Systemic Antibiotic Prophylaxis in Maxillofacial Trauma: A Scoping Review and Critical Appraisal
Abstract
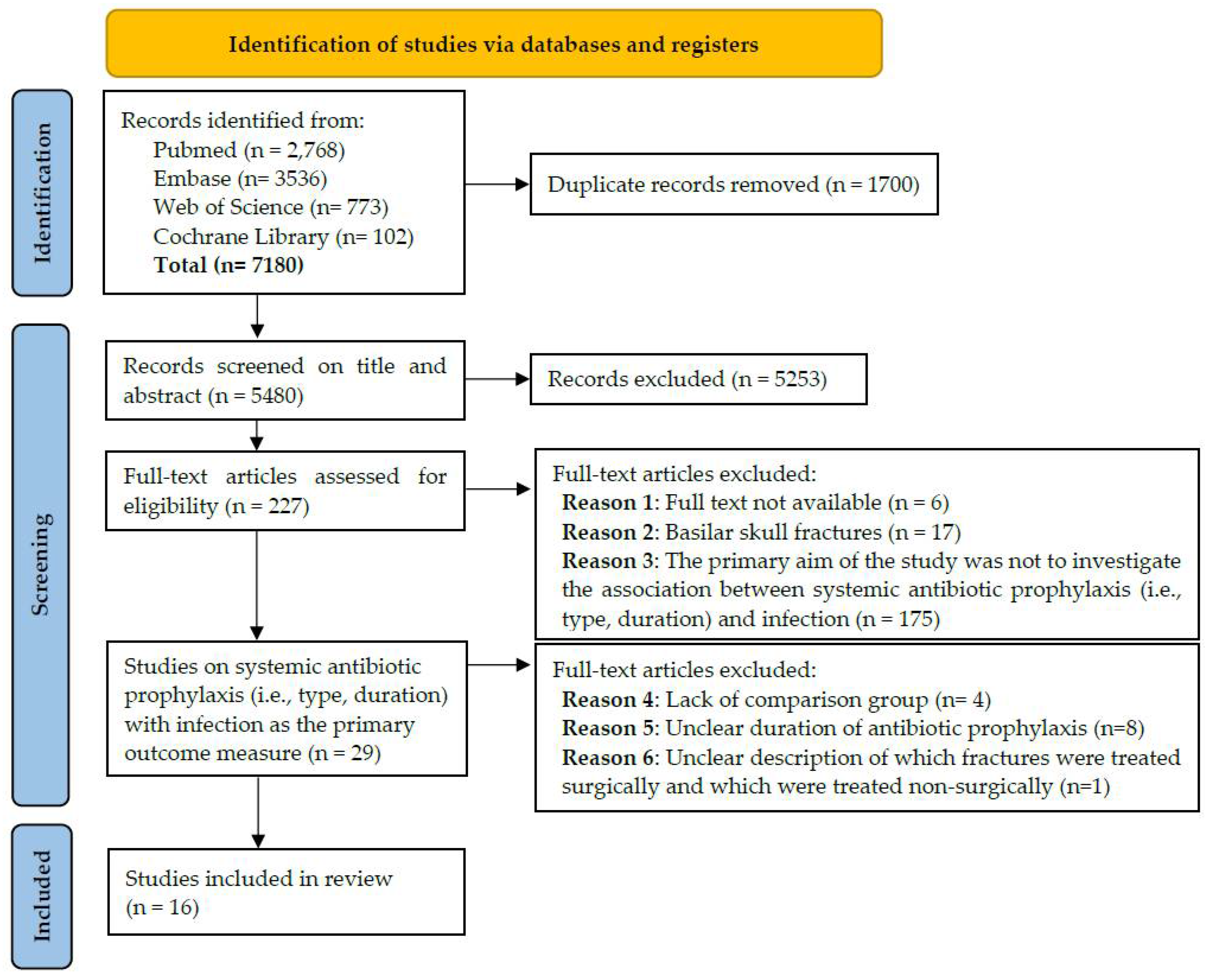
1. Introduction
2. Methods
2.1. Data Sources and Search Strategy
2.2. Inclusion/Exclusion Criteria
2.3. Study Selection
2.4. Data Extraction and Assessment of Evidence Level
3. Results
3.1. Study Design and Research Aims
3.2. Study Outcomes
3.2.1. Fractures of the Lower Facial Third
3.2.2. Fractures of the Midface and Upper Facial Third
3.2.3. Antibiotic Type
3.3. Outcome Description
3.4. Follow-Up Period
4. Discussion
4.1. Duration of Systemic Antibiotic Prophylaxis
4.2. Clinical Heterogeneity and the Limited Number of High-Quality Studies
4.2.1. Patient Characteristics
4.2.2. Antibiotic Type and Duration
4.2.3. Causal Pathogens
4.2.4. Fracture Type
4.2.5. Surgical Treatment
4.2.6. Outcome Description
4.2.7. Follow-Up Period
4.3. Discrepancy between Guidelines and Clinical Practice
4.4. Implications of Antibiotic Overuse
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dawoud, B.E.S.; Kent, S.; Henry, A.; Wareing, J.; Chaudry, H.; Kyzas, P. Use of antibiotics in traumatic mandibular fractures: A systematic review and meta-analysis. Br. J. Oral Maxillofac. Surg. 2021. [Google Scholar] [CrossRef] [PubMed]
- Badia, J.M.; Casey, A.L.; Petrosillo, N.; Hudson, P.M.; Mitchell, S.A.; Crosby, C. Impact of surgical site infection on healthcare costs and patient outcomes: A systematic review in six European countries. J. Hosp. Infect. 2017, 96, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, P.A. Use of antibiotics in the treatment of mandible fractures: A systematic review. J. Oral Maxillofac. Surg. 2011, 69, 1129–1145. [Google Scholar] [CrossRef] [PubMed]
- Jang, N.; Shin, H.W. Are postoperative prophylactic antibiotics in closed reduction of nasal bone fracture valuable?: Prospective study of 30 cases. Arch. Craniofac. Surg. 2019, 20, 89–93. [Google Scholar] [CrossRef]
- Metsemakers, W.J.; Morgenstern, M.; McNally, M.A.; Moriarty, T.F.; McFadyen, I.; Scarborough, M.; Athanasou, N.A.; Ochsner, P.E.; Kuehl, R.; Raschke, M.; et al. Fracture-related infection: A consensus on definition from an international expert group. Injury 2018, 49, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, J.O.; Jensen, S.S.; Schwartz, O.; Hillerup, Y. A systematic review of prophylactic antibiotics in the surgical treatment of maxillofacial fractures. J. Oral Maxillofac. Surg. 2006, 64, 1664–1668. [Google Scholar] [CrossRef] [PubMed]
- Delaplain, P.T.; Phillips, J.L.; Lundeberg, M.; Nahmias, J.; Kuza, C.M.; Sheehan, B.M.; Murphy, L.S.; Pejcinovska, M.; Grigorian, A.; Gabriel, V.; et al. No Reduction in Surgical Site Infection Obtained with Post-Operative Antibiotics in Facial Fractures, Regardless of Duration or Anatomic Location: A Systematic Review and Meta-Analysis. Surg. Infect. 2020, 21, 112–121. [Google Scholar] [CrossRef]
- Djulbegovic, B.; Guyatt, G.H. Progress in evidence-based medicine: A quarter century on. Lancet 2017, 390, 415–423. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Anderson, D.J.; Sexton, D.J.; Post, T. Antimicrobial Prophylaxis for Prevention of Surgical Site Infection in Adults. UpToDate. 2016. Available online: https://www.uptodate.com/contents/antimicrobial-prophylaxis-for-prevention-of-surgical-site-infection-in-adults (accessed on 17 January 2022).
