A Cross-Sectional Study of Veterinarians in Germany on the Impact of the TÄHAV Amendment 2018 on Antimicrobial Use and Development of Antimicrobial Resistance in Dogs and Cats
Abstract
:1. Introduction
2. Results
2.1. Practice Information
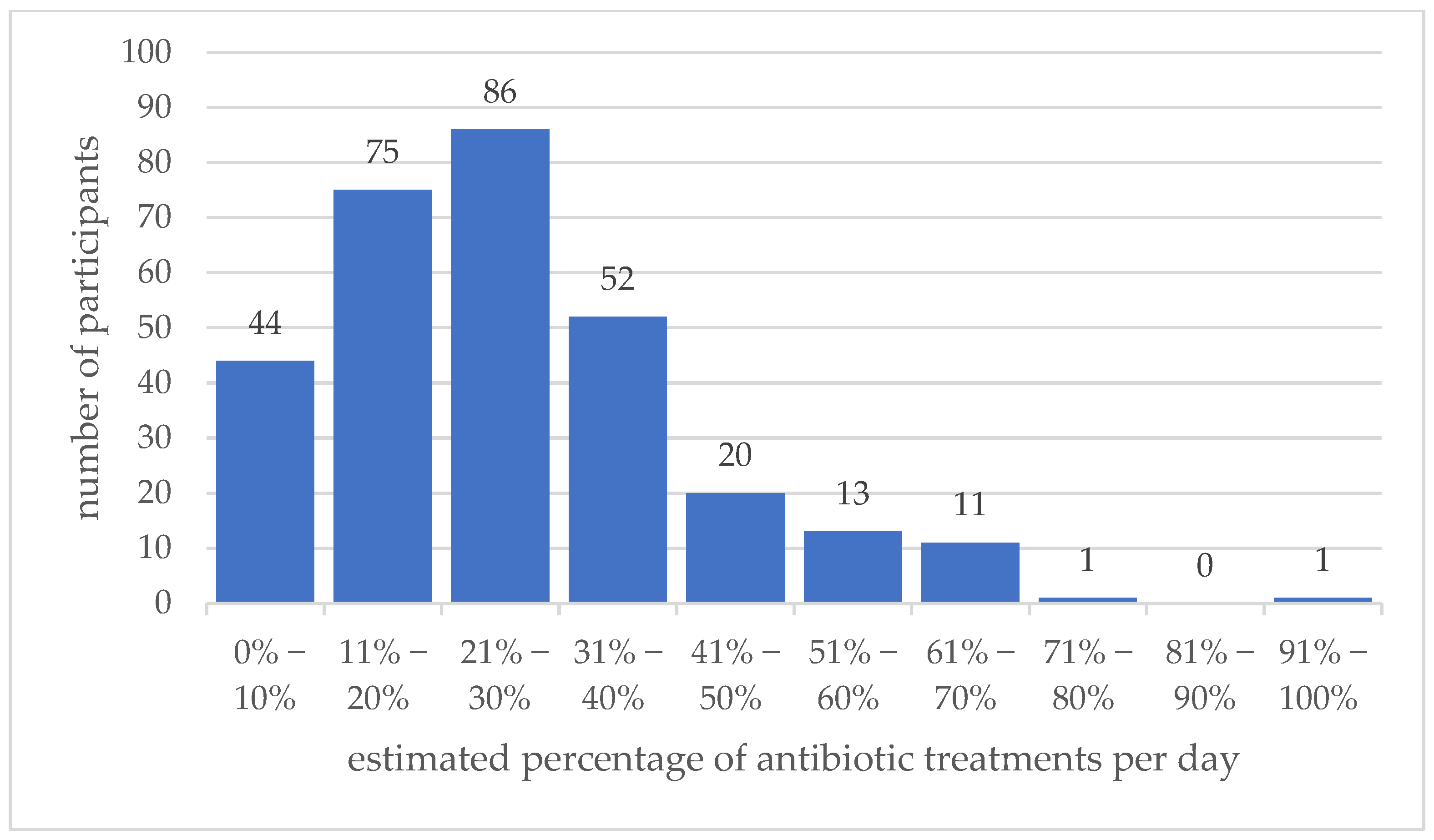
2.2. Antibiotic Use
3. Discussion
Limitations
4. Materials and Methods
4.1. Questionnaire
4.2. Implementation
4.3. Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richter, A.; Feßler, A.T.; Böttner, A.; Köper, L.M.; Wallmann, J.; Schwarz, S. Reasons for antimicrobial treatment failures and predictive value of in-vitro susceptibility testing in veterinary practice: An overview. Vet. Microbiol. 2020, 245, 108694. [Google Scholar] [CrossRef] [PubMed]
- Bundestierärztekammer. Leitlinien für den sorgfältigen Umgang mit antibakteriell wirksamen Tierarzneimitteln. Dt TÄBl 2015, 58, 2. [Google Scholar]
- Guardabassi, L.; Butaye, P.; Dockrell, D.H.; Fitzgerald, J.R.; Kuijper, E.J. One Health: A multifaceted concept combining diverse approaches to prevent and control antimicrobial resistance. Clin. Microbiol. Infect. 2020, 26, 1604–1605. [Google Scholar] [CrossRef] [PubMed]
- Guardabassi, L.; Schwarz, S.; Lloyd, D.H. Pet animals as reservoirs of antimicrobial-resistant bacteria. J. Antimicrob. Chemother. 2004, 54, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Hopman, N.E.M.; Portengen, L.; Hulscher, M.; Heederik, D.J.J.; Verheij, T.J.M.; Wagenaar, J.A.; Prins, J.M.; Bosje, T.; Schipper, L.; van Geijlswijk, I.M.; et al. Implementation and evaluation of an antimicrobial stewardship programme in companion animal clinics: A stepped-wedge design intervention study. PLoS ONE 2019, 14, e0225124. [Google Scholar] [CrossRef] [PubMed]
