidrug-resistant Klebsiella pneumoniae. J. Glob. Antimicrob. Resist. 2018, 15, 127–135. [Google Scholar] [CrossRef]
- Aygun, F.; Aygun, F.D.; Varol, F.; Durak, C.; Cokugraş, H.; Camcioglu, Y.; Cam, H. Can Nebulised Colistin Therapy Improve Outcomes in Critically Ill Children with Multi-Drug Resistant Gram-Negative Bacterial Pneumonia? Antibiotics 2019, 8, 40. [Google Scholar] [CrossRef] [Green Version]
- McKinnell, J.A.; Dwyer, J.P.; Talbot, G.H.; Connolly, L.E.; Friedland, I.; Smith, A.; Jubb, A.M.; Serio, A.W.; Krause, K.M.; Daikos, G.L.; et al. Plazomicin for Infections Caused by Carbapenem-Resistant Enterobacteriaceae. N. Engl. J. Med. 2019, 380, 791–793. [Google Scholar] [CrossRef]
- Plachouras, D.; Karvanen, M.; Friberg, L.E.; Papadomichelakis, E.; Antoniadou, A.; Tsangaris, I.; Karaiskos, I.; Poulakou, G.; Kontopidou, F.; Armaganidis, A.; et al. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob. Agents Chemother. 2009, 53, 3430–3436. [Google Scholar] [CrossRef] [Green Version]
- Nation, R.L.; Garonzik, S.M.; Li, J.; Thamlikitkul, V.; Giamarellos-Bourboulis, E.J.; Paterson, D.L.; Turnidge, J.D.; Forrest, A.; Silveira, F.P. Updated US and European dose recommendations for intravenous colistin: How do they perform? Clin. Infect. Dis. 2016, 62, 552–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FDA Approved Drug Products. Label and Approval History for Coly-Mycin M, NDA 050108. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050108s030lbl.pdf (accessed on 4 January 2022).
- Antoniadou, A.; Kontopidou, F.; Poulakou, G.; Koratzanis, E.; Galani, I.; Papadomichelakis, E.; Kopterides, P.; Souli, M.; Armaganidis, A.; Giamarellou, H. Colistin-resistant isolates of Klebsiella pneumoniae emerging in intensive care unit patients: First report of a multiclonal cluster. J. Antimicrob. Chemother. 2007, 59, 786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchaim, D.; Chopra, T.; Pogue, J.M.; Perez, F.; Hujer, A.M.; Rudin, S.; Endimiani, A.; Navon-Venezia, S.; Hothi, J.; Slim, J.; et al. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob. Agents Chemother. 2011, 55, 593–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capone, A.; Giannella, M.; Fortini, D.; Giordano, A.; Meledandri, M.; Ballardini, M.; Venditti, M.; Bordi, E.; Capozzi, D.; Balice, M.P.; et al. High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortality. Clin. Microbiol. Infect. 2013, 19, E23–E30. [Google Scholar] [CrossRef] [Green Version]
- Caniaux, I.; van Belkum, A.; Zambardi, G.; Poirel, L.; Gros, M.F. MCR: Modern colistin resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 415–420. [Google Scholar] [CrossRef] [Green Version]
- Schmitz-Hübsch, T.; du Montcel, S.T.; Baliko, L.; Berciano, J.; Boesch, S.; Depondt, C.; Giunti, P.; Globas, C.; Infante, J.; Kang, J.S.; et al. Scale for the assessment and rating of ataxia: Development of a new clinical scale. Neurology 2006, 66, 1717–1720. [Google Scholar] [CrossRef]