- Asokan, C.; Ebenezer, V.; Ramalingam, B. Role of prophylactic antibiotics in the surgical treatment of maxillofacial fractures. Indian J. Public Health Res. Dev. 2019, 10, 2848–2852. [Google Scholar] [CrossRef]
- Blatt, S.; Al-Nawas, B. A systematic review of latest evidence for antibiotic prophylaxis and therapy in oral and maxillofacial surgery. Infection 2019, 47, 519–555. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, H.; Hennedige, A.; Patel, M. Review of prophylactic prescribing of antibiotics during the management of fractured mandibles. Surgeon 2021, 19, e519–e525. [Google Scholar] [CrossRef]
- Habib, A.M.; Wong, A.D.; Schreiner, G.C.; Satti, K.F.; Riblet, N.B.; Johnson, H.A.; Ossoff, J.P. Postoperative prophylactic antibiotics for facial fractures: A systematic review and meta-analysis. Laryngoscope 2019, 129, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Milic, T.; Raidoo, P.; Gebauer, D. Antibiotic prophylaxis in oral and maxillofacial surgery: A systematic review. Br. J. Oral Maxillofac. Surg. 2020, 59, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Mundinger, G.S.; Borsuk, D.E.; Okhah, Z.; Christy, M.R.; Bojovic, B.; Dorafshar, A.H.; Rodriguez, E.D. Antibiotics and facial fractures: Evidence-based recommendations compared with experience-based practice. Craniomaxillofac. Trauma Reconstr. 2014, 8, 64–78. [Google Scholar] [CrossRef]
- Shridharani, S.M.; Berli, J.; Manson, P.N.; Tufaro, A.P.; Rodriguez, E.D. The Role of Postoperative Antibiotics in Mandible Fractures: A Systematic Review of the Literature. Ann. Plast. Surg. 2015, 75, 353–357. [Google Scholar] [CrossRef]
- Puetzler, J.; Zalavras, C.; Moriarty, T.F.; Verhofstad, M.H.J.; Kates, S.L.; Raschke, M.J.; Rosslenbroich, S.; Metsemakers, W.J. Clinical practice in prevention of fracture-related infection: An international survey among 1197 orthopaedic trauma surgeons. Injury 2019, 50, 1208–1215. [Google Scholar] [CrossRef]
- Declercq, P.; Zalavras, C.; Mertens, B.; Van der Linden, L.; Nijs, S.; Spriet, I.; Metsemakers, W.J. Perioperative antibiotic prophylaxis in long bone open fractures: The need for randomized controlled trials. Arch. Orthop. Trauma. Surg. 2021. epub ahead of print. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Abubaker, A.O.; Rollert, M.K. Postoperative antibiotic prophylaxis in mandibular fractures: A preliminary randomized, double-blind, and placebo-controlled clinical study. J. Oral Maxillofac. Surg. 2001, 59, 1415–1419. [Google Scholar] [CrossRef] [PubMed]
- Baliga, S.D.; Bose, A.; Jain, S. The evaluation of efficacy of post-operative antibiotics in the open reduction of the zygomatic and mandibular fracture: A prospective trial. J. Maxillofac. Oral Surg. 2014, 13, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Campos, G.B.; Lucena, E.E.; da Silva, J.S.; Gomes, P.P.; Germano, A.R. Efficacy assessment of two antibiotic prophylaxis regimens in oral and maxillofacial trauma surgery: Preliminary results. Int. J. Clin. Exp. Med. 2015, 8, 2846–2852. [Google Scholar]
- Chole, R.A.; Yee, J. Antibiotic prophylaxis for facial fractures: A prospective, randomized clinical trial. Arch. Otolaryngol.—Head Neck Surg. 1987, 113, 1055–1057. [Google Scholar] [CrossRef]
