Antibacterial and Antioxidant Activity of Dysphania ambrosioides (L.) Mosyakin and Clemants Essential Oils: Experimental and Computational Approaches
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Profile of DAEOs Analyzed by Gas Chromatography–Mass Spectrometry
2.2. Molecular Docking Results
Prediction of a Protein Target-Based Antioxidant and Antibacterial Mechanisms In Silico
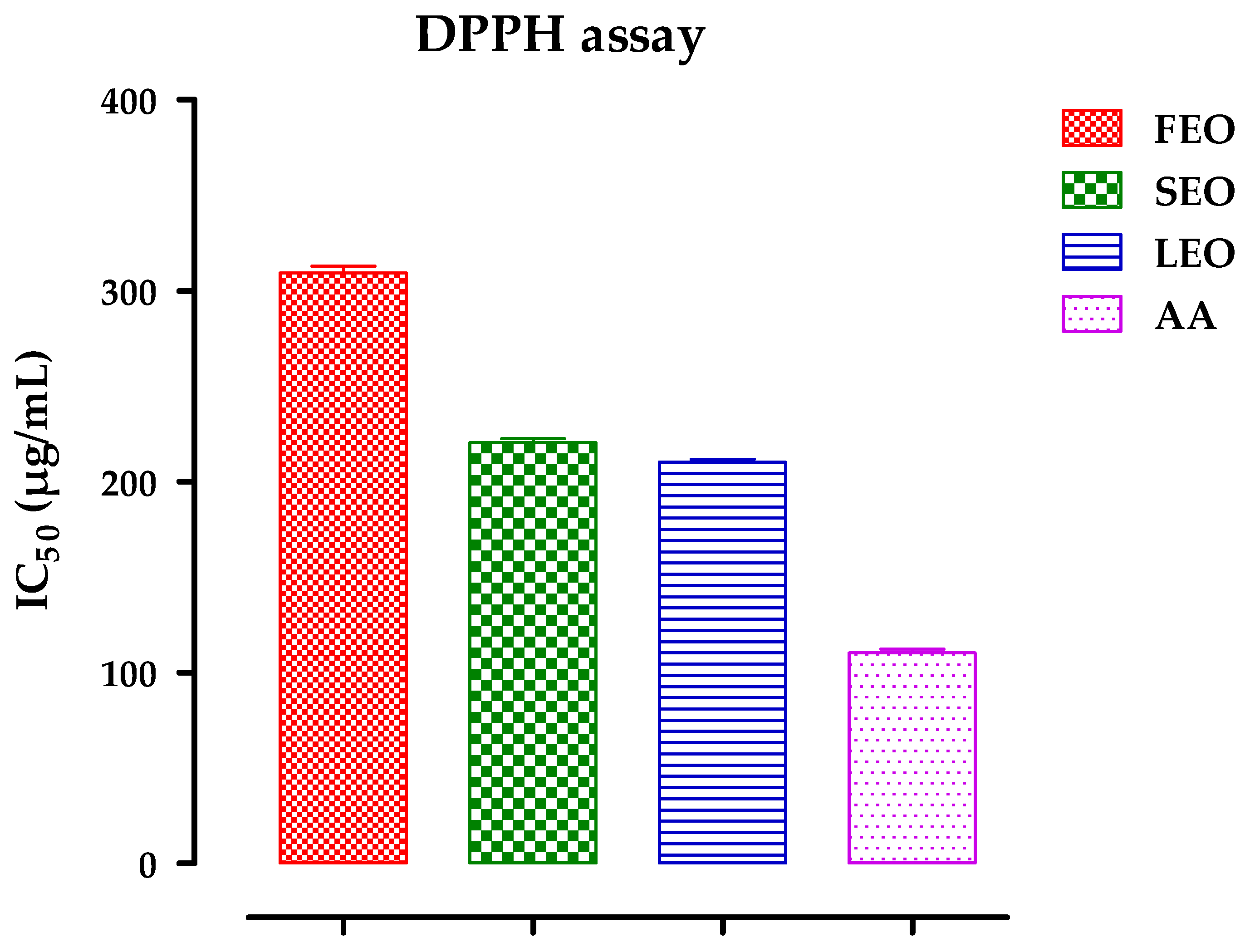
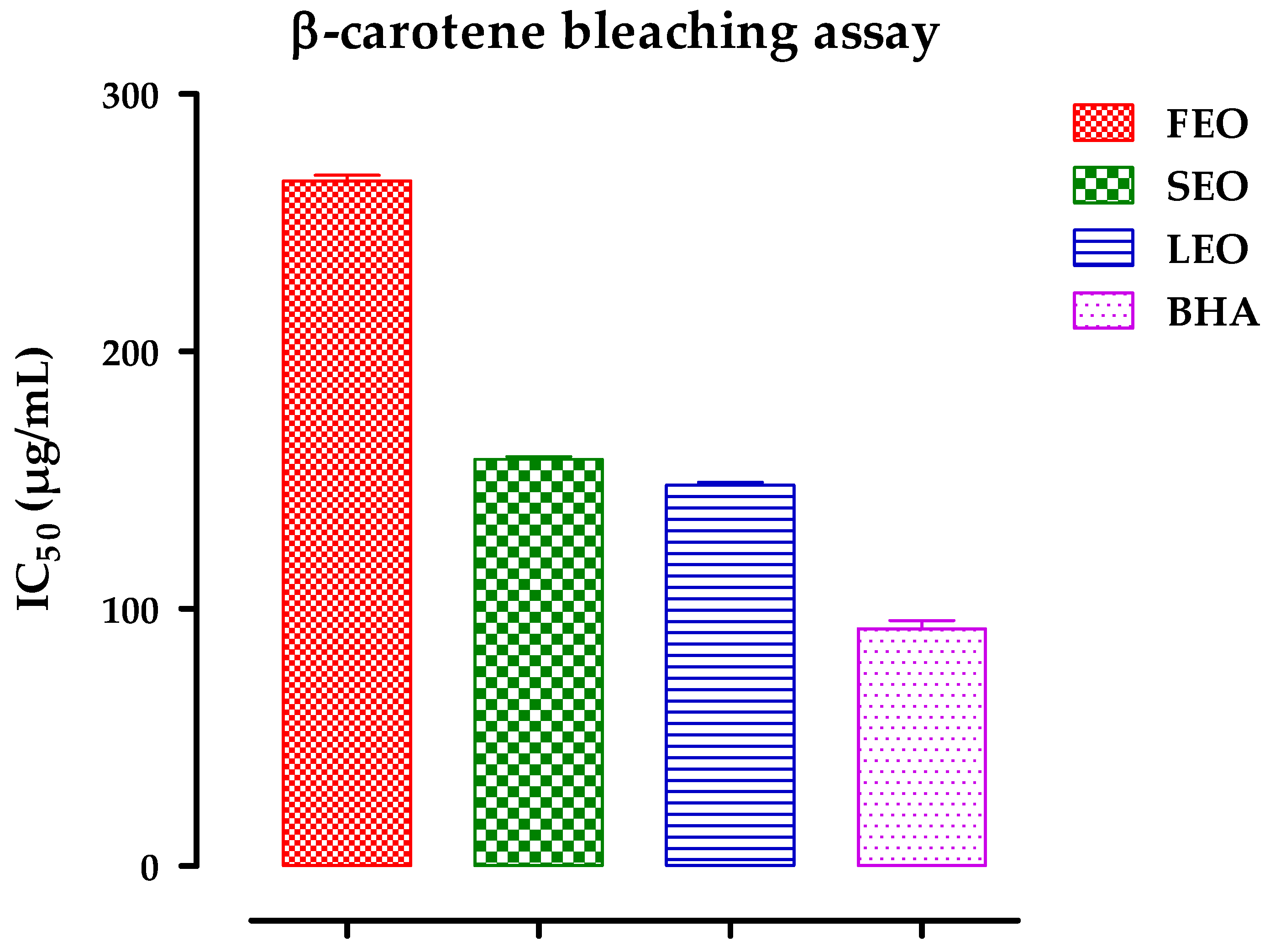
2.3. Antioxidant Activity
2.4. Antibacterial Activity
2.5. ADMET Analysis
3. Materials and Methods
3.1. Plant Material, Extraction, and Yielding of D. ambrosioides Essential Oils
3.2. GC–MS Analysis
3.3. Molecular Docking
3.4. Antioxidant Assays
3.4.1. The 2,2-diphenyl-1-picrylhydrazyl (DPPH) Assay
3.4.2. β-Carotene/Linoleic Acid Bleaching Assay
3.5. Bacterial Strains, Growth Media, and Chemicals
3.5.1. Bacterial Strains
3.5.2. Growth Medium
3.5.3. Determination of Minimum Inhibitory Concentrations (MIC)
3.5.4. Determination of Minimum Bactericidal Concentrations (MBC)
3.5.5. Chemicals
3.6. ADMET Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ergin, K.N.; Karakaya, S.; Göger, G.; Sytar, O.; Demirci, B.; Duman, H. Anatomical and Phytochemical Characteristics of Different Parts of Hypericum scabrum L. Extracts, Essential Oils, and Their Antimicrobial Potential. Molecules 2022, 27, 1228. [Google Scholar] [CrossRef] [PubMed]
- Guglielmi, P.; Pontecorvi, V.; Rotondi, G. Natural compounds and extracts as novel antimicrobial agents. Expert Opin. Ther. Pat. 2020, 30, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Bouhdid, S.; Skali, S.N.; Idaomar, M.; Zhiri, A.; Baudoux, D.; Amensour, M.; Abrini, J. Antibacterial and antioxidant activities of Origanum compactum essential oil. Afr. J. Biotechnol. 2008, 7, 1563–1570. [Google Scholar]
- Kwiatkowski, P.; Łopusiewicz, Ł.; Kostek, M.; Drozłowska, E.; Pruss, A.; Wojciuk, B.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Dołęgowska, B. The antibacterial activity of lavender essential oil alone and in combination with octenidine dihydrochloride against MRSA strains. Molecules 2019, 25, 95. [Google Scholar] [CrossRef] [PubMed]
- Wongsawan, K.; Chaisri, W.; Tangtrongsup, S.; Mektrirat, R. Bactericidal effect of clove oil against multidrug-resistant Streptococcus suis isolated from human patients and slaughtered pigs. Pathogens 2019, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Solórzano-Santos, F.; Miranda-Novales, M.G. Essential oils from aromatic herbs as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Burt, S.A.; van der Zee, R.; Koets, A.P.; de Graaff, A.M.; van Knapen, F.; Gaastra, W.; Haagsman, H.P.; Veldhuizen, E.J.A. Carvacrol induces heat shock protein 60 and inhibits synthesis of flagellin in Escherichia coli O157: H7. Appl. Environ. Microbiol. 2007, 73, 4484–4490. [Google Scholar] [CrossRef]
- Turgis, M.; Han, J.; Caillet, S.; Lacroix, M. Antimicrobial activity of mustard essential oil against Escherichia coli O157: H7 and Salmonella typhi. Food Control 2009, 20, 1073–1079. [Google Scholar] [CrossRef]