- Toutain, P.L.; Bousquet-Mélou, A.; Damborg, P.; Ferran, A.A.; Mevius, D.; Pelligand, L.; Veldman, K.T.; Lees, P. En Route towards European Clinical Breakpoints for Veterinary Antimicrobial Susceptibility Testing: A Position Paper Explaining the VetCAST Approach. Front. Microbiol. 2017, 8, 2344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guardabassi, L.; Apley, M.; Olsen, J.E.; Toutain, P.L.; Weese, S. Optimization of Antimicrobial Treatment to Minimize Resistance Selection. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Prescott, J.F. Antimicrobial use in food and companion animals. Anim. Health Res. Rev. 2008, 9, 127–133. [Google Scholar] [CrossRef]
- Guardabassi, L. Sixty years of antimicrobial use in animals: What is next? Vet. Rec. 2013, 173, 599–603. [Google Scholar] [CrossRef]
- Köck, R.; Daniels-Haardt, I.; Becker, K.; Mellmann, A.; Friedrich, A.W.; Mevius, D.; Schwarz, S.; Jurke, A. Carbapenem-resistant Enterobacteriaceae in wildlife, food-producing, and companion animals: A systematic review. Clin. Microbiol. Infect. 2018, 24, 1241–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano, C.; Rezusta, A.; Ferrer, I.; Pérez-Laguna, V.; Zarazaga, M.; Ruiz-Ripa, L.; Revillo, M.J.; Torres, C. Staphylococcus pseudintermedius Human Infection Cases in Spain: Dog-to-Human Transmission. Vector Borne Zoonotic Dis. 2017, 17, 268–270. [Google Scholar] [CrossRef]
- Pomba, C.; Rantala, M.; Greko, C.; Baptiste, K.E.; Catry, B.; Van Duijkeren, E.; Mateus, A.; Moreno, M.A.; Pyörälä, S.; Ružauskas, M.; et al. Public health risk of antimicrobial resistance transfer from companion animals. J. Antimicrob. Chemother. 2017, 72, 957–968. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO List of Critically Important Antimicrobials for Human Medicine; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Bundestierärztekammer. Die neue TÄHAV ist in Kraft. Dt TÄBl 2018, 66, 484–489. [Google Scholar]
- Bundesrat. Drucksache 759/17; Berlin, Germany, 2017. Available online: https://www.bundesrat.de/SharedDocs/drucksachen/2017/0701-0800/759-17.pdf?__blob=publicationFile&v=1 (accessed on 11 January 2022).