- Domingo, F.; Dale, E.; Gao, C.; Groves, C.; Stanley, D.; Maxwell, R.A.; Waldrop, J.L. A single-center retrospective review of postoperative infectious complications in the surgical management of mandibular fractures: Postoperative antibiotics add no benefit. J. Trauma Acute Care Surg. 2016, 81, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Lovato, C.; Wagner, J.D. Infection rates following perioperative prophylactic antibiotics versus postoperative extended regimen prophylactic antibiotics in surgical management of mandibular fractures. J. Oral Maxillofac. Surg. 2009, 67, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Miles, B.A.; Potter, J.K.; Ellis, E., III. The efficacy of postoperative antibiotic regimens in the open treatment of mandibular fractures: A prospective randomized trial. J. Oral Maxillofac. Surg. 2006, 64, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Mottini, M.; Wolf, R.; Soong, P.L.; Lieger, O.; Nakahara, K.; Schaller, B. The role of postoperative antibiotics in facial fractures: Comparing the efficacy of a 1-day versus a prolonged regimen. J. Trauma Acute Care Surg. 2014, 76, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Perepa, A.; Sinha, R.; Agarwal, A.; Khan, T.A. Protocol for Antibiotic Administration in Mandibular Trauma: A Prospective Clinical Trial. J. Maxillofac. Oral Surg. 2018, 17, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Reiss, B.; Rajjoub, L.; Mansour, T.; Chen, T.; Mumtaz, A. Antibiotic Prophylaxis in Orbital Fractures. Open Ophthalmol. J. 2017, 11, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Schaller, B.; Soong, P.L.; Zix, J.; Iizuka, T.; Lieger, O. The role of postoperative prophylactic antibiotics in the treatment of facial fractures: A randomized, double-blind, placebo-controlled pilot clinical study. Part 2: Mandibular fractures in 59 patients. Br. J. Oral Maxillofac. Surg. 2013, 51, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Soong, P.L.; Schaller, B.; Zix, J.; Iizuka, T.; Mottini, M.; Lieger, O. The role of postoperative prophylactic antibiotics in the treatment of facial fractures: A randomised, double-blind, placebo-controlled pilot clinical study. Part 3: Le Fort and zygomatic fractures in 94 patients. Br. J. Oral Maxillofac. Surg. 2014, 52, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Zix, J.; Schaller, B.; Iizuka, T.; Lieger, O. The role of postoperative prophylactic antibiotics in the treatment of facial fractures: A randomised, double-blind, placebo-controlled pilot clinical study. Part 1: Orbital fractures in 62 patients. Br. J. Oral Maxillofac. Surg. 2013, 51, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Zosa, B.M.; Ladhani, H.A.; Sajankila, N.; Elliott, C.W.; Claridge, J.A. Pre-Operative Antibiotic Agents for Facial Fractures: Is More than One Day Necessary? Surg. Infect. 2021, 22, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Malekpour, M.; Bridgham, K.; Neuhaus, N.; Widom, K.; Rapp, M.; Leonard, D.; Baro, S.; Dove, J.; Hunsinger, M.; Blansfield, J.; et al. Utility of Prophylactic Antibiotics in Nonoperative Facial Fractures. J. Craniofac. Surg. 2016, 27, 1677–1680. [Google Scholar] [CrossRef] [PubMed]
- Zallen, R.D.; Curry, J.T. A study of antibiotic usage in compound mandibular fractures. J. Oral Surg. 1975, 33, 431–434. [Google Scholar] [PubMed]