- Ames, B.N.; Lee, F.D.; Durston, W.E. An improved bacterial test system for the detection and classification of mutagens and carcinogens. Proc. Natl. Acad. Sci. USA 1973, 70, 782–786. [Google Scholar] [CrossRef]
- Elbouzidi, A.; Bencheikh, N.; Seddoqi, S.; Bouhrim, M.; Bouramdane, Y.; Addi, M. Investigation of the Allelopathic Effect of Matricaria chamomilla L. Parts’ Aqueous Extracts on Germination and Seedling Growth of Two Moroccan Varieties of Durum Wheat. Int. J. Agron. 2021, 2021, 4451181. [Google Scholar] [CrossRef]
- Bostanian, N.J.; Akalach, M.; Chiasson, H. Effects of a Chenopodium-based botanical insecticide/acaricide on Orius insidiosus (Hemiptera: Anthocoridae) and Aphidius colemani (Hymenoptera: Braconidae). Pest Manag. Sci. 2005, 61, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Bigoga, J.D.; Saahkem, P.A.; Ndindeng, S.A.; Ngondi, J.L.; Nyegue, M.; Oben, J.E.; Leke, R.G.F. Larvicidal and repellent potential of Chenopodium ambrosioides Linn essential oil against Anopheles gambiae Giles (Diptera: Culicidae). Open Entomol. J. 2013, 7, 16–22. [Google Scholar] [CrossRef]
- Zefzoufi, M.; Smaili, A.; Fdil, R.; Rifai, L.A.; Faize, L.; Koussa, T.; Makroum, K.; Ben Ali, A.; Tabyaoui, M.; Mouzdahir, A. Composition of essential oil of Moroccan Dysphania ambrosioides and its antimicrobial activity against bacterial and fungal phytopathogens. J. Plant Pathol. 2020, 102, 47–58. [Google Scholar] [CrossRef]
- Astani, A.; Reichling, J.; Schnitzler, P. Screening for antiviral activities of isolated compounds from essential oils. Evidence-based Complement. Altern. Med. 2011, 2011, 253643. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential oils as antimicrobial agents—Myth or real alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef]
- Stringaro, A.; Colone, M.; Angiolella, L. Antioxidant, antifungal, antibiofilm, and cytotoxic activities of Mentha spp. essential oils. Medicines 2018, 5, 112. [Google Scholar] [CrossRef]
- Ziyyat, A.; Legssyer, A.; Mekhfi, H.; Dassouli, A.; Serhrouchni, M.; Benjelloun, W. Phytotherapy of hypertension and diabetes in oriental Morocco. J. Ethnopharmacol. 1997, 58, 45–54. [Google Scholar] [CrossRef]
- Firenzuoli, F.; Jaitak, V.; Horvath, G.; Bassolé, I.H.N.; Setzer, W.N.; Gori, L. Essential oils: New perspectives in human health and wellness. Evidence-Based Complement. Altern. Med. 2014, 2014, 467363. [Google Scholar] [CrossRef]
- Kandsi, F.; Conte, R.; Marghich, M.; Lafdil, F.Z.; Alajmi, M.F.; Bouhrim, M.; Mechchate, H.; Hano, C.; Aziz, M.; Gseyra, N. Phytochemical Analysis, Antispasmodic, Myorelaxant, and Antioxidant Effect of Dysphania ambrosioides (L.) Mosyakin and Clemants Flower Hydroethanolic Extracts and Its Chloroform and Ethyl Acetate Fractions. Molecules 2021, 26, 7300. [Google Scholar] [CrossRef]