- Schnepf, A.; Kramer, S.; Wagels, R.; Volk, H.A.; Kreienbrock, L. Evaluation of Antimicrobial Usage in Dogs and Cats at a Veterinary Teaching Hospital in Germany in 2017 and 2018. Front. Vet. Sci. 2021, 8, 689018. [Google Scholar] [CrossRef] [PubMed]
- Bundestierärztekammer. Tierärztestatistik. Available online: https://www.bundestieraerztekammer.de/btk/statistik/ (accessed on 29 November 2021).
- Jessen, L.R.; Sørensen, T.M.; Lilja, Z.L.; Kristensen, M.; Hald, T.; Damborg, P. Cross-sectional survey on the use and impact of the Danish national antibiotic use guidelines for companion animal practice. Acta. Vet. Scand. 2017, 59, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Joosten, P.; Ceccarelli, D.; Odent, E.; Sarrazin, S.; Graveland, H.; Van Gompel, L.; Battisti, A.; Caprioli, A.; Franco, A.; Wagenaar, J.A.; et al. Antimicrobial Usage and Resistance in Companion Animals: A Cross-Sectional Study in Three European Countries. Antibiotics 2020, 9, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, S.; Kehrenberg, C.; Walsh, T.R. Use of antimicrobial agents in veterinary medicine and food animal production. Int. J. Antimicrob. Agents 2001, 17, 431–437. [Google Scholar] [CrossRef]
- Goggs, R.; Menard, J.M.; Altier, C.; Cummings, K.J.; Jacob, M.E.; Lalonde-Paul, D.F.; Papich, M.G.; Norman, K.N.; Fajt, V.R.; Scott, H.M.; et al. Patterns of antimicrobial drug use in veterinary primary care and specialty practice: A 6-year multi-institution study. J. Vet. Intern. Med. 2021, 35, 1496–1508. [Google Scholar] [CrossRef] [PubMed]
- Singleton, D.A.; Sánchez-Vizcaíno, F.; Dawson, S.; Jones, P.H.; Noble, P.J.M.; Pinchbeck, G.L.; Williams, N.J.; Radford, A.D. Patterns of antimicrobial agent prescription in a sentinel population of canine and feline veterinary practices in the United Kingdom. Vet. J. 2017, 224, 18–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escher, M.; Vanni, M.; Intorre, L.; Caprioli, A.; Tognetti, R.; Scavia, G. Use of antimicrobials in companion animal practice: A retrospective study in a veterinary teaching hospital in Italy. J. Antimicrob. Chemother. 2011, 66, 920–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moerer, M.; Merle, R.; Bäumer, W. Antibiotikaeinsatz und Resistenzentwicklung bei Hund und Katze unter dem Einfluss der TÄHAV-Novelle 2018—Ein Stimmungsbild Berliner Tierärzte. Berl. Munch. Tierarztl. Wochenschr. 2022, 135, 1–13. [Google Scholar] [CrossRef]
- Mateus, A.L.; Brodbelt, D.C.; Barber, N.; Stärk, K.D. Qualitative study of factors associated with antimicrobial usage in seven small animal veterinary practices in the UK. Prev. Vet. Med. 2014, 117, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Buckland, E.L.; O’Neill, D.; Summers, J.; Mateus, A.; Church, D.; Redmond, L.; Brodbelt, D. Characterisation of antimicrobial usage in cats and dogs attending UK primary care companion animal veterinary practices. Vet. Rec. 2016, 179, 489. [Google Scholar] [CrossRef] [Green Version]