- Esce, A.R.; Chavarri, V.M.; Joshi, A.B.; Meiklejohn, D.A. Evaluation of Antibiotic Prophylaxis for Acute Nonoperative Orbital Fractures. Ophthalmic Plast. Reconstr. Surg. 2021, 37, 462–464. [Google Scholar] [CrossRef] [PubMed]
- Gaal, A.; Bailey, B.; Patel, Y.; Smiley, N.; Dodson, T.; Kim, D.; Dillon, J. Limiting Antibiotics When Managing Mandible Fractures May Not Increase Infection Risk. J. Oral Maxillofac. Surg. 2016, 74, 2008–2018. [Google Scholar] [CrossRef] [PubMed]
- Gutta, R.; Tracy, K.; Johnson, C.; James, L.E.; Krishnan, D.G.; Marciani, R.D. Outcomes of mandible fracture treatment at an academic tertiary hospital: A 5-year analysis. J. Oral Maxillofac. Surg. 2014, 72, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Hindawi, Y.H.; Oakley, G.M.; Kinsella, C.R., Jr.; Cray, J.J.; Lindsay, K.; Scifres, A.M. Antibiotic duration and postoperative infection rates in mandibular fractures. J. Craniofac. Surg. 2011, 22, 1375–1377. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Lynham, A.; Wullschleger, M. Orbitozygomatic Fracture Repairs: Are Antibiotics Necessary? Craniomaxillofac. Trauma Reconstr. 2015, 8, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Lauder, A.; Jalisi, S.; Spiegel, J.; Stram, J.; Devaiah, A. Antibiotic prophylaxis in the management of complex midface and frontal sinus trauma. Laryngoscope 2010, 120, 1940–1945. [Google Scholar] [CrossRef] [PubMed]
- Linkugel, A.D.; Odom, E.B.; Bavolek, R.A.; Snyder-Warwick, A.K.; Patel, K.B. Systemic Preoperative Antibiotics with Mandible Fractures: Are They Indicated at the Time of Injury? Craniomaxillofac. Trauma Reconstr. 2018, 11, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Mamthashri, V.; Reddy, B.P. Comparison of Preoperative and Perioperative Antibiotic Prophylaxis Regimen in Compound Facial Fractures. J. Contemp. Dent. Pract. 2018, 19, 214–220. [Google Scholar] [CrossRef]
- Schaefer, E.H.; Caterson, E.J. Antibiotic selection for open reduction internal fixation of mandible fractures. J. Craniofac. Surg. 2013, 24, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Sethi, A.; Van Huekelom, E.; Mehra, P. Outcomes following use of a standard antibiotic protocol in the management of maxillofacial trauma patients. J. Oral Biol. Craniofacial Res. 2020, 10, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Wladis, E.J. Are post-operative oral antibiotics required after orbital floor fracture repair? Orbit 2013, 32, 30–32. [Google Scholar] [CrossRef]
- Zosa, B.M.; Elliott, C.W.; Kurlander, D.E.; Johnson, F.; Ho, V.P.; Claridge, J.A. Facing the facts on prophylactic antibiotics for facial fractures: 1 day or less. J. Trauma Acute Care Surg. 2018, 85, 444–450. [Google Scholar] [CrossRef]
- Adalarasan, S.; Mohan, A.; Pasupathy, S. Prophylactic antibiotics in maxillofacial fractures: A requisite? J. Craniofac. Surg. 2010, 21, 1009–1011. [Google Scholar] [CrossRef]
- Hammond, D.; Parmar, S.; Whitty, J.; McPhillips, M.; Wain, R. Prescription of antibiotics: Does it alter the outcome for patients who have fractures of the angle of the mandible? Br. J. Oral Maxillofac. Surg. 2017, 55, 958–961. [Google Scholar] [CrossRef]