- Mokni, R.E.; Youssef, F.S.; Jmii, H.; Khmiri, A.; Bouazzi, S.; Jlassi, I.; Jaidane, H.; Dhaouadi, H.; Ashour, M.L.; Hammami, S. The essential oil of Tunisian Dysphania ambrosioides and its antimicrobial and antiviral properties. J. Essent. Oil Bear. Plants 2019, 22, 282–294. [Google Scholar] [CrossRef]
- Fatokun, O.T.; Diyaolu, A.H.; Esievo, K.B.; Adamu, A.; Aboh, M.O.; Okhale, S.E. Chemical composition and antibacterial activity of the essential oil of Dysphania ambrosioides (L.) Mosyakin & Clemants from North Central Nigeria. J. Phytomedicine Ther. 2019, 18, 304–313. [Google Scholar]
- Kuete, V. Physical, hematological, and histopathological signs of toxicity induced by African medicinal plants. In Toxicological Survey of African Medicinal Plants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 635–657. [Google Scholar]
- Ávila-Blanco, M.E.; Rodríguez, M.G.; Moreno Duque, J.L.; Muñoz-Ortega, M.; Ventura-Juárez, J. Amoebicidal activity of essential oil of Dysphania ambrosioides (L.) Mosyakin & Clemants in an amoebic liver abscess hamster model. Evid.-Based Complement. Altern. Med. 2014, 2014, 930208. [Google Scholar]
- Hewis, L.G.; Daeli, G.B.C.; Tanoto, K.; Carlos, C.; Sahamastuti, A.A.T. A review of botany, phytochemical, and pharmacological effects of Dysphania ambrosioides. Indones. J. Life Sci. 2020, 2, 70–82. [Google Scholar]
- Bellakhdar, J. La Pharmacopée Marocaine Traditionnelle. Médecine arabe Ancienne et Savoirs Populaires; Fen, L., Ed.; Ibis Press: Paris, France; Rabat, Morocco, 1997. [Google Scholar]
- Fakchich, J.; Elachouri, M. Ethnobotanical survey of medicinal plants used by people in Oriental Morocco to manage various ailments. J. Ethnopharmacol. 2014, 154, 76–87. [Google Scholar]
- Bencheikh, N.; Elbouzidi, A.; Kharchoufa, L.; Ouassou, H.; Alami Merrouni, I.; Mechchate, H.; Es-Safi, I.; Hano, C.; Addi, M.; Bouhrim, M. Inventory of Medicinal Plants Used Traditionally to Manage Kidney Diseases in North-Eastern Morocco: Ethnobotanical Fieldwork and Pharmacological Evidence. Plants 2021, 10, 1966. [Google Scholar] [CrossRef] [PubMed]
- Zohra, T.; Ovais, M.; Khalil, A.T.; Qasim, M.; Ayaz, M.; Shinwari, Z.K. Extraction optimization, total phenolic, flavonoid contents, HPLC-DAD analysis and diverse pharmacological evaluations of Dysphania ambrosioides (L.) Mosyakin & Clemants. Nat. Prod. Res. 2019, 33, 136–142. [Google Scholar]
- Andrade, L.N.; Dos Reis Barreto de Oliveira, R.; De Sousa, D.P. A review on anti-inflammatory activity of phenylpropanoids found in essential oils. Molecules 2014, 19, 1459–1480. [Google Scholar]
- Cysne, D.N.; Fortes, T.S.; Reis, A.S.; de Paulo Ribeiro, B.; dos Santos Ferreira, A.; do Amaral, F.M.M.; Guerra, R.N.M.; Marinho, C.R.F.; Nicolete, R.; Nascimento, F.R.F. Antimalarial potential of leaves of Chenopodium ambrosioides L. Parasitol. Res. 2016, 115, 4327–4334. [Google Scholar] [CrossRef]
- Boutkhil, S.; El Idrissi, M.; Amechrouq, A.; Chbicheb, A.; Chakir, S.; El Badaoui, K. Chemical composition and antimicrobial activity of crude, aqueous, ethanol extracts and essential oils of Dysphania ambrosioides (L.) Mosyakin & Clemants. Acta Bot. Gall. 2009, 156, 201–209. [Google Scholar]