- Koch-Weser, J.; Sidel, V.W.; Federman, E.B.; Kanarek, P.; Finer, D.C.; Eaton, A.E. Adverse effects of sodium colistimethate. Manifestations and specific reaction rates during 317 courses of therapy. Ann. Intern. Med. 1970, 72, 857. [Google Scholar] [CrossRef]
- Imberti, R.; Cusato, M.; Villani, P.; Carnevale, L.; Iotti, G.A.; Langer, M.; Regazzi, M. Steady-state pharmacokinetics and BAL concentration of colistin in critically ill patients after IV colistin methanesulfonate administration. Chest 2010, 138, 1333–1339. [Google Scholar] [CrossRef]
- Grgurich, P.E.; Hudcova, J.; Lei, Y.; Sarwar, A.; Craven, D.E. Management and prevention of ventilator-associated pneumonia caused by multidrug-resistant pathogens. Expert Rev. Respir. Med. 2012, 6, 533–555. [Google Scholar] [CrossRef]
- Kwa, A.L.; Loh, C.; Low, J.G.; Kurup, A.; Tam, V.H. Nebulized colistin in the treatment of pneumonia due to multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Clin. Infect. Dis. 2005, 41, 754–757. [Google Scholar] [CrossRef]
- Berlana, D.; Llop, J.M.; Fort, E.; Badia, M.B.; Jódar, R. Use of colistin in the treatment of multiple-drug-resistant gram-negative infections. Am. J. Health Syst. Pharm. 2005, 62, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Daikos, G.L.; Durante-Mangoni, E.; Yahav, D.; Carmeli, Y.; Benattar, Y.D.; Skiada, A.; Andini, R.; Eliakim-Raz, N.; Nutman, A.; et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: An open-label, randomised controlled trial. Lancet Infect. Dis. 2018, 18, 391–400. [Google Scholar] [CrossRef]
- Falagas, M.E.; Siempos, I.I.; Rafailidis, P.I.; Korbila, I.P.; Ioannidou, E.; Michalopoulos, A. Inhaled colistin as monotherapy for multidrug- resistant gram (−) nosocomial pneumonia: A case series. Respir. Med. 2009, 103, 707–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naesens, R.; Vlieghe, E.; Verbrugghe, W.; Jorens, P.; Ieven, M. A retrospective observational study on the efficacy of colistin by inhalation as compared to parenteral administration for the treatment of nosocomial pneumonia associated with multidrug-resistant Pseudomonas aeruginosa. BMC Infect. Dis. 2011, 11, 317. [Google Scholar] [CrossRef] [Green Version]

| Cohort | N | Dosing Period 1 (IV) | Washout | Dosing Period 2 (aerosolized) | Washout | Dosing Period 3 (combination IV and aerosolized) |
|---|---|---|---|---|---|---|
| 1 | 9 | 2.5 mg/kg colistin base activity q12h × 2 doses (total exposure: 5 mg/kg) | At least 3 days | 75 mg colistin base activity q12h × 2 doses | At least 3 days | IV: 2.5 mg/kg colistin base activity q12h × 2 doses Aerosol: 75 mg colistin base activity q12h × 2 doses |
| 2 | 9 | 2.5 mg/kg colistin base activity q8h × 3 doses (total exposure: 7.5 mg/kg) | At least 3 days | 75 mg colistin base activity q8h × 3 doses | At least 3 days | IV: 2.5 mg/kg colistin base activity q8h × 3 doses Aerosol: 75 mg colistin base activity q8h × 3 doses |