- Knepil, G.J.; Loukota, R.A. Outcomes of prophylactic antibiotics following surgery for zygomatic bone fractures. J. Craniomaxillofac. Surg. 2010, 38, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Pessino, K.; Cook, T.; Layliev, J.; Bradley, J.P.; Bastidas, N. Excluding Antibiotics in the Management of Nonoperative Orbital and Zygomatic Fractures. Ann. Plast. Surg. 2021, 86, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.S.; Dodson, K.M.; Goldman, R.A. Prophylactic antibiotic therapy for fractures of the maxillary sinus. Ear Nose Throat J. 2015, 94, 170–177. [Google Scholar] [PubMed]
- Wick, E.H.; Deutsch, B.; Kallogjeri, D.; Chi, J.J.; Branham, G.H. Effectiveness of Prophylactic Preoperative Antibiotics in Mandible Fracture Repair: A National Database Study. Otolaryngol.–Head Neck Surg. 2021, 165, 798–808. [Google Scholar] [CrossRef]
- Eddine, S.B.Z.; Cooper–Johnson, K.; Ericksen, F.; Brookes, C.C.; Peppard, W.J.; Revolinski, S.L.; Carver, T.W. Antibiotic Duration and Outcome Complications for Surgical Site Infection Prevention in Traumatic Mandible Fracture. J. Surg. Res. 2020, 247, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, S.; Shi, Y.; Lu, Y.; Yu, B. Children with open tibial fractures show significantly lower infection rates than adults: Clinical comparative study. Int. Orthop. 2019, 43, 713–718. [Google Scholar] [CrossRef]
- Leekha, S.; Terrell, C.L.; Edson, R.S. General principles of antimicrobial therapy. Mayo Clin. Proc. 2011, 86, 156–167. [Google Scholar] [CrossRef]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am. J. Infect. Control. 1999, 27, 97–132. [Google Scholar] [CrossRef]
- Onsea, J.; Depypere, M.; Govaert, G.; Kuehl, R.; Vandendriessche, T.; Morgenstern, M.; McNally, M.; Trampuz, A.; Metsemakers, W.J. Accuracy of Tissue and Sonication Fluid Sampling for the Diagnosis of Fracture-Related Infection: A Systematic Review and Critical Appraisal. J. Bone Jt. Infect. 2018, 3, 173–181. [Google Scholar] [CrossRef]
- Trampuz, A.; Zimmerli, W. Diagnosis and treatment of infections associated with fracture-fixation devices. Injury 2006, 37 (Suppl. 2), S59–S66. [Google Scholar] [CrossRef]
- AO Surgery Reference—CMF—Trauma—Mandible. Available online: https://surgeryreference.aofoundation.org/cmf/trauma/mandible (accessed on 16 January 2022).
- Zalavras, C.G.; Marcus, R.E.; Levin, L.S.; Patzakis, M.J. Management of open fractures and subsequent complications. Instr. Course Lect. 2008, 57, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.L.; Moriarty, T.F.; Zalavras, C.; Morgenstern, M.; Jaiprakash, A.; Crawford, R.; Burch, M.A.; Boot, W.; Tetsworth, K.; Miclau, T.; et al. The influence of biomechanical stability on bone healing and fracture-related infection: The legacy of Stephan Perren. Injury 2021, 52, 43–52. [Google Scholar] [CrossRef]
- Andreasen, J.O.; Storgård Jensen, S.; Kofod, T.; Schwartz, O.; Hillerup, S. Open or closed repositioning of mandibular fractures: Is there a difference in healing outcome? A systematic review. Dent. Traumatol. 2008, 24, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Metsemakers, W.J.; Moriarty, T.F.; Morgenstern, M.; Kuehl, R.; Borens, O.; Kates, S.; Richards, R.G.; Verhofstad, M. Letter to the Editor: New Definition for Periprosthetic Joint Infection: From the Workgroup of the Musculoskeletal Infection Society. Clin. Orthop. Relat. Res. 2016, 474, 2726–2727. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention, National Healthcare Safety Network. Surveillance for Surgical Site Infection Events. 2022. Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf (accessed on 10 March 2022).