- Weese, J.S.; Blondeau, J.; Boothe, D.; Guardabassi, L.G.; Gumley, N.; Papich, M.; Jessen, L.R.; Lappin, M.; Rankin, S.; Westropp, J.L.; et al. International Society for Companion Animal Infectious Diseases (ISCAID) guidelines for the diagnosis and management of bacterial urinary tract infections in dogs and cats. Vet. J. 2019, 247, 8–25. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortazar Schmidt, C.; Herskin, M.; Michel, V.; et al. Assessment of animal diseases caused by bacteria resistant to antimicrobials: Dogs and cats. EFSA J. 2021, 19, e06680. [Google Scholar] [CrossRef]
- De Briyne, N.; Atkinson, J.; Pokludová, L.; Borriello, S.P. Antibiotics used most commonly to treat animals in Europe. Vet. Rec. 2014, 175, 325. [Google Scholar] [CrossRef] [Green Version]
- Kvaale, M.K.; Grave, K.; Kristoffersen, A.B.; Norström, M. The prescription rate of antibacterial agents in dogs in Norway—Geographical patterns and trends during the period 2004–2008. J. Vet. Pharmacol. Ther. 2013, 36, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Mateus, A.; Brodbelt, D.C.; Barber, N.; Stärk, K.D. Antimicrobial usage in dogs and cats in first opinion veterinary practices in the UK. J. Small Anim. Pract. 2011, 52, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Poveda, B.; Moreno, M.A. Antimicrobial Prescriptions for Dogs in the Capital of Spain. Front. Vet. Sci. 2018, 5, 309. [Google Scholar] [CrossRef]
- Bundesamt für Verbraucherschutz und Lebensmittelsicherheit. Resistenzsituation Bei Klinisch Wichtigen Tierpathogenen Bakterien—BVL-Report 15.6 Bericht zur Resistenzmonitoringstudie 2019; Bundesamt für Verbraucherschutz und Lebensmittelsicherheit: Berlin, Germany, 2021; p. 98. Available online: https://www.bvl.bund.de/SharedDocs/Berichte/07_Resistenzmonitoringstudie/Bericht_Resistenzmonitoring_2019.pdf?__blob=publicationFile&v=2 (accessed on 4 April 2022).
- Beever, L.; Bond, R.; Graham, P.A.; Jackson, B.; Lloyd, D.H.; Loeffler, A. Increasing antimicrobial resistance in clinical isolates of Staphylococcus intermedius group bacteria and emergence of MRSP in the UK. Vet. Rec. 2015, 176, 172. [Google Scholar] [CrossRef] [PubMed]
- Singleton, D.A.; Pinchbeck, G.L.; Radford, A.D.; Arsevska, E.; Dawson, S.; Jones, P.H.; Noble, P.-J.M.; Williams, N.J.; Sánchez-Vizcaíno, F. Factors Associated with Prescription of Antimicrobial Drugs for Dogs and Cats, United Kingdom, 2014–2016. Emerg. Infect. Dis. 2020, 26, 1778–1791. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S. Investigation of antimicrobial use and the impact of antimicrobial use guidelines in a small animal veterinary teaching hospital: 1995–2004. J. Am. Vet. Med. Assoc. 2006, 228, 553–558. [Google Scholar] [CrossRef]
- De Briyne, N.; Atkinson, J.; Pokludová, L.; Borriello, S.P.; Price, S. Factors influencing antibiotic prescribing habits and use of sensitivity testing amongst veterinarians in Europe. Vet. Rec. 2013, 173, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, C.; De Jong, A.; Moyaert, H.; El Garch, F.; Janes, R.; Klein, U.; Morrissey, I.; Thiry, J.; Youala, M. Antimicrobial susceptibility monitoring of dermatological bacterial pathogens isolated from diseased dogs and cats across Europe (ComPath results). J. Appl. Microbiol. 2016, 121, 1254–1267. [Google Scholar] [CrossRef]
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Available online: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf (accessed on 27 October 2021).