- Brahim, M.A.S.; Fadli, M.; Hassani, L.; Boulay, B.; Markouk, M.; Bekkouche, K.; Abbad, A.; Ali, M.A.; Larhsini, M. Chenopodium ambrosioides var. ambrosioides used in Moroccan traditional medicine can enhance the antimicrobial activity of conventional antibiotics. Ind. Crops Prod. 2015, 71, 37–43. [Google Scholar] [CrossRef]
- Sá, R.D.; Galvão, M.A.M.; Ferreira, M.R.A.; Soares, L.A.L.; Randau, K.P. Chemical composition of the essential oil from leaves of Chenopodium ambrosioides L. grown in Recife-PE, Brazil. Rev. Bras. Farm 2014, 95, 855–866. [Google Scholar]
- de Lacerda Neto, L.J.; Ramos, A.G.; da Silva, R.E.; Pereira-de-Morais, L.; Silva, F.M.; da Costa, R.H.; Rodrigues Dantas, L.B.; da Costa, J.G.; Coutinho, H.D.; Kowalska, G.; et al. Myorelaxant Effect of the Dysphania ambrosioides Essential Oil on Sus scrofa domesticus Coronary Artery and Its Toxicity in the Drosophila melanogaster Model. Molecules 2021, 26, 2041. [Google Scholar] [CrossRef]
- Cavalli, J.; Tomi, F.; Bernardini, A.; Casanova, J. Combined analysis of the essential oil of Chenopodium ambrosioides by GC, GC-MS and 13C-NMR spectroscopy: Quantitative determination of ascaridole, a heat-sensitive compound. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2004, 15, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; Montalvo, A.M.; Scull, R.; Miranda, M.; Abreu, J. Activity, toxicity and analysis of resistance of essential oil from Chenopodium ambrosioides after intraperitoneal, oral and intralesional administration in BALB/c mice infected with Leishmania amazonensis: A preliminary study. Biomed. Pharmacother. 2007, 61, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, R.; Sarma, N.; Begum, T.; Pandey, S.K.; Lal, M. North-East Indian Chromolaena odorata (L. King Robinson) aerial part essential oil chemical composition, pharmacological activities-neurodegenerative inhibitory and toxicity study. J. Essent. Oil Bear. Plants 2020, 23, 1173–1191. [Google Scholar] [CrossRef]
- Tittal, R.K.; Yadav, P.; Lal, K.; Kumar, A. Synthesis, molecular docking and DFT studies on biologically active 1, 4-disubstituted-1, 2, 3-triazole-semicarbazone hybrid molecules. New J. Chem. 2019, 43, 8052–8058. [Google Scholar]
- Ebrahimipour, S.Y.; Sheikhshoaie, I.; Simpson, J.; Ebrahimnejad, H.; Dusek, M.; Kharazmi, N.; Eigner, V. Antimicrobial activity of aroylhydrazone-based oxido vanadium (v) complexes: In vitro and in silico studies. New J. Chem. 2016, 40, 2401–2412. [Google Scholar] [CrossRef]
- Trevisan, D.A.C.; da Silva, P.V.; Farias, A.B.P.; Campanerut-Sá, P.A.Z.; Ribeiro, T.; Faria, D.R.; de Mendonça, P.S.B.; de Mello, J.C.P.; Seixas, F.A.V.; Mikcha, J.M.G. Antibacterial activity of Barbatimão (Stryphnodendron adstringens) against Staphylococcus aureus: In vitro and in silico studies. Lett. Appl. Microbiol. 2020, 71, 259–271. [Google Scholar] [CrossRef]