- Zalavras, C.G.; Aerden, L.; Declercq, P.; Belmans, A.; Metsemakers, W.J. Ninety-Day Follow-up Is Inadequate for Diagnosis of Fracture-related Infections in Patients with Open Fractures. Clin. Orthop. Relat. Res. 2022, 480, 139–146. [Google Scholar] [CrossRef]
- Geller, A.I.; Lovegrove, M.C.; Shehab, N.; Hicks, L.A.; Sapiano, M.R.P.; Budnitz, D.S. National Estimates of Emergency Department Visits for Antibiotic Adverse Events Among Adults—United States, 2011–2015. J. Gen. Intern. Med. 2018, 33, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Branch-Elliman, W.; O’Brien, W.; Strymish, J.; Itani, K.; Wyatt, C.; Gupta, K. Association of Duration and Type of Surgical Prophylaxis with Antimicrobial-Associated Adverse Events. JAMA Surg. 2019, 154, 590–598. [Google Scholar] [CrossRef]
- Oppelaar, M.C.; Zijtveld, C.; Kuipers, S.; Oever, J.T.; Honings, J.; Weijs, W.; Wertheim, H.F.L. Evaluation of Prolonged vs. Short Courses of Antibiotic Prophylaxis Following Ear, Nose, Throat, and Oral and Maxillofacial Surgery: A Systematic Review and Meta-analysis. JAMA Otolaryngol.—Head Neck Surg. 2019, 145, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Declercq, P.; Zalavras, C.; Nijssen, A.; Mertens, B.; Mesure, J.; Quintens, J.; De Ridder, T.; Belmans, A.; Nijs, S.; Spriet, I.; et al. Impact of duration of perioperative antibiotic prophylaxis on development of fracture-related infection in open fractures. Arch. Orthop. Trauma. Surg. 2021, 141, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. 2019. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 17 January 2022).
| Author | Risk of Bias * | Open or Closed Fractures | Treatment | N | Antibiotic Type | Comparison Antibiotic Regimen for All Patients | Outcome (IR = Infection Incidence Rate) | Follow-Up |
|---|---|---|---|---|---|---|---|---|
| Randomized controlled trials | ||||||||
| Surgically treated fractures | ||||||||
| Campos 2015 (Part I) | High risk of bias | NR | Surgical (NS) | 31 | cefazolin | Postoperative Ab every 6 h for 24 h vs. no postoperative Ab All patients received Ab 20 min before surgery. | IR: 24 h postoperative Ab 5.6%, no postoperative Ab 38.5% Significant difference between the regimens. | 6 w |
| Chole 1987 (Part I) | High risk of bias | Open and closed | ORIF and CR-MMF | 79 | cefazolin | Ab 1 h preoperatively and 8 h later vs. no Ab | IR: Ab 13.2 %, no Ab 43.9% Significant difference between the regimens. | 30 d |
| Baliga 2014 (Part I) | High risk of bias | NR | ORIF | 30 | cefotaxime, metronidazole | 5 d postoperative Ab vs. no postoperative Ab All patients received Ab pre- and intra-operatively | IR: Ab group 3.3%; no Ab group 3.3% No significant difference between the regimens. | 3 w |
| Abubaker 2001 | High risk of bias | Open and closed | ORIF and CR-MMF | 30 | penicillin G and penicillin VK | 5 d postoperative Ab vs. no prolonged postoperative Ab All patients received Ab every 4 h preoperatively, intraoperatively and 12 h postoperatively | IR: 5 d postoperative Ab 14.3%, no prolonged postoperative Ab 12.5% No significant difference between the regimens. | 6 w |
| Perepa 2018 | High risk of bias | NR | ORIF | 144 | amoxicillin/ clavulanic acid, metronidazole | 5 d postoperative Ab vs. no prolonged postoperative Ab All patients received preoperative Ab and 1 dose iv postoperative | IR: 5 d postoperative Ab 20.5%, no prolonged postoperative Ab 20% No significant difference between the regimens. | 3 m |
| Schaller 2013 | High risk of bias | NR | ORIF | 59 | amoxicillin/ clavulanic acid | 5 d postoperative Ab vs. no prolonged postoperative Ab All patients received Ab from admission until 24 h postoperatively | IR: 5 d postoperative Ab 20%, no prolonged postoperative Ab 21% No significant difference between the regimens. | 6 m |
| Miles 2006 | High risk of bias | Open and closed | ORIF | 181 | penicillin G, metronidazole, cephalosporins, cefazolin, clindamycin | 5–7 d postoperative Ab vs. no postoperative Ab All patients received Ab from diagnosis until surgery and intraoperatively | IR: 5–7 d postoperative Ab 9.9%, no postoperative Ab 14.0% No significant difference between the regimens. | 5 w |