- Nocera, F.P.; Ambrosio, M.; Fiorito, F.; Cortese, L.; De Martino, L. On Gram-Positive- and Gram-Negative-Bacteria-Associated Canine and Feline Skin Infections: A 4-Year Retrospective Study of the University Veterinary Microbiology Diagnostic Laboratory of Naples, Italy. Animals 2021, 11, 1603. [Google Scholar] [CrossRef] [PubMed]
- Mayring, P. Zum Verhältnis qualitativer und quantitativer Analyse. In Methoden der Umweltbildungsforschung; Bolscho, D., Ed.; VS Verlag für Sozialwissenschaften: Opladen, Germany, 1999; pp. 13–25. [Google Scholar]
| Absolute Number | Percentages | 95% CI | |
|---|---|---|---|
| Do you use topical or systemic antibiotics more frequently? | |||
| Topical more frequent | 19 | 6.3% | 4.05–9.59% |
| Systemic more frequent | 186 | 61.4% | 55.79–66.69% |
| About equally often | 98 | 32.3% | 27.33–37.8% |
| Are penicillins the antibiotics you use most often? | |||
| I agree | 283 | 93.3% | 90.03–95.69% |
| I partly agree | 12 | 4% | 2.28–6.79% |
| I disagree | 8 | 2.7% | 1.34–5.12% |
| Are HPCIA the antibiotics you rarely use? | |||
| I agree | 259 | 85.6% | 81.07–89% |
| I partly agree | 26 | 8.6% | 5.92–12.28% |
| I disagree | 18 | 5.8% | 3.79–9.19% |
| Do you use HPCIA less frequently since the 2018 amendment to the TÄHAV? | |||
| I agree | 240 | 79.2% | 74.29–83.4% |
| I partly agree | 31 | 10.2% | 7.3–14.16% |
| I disagree | 32 | 41.6% | 7.58–14.53% |
| Did the 2018 amendment to the TÄHAV lead to a general reduction in antimicrobial treatments? | |||
| I agree | 108 | 35.6% | 30.46–41.19% |
| I partly agree | 69 | 22.8% | 18.41–27.82% |
| I disagree | 126 | 41.6% | 36.17–47.21% |
| Have you had more AST done since the introduction of the TÄHAV amendment? | |||
| I agree | 190 | 62.7% | 57.13–67.96% |
| I partly agree | 60 | 19.8% | 15.71–24.66% |
| I disagree | 53 | 17.5% | 14–22% |
| Do you generally use penicillins as empirical treatment, due to the TÄHAV amendment? | |||
| I agree | 230 | 75.9% | 70.79–80.38% |
| I partly agree | 37 | 12.2% | 8.99–16.38% |
| I disagree | 36 | 11.9% | 8.71–16.01% |
| After AST, do penicillins more frequently need to be replaced by another substance, compared to other antimicrobial agents? | |||
| I agree | 73 | 24.1% | 19.62–29.21% |
| I partly agree | 103 | 34% | 28.89–39.5% |
| I disagree | 127 | 41.9% | 36.49–47.54% |
| Have you frequently used AST alongside with treatment or only when a change of antibiotic is needed? | |||
| Parallel with treatment | 135 | 44.7% | 39.2–50.34% |
| To change an antibiotic | 62 | 20.5% | 16.36–25.44% |
| Equally often parallel and to change the agent | 105 | 34.8% | 29.62–40.3% |
| For the treatment of which diseases do you need AST particularly often? | |||
| Otitis externa | 190 | 62.7% | 57.13–67.96% |
| Cystitis | 168 | 55.4% | 49.82–60.94% |