- Priyadarshi, A.; Kim, E.E.; Hwang, K.Y. Structural insights into Staphylococcus aureus enoyl-ACP reductase (FabI), in complex with NADP and triclosan. Proteins Struct. Funct. Bioinform. 2010, 78, 480–486. [Google Scholar] [CrossRef]
- Semidalas, C.; Semidalas, E.; Matsoukas, M.T.; Nixarlidis, C.; Zoumpoulakis, P. In silico studies reveal the mechanisms behind the antioxidant and anti-inflammatory activities of hydroxytyrosol. Med. Chem. Res. 2016, 25, 2498–2511. [Google Scholar] [CrossRef]
- Feng, R.; Zhou, X.; Or, P.M.Y.; Ma, J.-Y.; Tan, X.-S.; Fu, J.; Ma, C.; Shi, J.-G.; Che, C.-T.; Wang, Y. Enzyme kinetic and molecular docking studies on the metabolic interactions of 1-hydroxy-2, 3, 5-trimethoxy-xanthone, isolated from Halenia elliptica D. Don, with model probe substrates of human cytochrome P450 enzymes. Phytomedicine 2012, 19, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Li, J.; Sivakumar, D.; Kim, T.-S.; Patel, S.K.S.; Kalia, V.C.; Kim, I.-W.; Zhang, Y.-W.; Lee, J.-K. NADH oxidase from Lactobacillus reuteri: A versatile enzyme for oxidized cofactor regeneration. Int. J. Biol. Macromol. 2019, 123, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Sutomo, S.; Pratama, M.R.F. Measuring the potential antioxidant activity of methyl gallate: Molecular docking study. Thai J. Pharm. Sci. 2020, 44, 7300. [Google Scholar]
- Kikuno, R.; Daiyasu, H.; Toh, H. 1.10—Molecular Evolution of Proteins Involved in the Arachidonic Acid Cascade; Barton, S.D., Nakanishi, K., Meth-Cohn, O.B.T.-C.N.P.C., Eds.; Pergamon: Oxford, UK, 1999; pp. 273–284. ISBN 978-0-08-091283-7. [Google Scholar]
- da Silva Costa, J.; da Silva Ramos, R.; da Silva Lopes Costa, K.; do Socorro Barros Brasil, D.; de Paula da Silva, C.H.T.; Ferreira, E.F.B.; dos Santos Borges, R.; Campos, J.M.; da Cruz Macêdo, W.J.; dos Santos, C.B.R. An in silico study of the antioxidant ability for two caffeine analogs using molecular docking and quantum chemical methods. Molecules 2018, 23, 2801. [Google Scholar] [CrossRef]
- Janakiramudu, D.B.; Subba Rao, D.; Srikanth, C.; Madhusudhana, S.; Sreenivasa Murthy, P.; Nagalakshmidevamma, M.; Chalapathi, P.V.; Naga Raju, C. Sulfonamides and carbamates of 3-fluoro-4-morpholinoaniline (linezolid intermediate): Synthesis, antimicrobial activity and molecular docking study. Res. Chem. Intermed. 2018, 44, 469–489. [Google Scholar] [CrossRef]
- Kim, O.K.; Barrett, J.F.; Ohemeng, K. Advances in DNA gyrase inhibitors. Expert Opin. Investig. Drugs 2001, 10, 199–212. [Google Scholar] [CrossRef]
- Patil, M.; Poyil, A.N.; Joshi, S.D.; Patil, S.A.; Patil, S.A.; Bugarin, A. Design, synthesis, and molecular docking study of new piperazine derivative as potential antimicrobial agents. Bioorg. Chem. 2019, 92, 103217. [Google Scholar] [CrossRef]
- Rafi, S.; Novichenok, P.; Kolappan, S.; Zhang, X.; Stratton, C.F.; Rawat, R.; Kisker, C.; Simmerling, C.; Tonge, P.J. Structure of acyl carrier protein bound to FabI, the FASII enoyl reductase from Escherichia coli. J. Biol. Chem. 2006, 281, 39285–39293. [Google Scholar] [CrossRef]