| 3 | 9 | 3.3 mg/kg colistin base activity q8h × 3 doses (total exposure: 10 mg/kg) | At least 3 days | 75 mg colistin base activity q6h × 4 doses | At least 3 days | IV: 3.3 mg/kg colistin base activity q8h × 3 doses Aerosol: 75 mg colistin base activity q6h × 4 doses |
| Characteristics | Pharmacokinetic Population | Safety Population | ||||
|---|---|---|---|---|---|---|
| Cohort 1 (N = 9) | Cohort 2 (N = 8) | Cohort 3 (N = 12) | Cohort 1 (N = 9) | Cohort 2 (N = 10) | Cohort 3 (N = 12) | |
| Age (years) | ||||||
| n | 9 | 8 | 12 | 9 | 10 | 12 |
| Mean (SD) | 23.9 (4.4) | 24.9 (4.0) | 24.9 (5.1) | 23.9 (4.4) | 25.2 (4.7) | 24.9 (5.1) |
| Median | 21.0 | 23.5 | 22.5 | 21.0 | 23.5 | 22.5 |
| Min, Max | 19, 30 | 21, 31 | 19, 38 | 19, 30 | 20, 33 | 19, 38 |
| Gender | ||||||
| Male | 6 (66.7%) | 2 (25.0%) | 8 (66.7%) | 6 (66.7%) | 3 (30.0%) | 8 (66.7%) |
| Female | 3 (33.3%) | 6 (75.0%) | 4 (33.3%) | 3 (33.3%) | 7 (70.0%) | 4 (33.3%) |
| Unknown | 0 | 0 | 0 | 0 | 0 | 0 |
| Race | ||||||
| White | 7 (77.8%) | 6 (75.0%) | 8 (66.7%) | 7 (77.8%) | 7 (70.0%) | 8 (66.7%) |
| Black/African American | 0 | 1 (12.5%) | 1 (8.3%) | 0 | 2 (20.0%) | 1 (8.3%) |
| American Indian/Alaskan Native | 0 | 0 | 0 | 0 | 0 | 0 |
| Native Hawaiian /Other Pacific Islander | 0 | 0 | 0 | 0 | 0 | 0 |
| Asian | 2 (22.2%) | 1 (12.5%) | 3 (25.0%) | 2 (22.2%) | 1 (10.0%) | 3 (25.0%) |
| Other | 0 | 0 | 0 | 0 | 0 | 0 |
| Ethnicity | ||||||
| Hispanic or Latino | 1 (11.1%) | 0 | 2 (16.7%) | 1 (11.1%) | 0 | 2 (16.7%) |
| Non-Hispanic or Latino | 8 (88.9%) | 8 (100.0%) | 10 (83.3%) | 8 (88.9%) | 10 (100.0%) | 10 (83.3%) |
| Characteristics | Cohort 1 (N = 9) | Cohort 2 (N = 10) | Cohort 3 (N = 12) | Overall (N = 31) |
|---|---|---|---|---|
| Total Number of Adverse Events | 60 | 108 | 119 | 287 |
| Number of Subjects with at Least One Adverse Event | 9 (100.0%) | 10 (100.0%) | 11 (91.7%) | 30 (96.8%) |
| Investigations | 7 (77.8%) | 6 (60.0%) | 11 (91.7%) | 24 (77.4%) |
| Blood calcium decreased | 2 (22.2%) | 0 | 4 (33.3%) | 6 (19.4%) |
| Blood creatinine increased | 0 | 1 (10.0%) | 4 (33.3%) | 5 (16.1%) |
| Lymphocyte count decreased | 2 (22.2%) | 1 (10.0%) | 1 (8.3%) | 4 (12.9%) |
| Prothrombin time prolonged | 3 (33.3%) | 3 (30.0%) | 1 (8.3%) | 7 (22.6%) |
| Red blood cells urine | 0 | 1 (10.0%) | 3 (25.0%) | 4 (12.9%) |
| White blood cells urine positive | 0 | 2 (20.0%) | 7 (58.3%) | 9 (29.0%) |
| Nervous system disorders | 6 (66.7%) | 10 (100.0%) | 11 (91.7%) | 27 (87.1%) |
| Ataxia | 0 | 7 (70.0%) | 10 (83.3%) | 17 (54.8%) |
| Dizziness | 0 | 3 (30.0%) | 2 (16.7%) | 5 (16.1%) |
| Headache | 5 (55.6%) | 5 (50.0%) | 4 (33.3%) | 14 (45.2%) |
| Paresthesia | 5 (55.6%) | 10 (100.0%) | 9 (75.0%) | 24 (77.4%) |
| Renal and urinary disorders | 1 (11.1%) | 2 (20.0%) | 7 (58.3%) | 10 (32.3%) |
| Glycosuria | 0 | 0 | 5 (41.7%) | 5 (16.1%) |
| Proteinuria | 1 (11.1%) | 2 (20.0%) | 7 (58.3%) | 10 (32.3%) |
| Respiratory, thoracic and mediastinal disorders | 3 (33.3%) | 3 (30.0%) | 2 (16.7%) | 8 (25.8%) |