| Retrospective cohort studies | ||||||||
| Surgically treated fractures | ||||||||
| Lovato 2009 | Serious risk of bias | Open and closed | ORIF and CR-MMF | 150 | cefazolin, cefalexin, cefepime, cefotetan, clindamycin, doxycycline, imipenem, penicillin, amoxicillin/clavulanic acid, amoxicillin/sulbactam, piperacillin/tazobactam, unknown (18%) | ≤24 h postoperative Ab vs. >24 h postoperative Ab (24 h–10 d) All patients received perioperative Ab for ≤24 h | IR: <24 h postoperative Ab 13.33%, >24 h postoperative Ab 10.67% No significant difference between the regimens. | 6 w |
| Mottini 2014 (Part I) | Serious risk of bias | NR | NR | 115 | amoxicillin/ clavulanic acid or clindamycin | ≥5 d postoperative Ab vs. no prolonged postoperative Ab All patients received Ab from admission until 24 h postoperatively. | IR: ≥5 d postoperative Ab 9.59%, no prolonged postoperative Ab 11.90% No significant difference between the regimens. | 6 m |
| Domingo 2016 | Critical risk of bias | Open and closed | ORIF and CR-MMF | 359 | cefazolin, cefalexin, cefadroxil, cefepime ceftriaxone, amoxicillin, amoxicillin/clavulanic acid, amoxicillin/sulbactam, moxifloxacin, levofloxacin, ciprofloxacin, bactrim, clindamycin, combinations | Postoperative Ab (1–3 d, 4–7 d, >7 d, or unknown) vs. no postoperative Ab Patients received variable or no preoperative Ab. | IR: postoperative Ab 14.7%, no postoperative Ab 9.6% No significant difference between the regimens. | 4 w |
| Zosa 2021 (Part I) | Serious risk of bias | Open and closed | NR | 42 | First- generation cephalosporins, β-lactam antibiotics with β-lactamase inhibitors, clindamycin, penicillin, vancomycin, tetracycline, and combinations. | <24 h Ab (incl. single-dose or no Ab) vs. >24 h Ab (median 4 d (range 1–14 d)) | IR: <24 h Ab 4.0%, >24 h Ab 29.4% Significantly higher infection rates for longer courses. | 1–30 m (8 m) (Follow-up rate 93.6%) |
| Author | Risk of Bias * | Fracture Location | Open or Closed Fractures | N | Antibiotic Type | Comparison Antibiotic Regimen for All Patients | Outcome (IR = Infection Incidence Rate) | Follow-Up |
|---|---|---|---|---|---|---|---|---|
| Randomized controlled trials | ||||||||
| Surgically treated fractures | ||||||||
| Campos 2015 (Part II) | High risk of bias | Midface, upper face | NR | 44 | cefazolin | Postoperative Ab every 6 h for 24 h vs. no postoperative Ab All patients received Ab 20 min before surgery. | IR: 24 h postoperative Ab 0%, no postoperative Ab Ab 3.3% No significant difference between the regimens | 6 w |
| Chole 1987 (Part II) | High risk of bias | Zygoma, Le Fort | NR | 101 | cefazolin | 1 h preoperative and 8 h later vs. no Ab | IR: 0% No significant difference between the regimens. | 30 d |
| Baliga 2014 (Part II) | High risk of bias | Zygoma | NR | 30 | cefotaxime, metronidazole | 5 d postoperative Ab vs. no postoperative Ab All patients received Ab pre- and intra-operatively | IR: 0.00% No significant difference between the regimens. | 3 w |
| Jang 2019 | High risk of bias | Nasal bone | Closed | 30 | cefazedone, cephalexin | Ab 4 d postoperatively vs. no postoperative Ab All patients received one dose of Ab at induction. | IR: 0% No significant difference between the regimens. | 30 d |
| Zix 2013 | High risk of bias | Orbit | NR | 62 | amoxicillin/ clavulanic acid | 5 d postoperative Ab vs. no prolonged postoperative Ab All patients received Ab from admission until 24 h postoperatively. | IR: 5 d postoperative Ab 6.8%, no prolonged postoperative Ab 3.2% No significant difference between the regimens. | 6 m |
| Soong 2014 | High risk of bias | Le Fort, zygoma | NR | 94 | amoxicillin/ clavulanic acid | 5 d postoperative Ab vs. no prolonged postoperative Ab All patients received Ab from admission until 24 h postoperatively. | IR: 5 d postoperative Ab 4.4%, no prolonged postoperative Ab 4.1% No significant difference between the regimens. | 6 m |
| Retrospective cohort studies | ||||||||
| Surgically treated fractures | ||||||||