- Heath, R.J.; Rock, C.O. Enoyl-Acyl Carrier Protein Reductase (fabI) Plays a Determinant Role in Completing Cycles of Fatty Acid Elongation in Escherichia coli (∗). J. Biol. Chem. 1995, 270, 26538–26542. [Google Scholar] [CrossRef] [PubMed]
- Kalamorz, F.; Reichenbach, B.; März, W.; Rak, B.; Görke, B. Feedback control of glucosamine-6-phosphate synthase GlmS expression depends on the small RNA GlmZ and involves the novel protein YhbJ in Escherichia coli. Mol. Microbiol. 2007, 65, 1518–1533. [Google Scholar] [CrossRef]
- Milewski, S. Glucosamine-6-phosphate synthase—The multi-facets enzyme. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 2002, 1597, 173–192. [Google Scholar] [CrossRef]
- Lešnik, S.; Bren, U. Mechanistic Insights into Biological Activities of Polyphenolic Compounds from Rosemary Obtained by Inverse Molecular Docking. Foods 2022, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Vijesh, A.M.; Isloor, A.M.; Telkar, S.; Arulmoli, T.; Fun, H.-K. Molecular docking studies of some new imidazole derivatives for antimicrobial properties. Arab. J. Chem. 2013, 6, 197–204. [Google Scholar] [CrossRef]
- Man, A.; Santacroce, L.; Iacob, R.; Mare, A.; Man, L. Antimicrobial activity of six essential oils against a group of human pathogens: A comparative study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef]
- Alitonou, G.A.; Sessou, P.; Tchobo, P.F.; Noudogbessi, J.-P.; Avlessi, F.; Yehouenou, B.; Menut, C.; Villeneuve, P.; Sohounhloue, D.C.K. Chemical composition and biological activities of essential oils of Chenopodium ambrosioides L. collected in two areas of Benin. Int. J. Biosci. 2012, 2, 58–66. [Google Scholar]
- Baumgart, A.M.K. Avaliação do Potencial Antimicrobiano das Espécies Vegetais Cipura Paludosa e Chenopodium ambrosioides 2014. Available online: http://siaibib01.univali.br/pdf/Ana%20Milda%20Karsten%20Baumgart.pdf (accessed on 19 February 2022).
- Abd-ElGawad, A.M.; El-Amier, Y.A.; Bonanomi, G.; Gendy, A.E.-N.G.E.; Elgorban, A.M.; Alamery, S.F.; Elshamy, A.I. Chemical Composition of Kickxia aegyptiaca Essential Oil and Its Potential Antioxidant and Antimicrobial Activities. Plants 2022, 11, 594. [Google Scholar] [CrossRef]
- Mahmoudzadeh, M.; Hosseini, H.; Shahraz, F.; Akhondzadeh-Basti, A.; Khaneghah, A.M.; Azizkhani, M.; Sant’ana, A.D.S.; Haghshenas, M.; Mahmoudzadeh, L. Essential oil composition and antioxidant capacity of Carum copticum and its antibacterial effect on Staphylococcus aureus, Enterococcus faecalis and Escherichia coli O157: H7. J. Food Process. Preserv. 2017, 41, e12938. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Martin, Y.C. A bioavailability score. J. Med. Chem. 2005, 48, 3164–3170. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. PkCSM: Predicting Small-Molecule Pharmacokinetic Properties Using Graph-Based Signatures (Theory-How to Enterpret PkCSM Result). J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, M.C.; Tristani-Firouzi, M. hERG potassium channels and cardiac arrhythmia. Nature 2006, 440, 463–469. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef]