| Cough | 1 (11.0%) | 3 (30.0%) | 2 (16.7%) | 6 (19.4%) |
| Time Point | Biomarker | Correlation Coefficient | p-Value |
|---|---|---|---|
| Before First Dose | Cystatin | 0.15 | 0.63 |
| Microglobulin | 0.32 | 0.34 | |
| NGAL | −0.25 | 0.43 | |
| 0.5 hr After First Dose | Cystatin | 0.03 | 0.93 |
| Microglobulin | 0.25 | 0.49 | |
| NGAL | −0.26 | 0.43 |
| SARA Test Parameter | Subject SARA Scores 1 | ||||
|---|---|---|---|---|---|
| 20012078 | 20012079 | 20012080 | 20012081 | 20012082 | |
| Gait 2 | 1 | 2 | 3 | 1 | 1 |
| Stance 3 | 0 | 2 | 2 | 0 | 1 |
| Sitting 4 | 0 | 0 | 0 | 0 | 0 |
| Speech disturbance 4 | 0 | 0 | 0 | 0 | 0 |
| Finger chase—right 5 | 0 | 0 | 0 | 0.5 | 0 |
| Finger chase—left 5 | 0 | 0.5 | 0 | 0 | 0 |
| Nose–finger—right 6 | 0 | 0 | 0.5 | 0 | 0 |
| Nose–finger—left 6 | 0 | 0 | 0.5 | 0 | 0 |
| Fast alternating hand movements—right 4 | 0 | 0 | 0 | 0 | 0 |
| Fast alternating hand movements—left 4 | 0 | 0 | 0 | 0 | 0 |
| Heel–shin slide—right 4 | 0 | 0 | 0 | 0 | 0 |
| Heel–shin slide—left 4 | 0 | 0 | 0 | 0 | 0 |
| Cumulative SARA Score | 1 | 4.5 | 6 | 1.5 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yendewa, G.A.; Griffiss, J.M.; Gray, W.A.; Healen, A.; Proskin, H.M.; Fulton, S.A.; O’Riordan, M.A.; Hoppel, C.; Blumer, J.L.; Salata, R.A. Dosing Colistimethate Every 8 h Results in Higher Plasma Concentrations of Active Colistin Than Every 12-Hourly Dosing without Increase in Nephrotoxicity: A Phase 1 Pharmacokinetics Trial in Healthy Adult Volunteers. Antibiotics 2022, 11, 490. https://doi.org/10.3390/antibiotics11040490
Yendewa GA, Griffiss JM, Gray WA, Healen A, Proskin HM, Fulton SA, O’Riordan MA, Hoppel C, Blumer JL, Salata RA. Dosing Colistimethate Every 8 h Results in Higher Plasma Concentrations of Active Colistin Than Every 12-Hourly Dosing without Increase in Nephrotoxicity: A Phase 1 Pharmacokinetics Trial in Healthy Adult Volunteers. Antibiotics. 2022; 11(4):490. https://doi.org/10.3390/antibiotics11040490
Chicago/Turabian StyleYendewa, George A., John McLeod Griffiss, Wesley A. Gray, Amanda Healen, Howard M. Proskin, Scott A. Fulton, Mary Ann O’Riordan, Charles Hoppel, Jeffrey L. Blumer, and Robert A. Salata. 2022. "Dosing Colistimethate Every 8 h Results in Higher Plasma Concentrations of Active Colistin Than Every 12-Hourly Dosing without Increase in Nephrotoxicity: A Phase 1 Pharmacokinetics Trial in Healthy Adult Volunteers" Antibiotics 11, no. 4: 490. https://doi.org/10.3390/antibiotics11040490
APA StyleYendewa, G. A., Griffiss, J. M., Gray, W. A., Healen, A., Proskin, H. M., Fulton, S. A., O’Riordan, M. A., Hoppel, C., Blumer, J. L., & Salata, R. A. (2022). Dosing Colistimethate Every 8 h Results in Higher Plasma Concentrations of Active Colistin Than Every 12-Hourly Dosing without Increase in Nephrotoxicity: A Phase 1 Pharmacokinetics Trial in Healthy Adult Volunteers. Antibiotics, 11(4), 490. https://doi.org/10.3390/antibiotics11040490