| Reiss 2017 | High risk of bias | Orbit | NR | 172 | cefazolin, cephalexin, cefdinir, ceftriaxone, penicillin, amoxicillin, amoxicillin/clavulante, ampicillin/sulbactam, piperacillin/tazobactam, clindamycin ciprofloxacin, levofloxacin, azithromycin, vancomycin | No Ab, vs. one dose of Ab, vs. 5–7 days of Ab, vs. 10–14 d of Ab | IR: 0.00% No significant difference between the regimens. | <1 w–>3 m |
| Mottini 2014 (Part II) | Serious risk of bias | Zygoma, orbit, Le Fort | NR | 339 | amoxicillin/ clavulanic acid or clindamycin | ≥5 d postoperative Ab vs. no prolonged postoperative Ab All patients received Ab from admission until 24 h postoperatively. | IR: ≥5 d postoperative Ab 0.4%, no prolonged postoperative Ab 0%, No significant difference between the regimens. | 6 m |
| Zosa 2021 (Part II) | Serious risk of bias | Midface | Open and closed | 49 | First- generation cephalosporins, β-lactam antibiotics with β-lactamase inhibitors, clindamycin, penicillin, vancomycin, tetracycline, and combinations. | <24 h Ab (incl. single-dose or no Ab) vs. >24 h Ab (median 4 d (range 1–14 d)) | IR: <24 h Ab 7.3%, >24 h Ab 12.5% No significant difference between the regimens. | 1–30 m (8 m) (Follow-up rate 93.6%) |
| Conservatively (non-surgically) treated fractures | ||||||||
| Malekpour 2016 | High risk of bias | Maxilla, orbit | Closed | 289 | ampicillin/sulbactam, amoxicillin/clavulanate, or combinations, clindamycin | No Ab, vs. 1–5 d of Ab, vs. >5 days of Ab | IR: 0.00% No significant difference between the regimens. | 2 w |
| Author | Outcome Description: Definition of Infection |
|---|---|
| Lovato 2009 Reiss 2017 | Not reported |
| Domingo 2016 Perepa 2018 Schaller 2013 Mottini 2014 Soong 2014 Zix 2013 | CDC guidelines |
| Miles 2006 Perepa 2018 | Clinical criteria: Grade I: Erythema around suture line < 1 cm; Grade II: 1–5 cm of erythema; Grade III: > 5 cm of erythema and induration; Grade IV: Purulent drainage spontaneously or by incision; Grade V: Fistulae Radiological criteria: Grade I: Ossification of fracture site/no change from initial injury; Grade II: Radiolucenties localized to hardware or necrotic tooth; Grade III: Generalized radiolucenties of fracture or hardware |
| Abubaker 2001 Baliga 2014 Campos 2015 | Purulent drainage from the surgical or fracture site, increased facial Swelling beyond postoperative day 7, fistula formation at the surgical or fracture site, with evidence of drainage, fever associated with local evidence of infection (swelling, erythema, or tenderness). |
| Jang 2019 | Nasal bone infection: Heating sensation, swelling, persistent pain, purulent nasal drainage, septal abscess, vital sign showing general signs of infection (not specified). |
| Malekpour 2016 | Warmth, redness, abscess, fever, purulent drainage, or patients who were started on antibiotics at follow-up. |
| Chole 1987 | The fracture site, incision, or adjacent area showed clinical signs of infection, including purulent drainage, abscess formation, or cellulitis. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goormans, F.; Coropciuc, R.; Vercruysse, M.; Spriet, I.; Willaert, R.; Politis, C. Systemic Antibiotic Prophylaxis in Maxillofacial Trauma: A Scoping Review and Critical Appraisal. Antibiotics 2022, 11, 483. https://doi.org/10.3390/antibiotics11040483
Goormans F, Coropciuc R, Vercruysse M, Spriet I, Willaert R, Politis C. Systemic Antibiotic Prophylaxis in Maxillofacial Trauma: A Scoping Review and Critical Appraisal. Antibiotics. 2022; 11(4):483. https://doi.org/10.3390/antibiotics11040483
Chicago/Turabian StyleGoormans, Femke, Ruxandra Coropciuc, Maximilien Vercruysse, Isabel Spriet, Robin Willaert, and Constantinus Politis. 2022. "Systemic Antibiotic Prophylaxis in Maxillofacial Trauma: A Scoping Review and Critical Appraisal" Antibiotics 11, no. 4: 483. https://doi.org/10.3390/antibiotics11040483
APA StyleGoormans, F., Coropciuc, R., Vercruysse, M., Spriet, I., Willaert, R., & Politis, C. (2022). Systemic Antibiotic Prophylaxis in Maxillofacial Trauma: A Scoping Review and Critical Appraisal. Antibiotics, 11(4), 483. https://doi.org/10.3390/antibiotics11040483