- Jianu, C.; Goleț, I.; Stoin, D.; Cocan, I.; Bujancă, G.; Mișcă, C.; Mioc, M.; Mioc, A.; Șoica, C.; Lukinich-Gruia, A.T.; et al. Chemical Profile of Ruta graveolens, Evaluation of the Antioxidant and Antibacterial Potential of Its Essential Oil, and Molecular Docking Simulations. Appl. Sci. 2021, 11, 11753. [Google Scholar] [CrossRef]
- Rădulescu, M.; Jianu, C.; Lukinich-Gruia, A.T.; Mioc, M.; Mioc, A.; Șoica, C.; Stana, L.G. Chemical composition, in vitro and in silico antioxidant potential of Melissa officinalis subsp. officinalis essential oil. Antioxidants 2021, 10, 1081. [Google Scholar] [CrossRef]
- Lountos, G.T.; Jiang, R.; Wellborn, W.B.; Thaler, T.L.; Bommarius, A.S.; Orville, A.M. The Crystal Structure of NAD(P)H Oxidase from Lactobacillus sanfranciscensis: Insights into the Conversion of O2 into Two Water Molecules by the Flavoenzyme. Biochemistry 2006, 45, 9648–9659. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, Y.; Wang, X.; Yan, H.; Liu, E.; Gao, X. Interaction between the Natural Components in Danhong Injection (DHI) with Serum Albumin (SA) and the Influence of the Coexisting Multi-Components on the SaB-BSA Binding System: Fluorescence and Molecular Docking Studies. PLoS ONE 2015, 10, e0128919. [Google Scholar] [CrossRef][Green Version]
- Zrouri, H.; Elbouzidi, A.; Bouhrim, M.; Bencheikh, N.; Kharchoufa, L.; Ouahhoud, S.; Ouassou, H.; El Assri, S.; Choukri, M. Phytochemical analysis, antioxidant activity, and nephroprotective effect of the Raphanus sativus aqueous extract. Mediterr. J. Chem. 2021, 11, 84–94. [Google Scholar] [CrossRef]
- Hammami, S.; Jmii, H.; Mokni, R.E.; Khmiri, A.; Faidi, K.; Dhaouadi, H.; Aouni, M.H.; Aouni, M.; Joshi, R.K. Essential Oil Composition, Antioxidant, Cytotoxic and Antiviral Activities of Teucrium pseudochamaepitys Growing Spontaneously in Tunisia. Molecules 2015, 20, 20426–20433. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zuo, G.-L.; Wang, C.-Y.; Kim, H.Y.; Lim, S.S.; Tong, S.-Q. An Off-Line DPPH-GC-MS Coupling Countercurrent Chromatography Method for Screening, Identification, and Separation of Antioxidant Compounds in Essential Oil. Antioxidants 2020, 9, 702. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Calderon, O.; Chacaltana-Ramos, L.J.; Huayanca-Gutiérrez, I.C.; Algarni, M.A.; Alqarni, M.; Batiha, G.E.-S. Chemical Constituents, In Vitro Antioxidant Activity and In Silico Study on NADPH Oxidase of Allium sativum L. (Garlic) Essential Oil. Antioxidants 2021, 10, 1844. [Google Scholar] [CrossRef] [PubMed]
- Mann, C.M.; Markham, J.L. A new method for determining the minimum inhibitory concentration of essential oils. J. Appl. Microbiol. 1998, 84, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Badavath, V.N.; Sinha, B.N.; Jayaprakash, V. Design, in-silico docking and predictive ADME properties of novel pyrazoline derivatives with selective human MAO inhibitory activity. Int. J. Pharm. Pharm. Sci. 2015, 7, 277–282. [Google Scholar]