| Wounds | 132 | 43.6% | 38.1–49.19% |
| Pyoderma | 88 | 29% | 24.22–34.39% |
| Respiratory Infections | 71 | 23.4% | 19.01–28.52% |
| Diarrhea | 47 | 15.5% | 11.87–20.02% |
| From which species are samples most frequently sent in for resistance testing? | |||
| Cats | 19 | 6.3% | 4.05–9.59% |
| Dogs | 116 | 38.3% | 32.99–43.87% |
| Cats and Dogs equally often | 168 | 55.4% | 49.82–60.94% |
| What are owners’ reactions to additional costs due to antibiogram requirements? | |||
| They agree with submitting a sample. | 159 | 52.5% | 46.86–58.03% |
| They partly agree with submitting a sample. | 107 | 35.3% | 30.15–40.85% |
| They disagree with submitting a sample. | 37 | 12.2% | 8.99–16.38% |
| Cystitis | Otitis Externa | Pyoderma | Bite Wounds | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absolute Number | Percentage | 95% CI | Absolute Number | Percentage | 95% CI | Absolute Number | Percentage | 95% CI | Absolute Number | Percentage | 95% CI | ||
| Antibiotic use | Always (80–100%) | 53 | 18.30% | 14.3–23.21% | 95 | 31.70% | 26.66–37.13% | 68 | 23.40% | 18.81–28.46% | 208 | 71.70% | 66.28–76.6% |
| Frequent (60–79%) | 95 | 32.90% | 27.71–38.48% | 111 | 37% | 31.73–42.6% | 89 | 30.60% | 25.48–35.98% | 59 | 20.30% | 16.11–25.35% | |
| Partly (40–59%) | 74 | 25.60% | 20.92–30.93% | 56 | 18.70% | 14.66–23.46% | 71 | 24.40% | 19.75–29.55% | 16 | 5.50% | 3.42–8.77% | |
| Rarely to never (0–39%) | 67 | 23.20% | 18.69–28.38% | 38 | 12.70% | 9.37–16.91% | 63 | 21.60% | 17.24–26.65% | 7 | 2.40% | 1.17–4.9% | |
| AST | Always (80–100%) | 36 | 12.50% | 9.14–16.76% | 18 | 6% | 3.83–9.28% | 18 | 6.20% | 3.93–9.53% | 13 | 4.50% | 2.64–7.52% |
| Frequent (60–79%) | 41 | 14.20% | 10.63–18.68% | 21 | 7% | 4.62–10.46% | 21 | 7.20% | 4.75–10.74% | 11 | 3.80% | 2.13–6.66% | |
| Partly (40–59%) | 66 | 22.80% | 18.37–28.01% | 42 | 14% | 10.53–18.38% | 30 | 10.30% | 7.29–14.29% | 28 | 9.70% | 6.76–13.6% | |
| Rarely (20–39%) | 105 | 36.30% | 31–42.02% | 147 | 49% | 43.39–54.63% | 150 | 51.40% | 45.66–57.05% | 95 | 32.80% | 27.61–38.36% | |
| Never (0–19%) | 41 | 14.20% | 10.63–18.68% | 72 | 24% | 19.52–29.14% | 73 | 25% | 20.38–30.27% | 143 | 49.30% | 43.6–55.04% | |
| Absolute Number | Percentage | 95% CI | |
|---|---|---|---|
| Active ingredient | |||
| Penicillins | 216 | 74.7% | 69.43–79.4% |
| Fluoroquinolones | 51 | 17.6% | 13.68–22.46% |
| Trimethoprim-Sulfonamide | 18 | 6.2% | 3.98–9.63% |
| Others | 16 | 5.5% | 3.44–8.8% |
| Loss of antibiotic therapy success | |||
| No | 219 | 75.8% | 70.52–80.36% |
| Yes | 70 | 24.2% | 19.64–29.48% |
| Penicillins | 58/70 | 82.9% | 72.38–89.91% |
| Fluoroquinolones | 11/70 | 15.7% | 9.01–25.99% |
| Trimethoprim-Sulfonamide | 10/70 | 14.3% | 7.95–24.34% |
| Absolute Number | Percentage | 95% CI | |
|---|---|---|---|
| Active ingredient | |||
| Polymyxin B | 135 | 45% | 39.47–50.66% |
| Gentamicin | 85 | 28.3% | 23.53–33.68% |
| Florfenicol | 63 | 21% | 16.77–25.96% |
| Others | 17 | 5.7% | 3.57–8.89% |
| Loss of antibiotic therapy success | |||
| No | 240 | 80% | 75.11–84.13% |
| Yes | 60 | 20% | 15.87–24.89% |
| Polymyxin B | 42/60 | 70% | 57.49–80.1% |
| Gentamicin | 14/60 | 23.3% | 14.44–35.44% |
| Marbofloxacin | 9/60 | 15% | 8.1–26.11% |
| Florfenicol | 4/60 | 6.7% | 2.62–15.93% |
| Absolute Number | Percentage | 95% CI | |
|---|---|---|---|
| Active ingredient for superficial pyoderma | |||
| Fusidic acid | 103 | 35.4% | 30.02–40.91% |
| Polymyxin B | 64 | 22% | 17.55–27.01% |
| Neomycin | 56 | 19.2% | 15.07–24.08% |
| Active ingredient for deep pyoderma | |||
| Penicillins | 164 | 56.3% | 50.43–61.74% |
| Cephalosporins | 113 | 38.7% | 33.29–44.4% |
| Loss of antibiotic therapy success | |||
| No | 253 | 86.6% | 82.26–90.07% |
| Yes | 39 | 13.4% | 9.93–17.74% |
| Penicillins | 34/39 | 87.2% | 73.29–94.4% |
| Cephalosporins | 10/39 | 25.6% | 14.57–41.08% |
| Fluoroquinolones | 3/39 | 7.7% | 2.65–20.32% |
| Absolute Number | Percentage | 95% CI | |
|---|---|---|---|
| Active ingredient | |||
| Penicillins | 274 | 94.5% | 91.23–96.58% |
| Others | 16 | 5.5% | 3.42–8.77% |
| Loss of antibiotic therapy success | |||
| No | 263 | 86.6% | 86.79–93.52% |
| Yes | 27 | 13.4% | 6.48–13.21% |
| Penicillins | 23/27 | 85.2% | 67.52–94.08% |
| Cefovecin | 6/27 | 22.2% | 10.61–40.76% |
| Fluoroquinolones | 4/27 | 14.8% | 5.92–32.48% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moerer, M.; Merle, R.; Bäumer, W. A Cross-Sectional Study of Veterinarians in Germany on the Impact of the TÄHAV Amendment 2018 on Antimicrobial Use and Development of Antimicrobial Resistance in Dogs and Cats. Antibiotics 2022, 11, 484. https://doi.org/10.3390/antibiotics11040484
Moerer M, Merle R, Bäumer W. A Cross-Sectional Study of Veterinarians in Germany on the Impact of the TÄHAV Amendment 2018 on Antimicrobial Use and Development of Antimicrobial Resistance in Dogs and Cats. Antibiotics. 2022; 11(4):484. https://doi.org/10.3390/antibiotics11040484
Chicago/Turabian StyleMoerer, Marianne, Roswitha Merle, and Wolfgang Bäumer. 2022. "A Cross-Sectional Study of Veterinarians in Germany on the Impact of the TÄHAV Amendment 2018 on Antimicrobial Use and Development of Antimicrobial Resistance in Dogs and Cats" Antibiotics 11, no. 4: 484. https://doi.org/10.3390/antibiotics11040484
APA StyleMoerer, M., Merle, R., & Bäumer, W. (2022). A Cross-Sectional Study of Veterinarians in Germany on the Impact of the TÄHAV Amendment 2018 on Antimicrobial Use and Development of Antimicrobial Resistance in Dogs and Cats. Antibiotics, 11(4), 484. https://doi.org/10.3390/antibiotics11040484