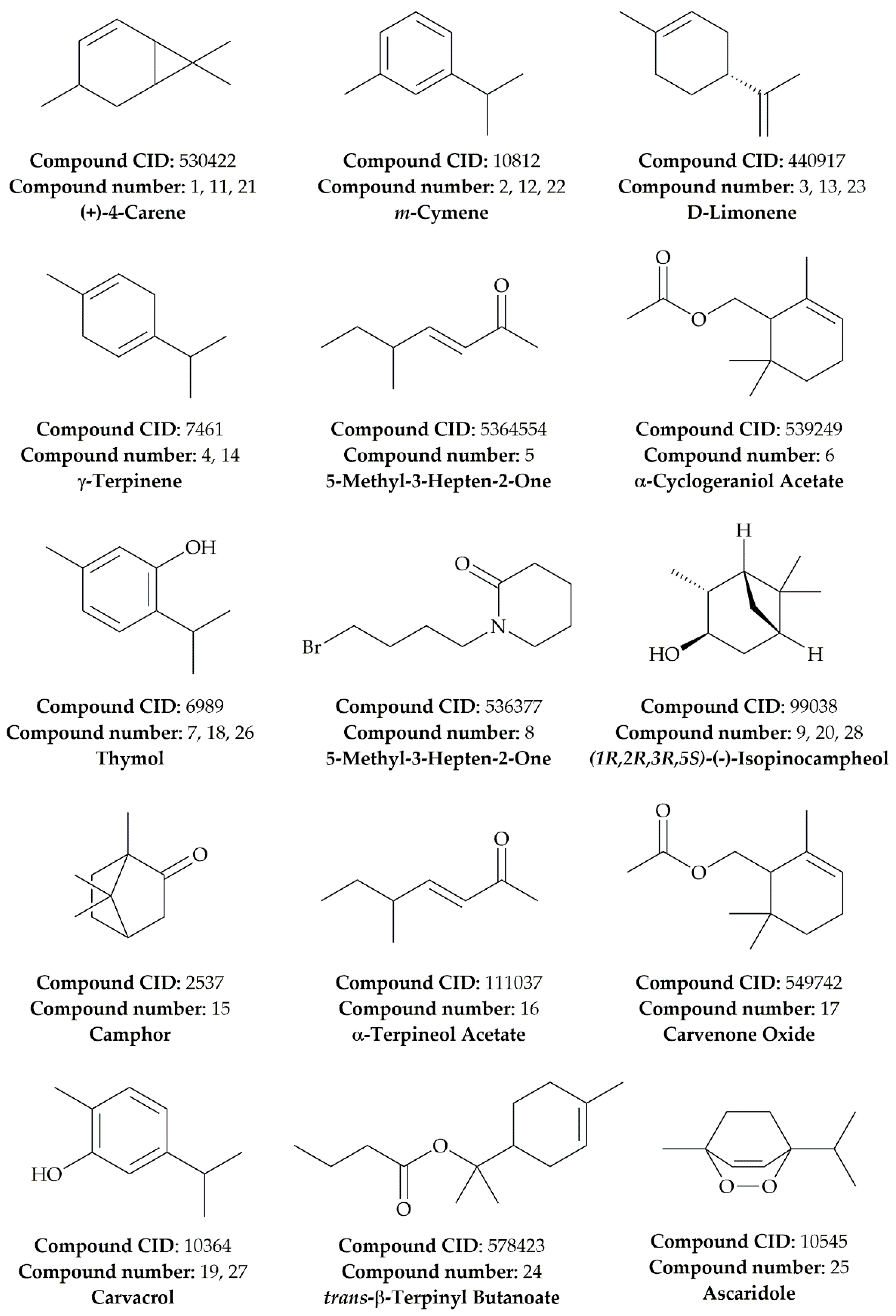

| Compound Number | Compound Name | Formula | Mol. Wt. | RT (min) | Peak Area (%) |
|---|---|---|---|---|---|
| Stem Essential Oil (SEO) | |||||
| 1 | (+)-4-Carene | C10H16 | 136.23 | 6.467 | 50.5 |
| 2 | m-Cymene | C10H14 | 134.22 | 6.592 | 3.13 |
| 3 | D-Limonene | C10H16 | 136.23 | 6.667 | 0.97 |
| 4 | γ-Terpinene | C10H16 | 136.23 | 7.158 | 0.68 |
| 5 | 3-Hepten-2-one, 5-Methyl | C8H14O | 126.20 | 8.2330 | 2.06 |
| 6 | α-Cyclogeraniol Acetate | C12H20O2 | 196.29 | 10.108 | 22.64 |
| 7 | Thymol | C10H14O | 150.22 | 10.800 | 7.16 |
| 8 | 1-(4-Bromobutyl)-2-Piperidinone | C9H16BrNO | 234.13 | 10.958 | 3.98 |
| 9 | (1R,2R,3R,5S)-(-)-Isopinocampheol | C10H18O | 154.25 | 11.108 | 8.87 |
| Leaves Essential Oil (LEO) | |||||
| 10 | α-Terpinene | C10H16 | 136.23 | 6.367 | 5.67 |
| 11 | (+)-4-Carene | C10H16 | 136.23 | 6.467 | 46.2 |
| 12 | m-Cymene | C10H14 | 134.22 | 6.592 | 20.74 |
| 13 | D-Limonene | C10H16 | 136.23 | 6.667 | 1.38 |
| 14 | γ-Terpinene | C10H16 | 136.23 | 7.158 | 0.69 |
| 15 | Camphor | C10H16O | 152.23 | 8.642 | 0.20 |
| 16 | α-Terpineol Acetate | C12H20O2 | 196.29 | 10.108 | 14.5 |
| 17 | Carvenone Oxide | C10H16O2 | 168.23 | 10.367 | 0.54 |
| 18 | Thymol | C10H14O | 150.22 | 10.800 | 3.76 |
| 19 | Carvacrol | C10H14O | 150.22 | 10.958 | 1.73 |
| 20 | (1R,2R,3R,5S)-(-)-Isopinocampheol | C10H18O | 154.25 | 11.108 | 4.42 |
| Flowers Essential Oil (FEO) | |||||
| 21 | (+)-4-Carene | C10H16 | 136.23 | 6.467 | 28.05 |
| 22 | m-Cymene | C10H14 | 134.22 | 6.592 | 8.15 |
| 23 | D-Limonene | C10H16 | 136.23 | 6.658 | 1.12 |
| 24 | trans-β-Terpinyl Butanoate | C14H24O2 | 224.34 | 10.117 | 31.13 |
| 25 | Ascaridole | C10H16O2 | 168.23 | 10.367 | 1.16 |
| 26 | Thymol | C10H14O | 150.22 | 10.800 | 7.79 |
| 27 | Carvacrol | C10H14O | 150.22 | 10.950 | 4.53 |
| 28 | (1R,2R,3R,5S)-(-)-Isopinocampheol | C10H18O | 154.25 | 11.117 | 18.06 |
| Ligand | Antioxidant Proteins PDB IDs | Antibacterial Proteins PDB IDs | |||||
|---|---|---|---|---|---|---|---|
| 1N8Q | 1OG5 | 2CDU | 4JK4 | 1KZN | 3GNS | 2VF5 | |
| Free Binding Energy ∆G (kcal/mol) 1 | |||||||
| Native Ligand | −6.0 | −6.6 | −8.6 | −5.3 | −9.6 | −6.0 | −7.2 |
| (+)-4-Carene | −6.1 | −5.8 | −5.8 | −6.2 | −5.0 | −5.0 | −5.1 |
| m-Cymene | −5.5 | −5.9 | −5.8 | −7.4 | −4.8 | −4.9 | −5.1 |
| D-Limonene | −6.0 | −6.3 | −5.6 | −6.3 | −5.8 | −4.7 | −5.0 |
| γ-Terpinene | −5.1 | −6.1 | −5.6 | −6.4 | −5.8 | −4.7 | −5.0 |
| 3-Hepten-2-one, 5-Methyl | −4.5 | −4.8 | −5.0 | −5.9 | −4.8 | −4.3 | −4.1 |
| α-Cyclogeraniol Acetate | −5.8 | −6.2 | −5.6 | −6.1 | −5.8 | −5.5 | −6.2 |
| Thymol | −5.3 | −6.0 | −5.5 | −6.2 | −6.2 | −5.1 | −5.2 |
| 1-(4-Bromobutyl)-2-Piperidinone | −4.8 | −5.4 | −5.1 | −5.4 | −4.7 | −4.0 | −4.7 |
| (1R,2R,3R,5S)-(-)-Isopinocampheol | −5.2 | −5.7 | −5.9 | −6.1 | −4.7 | −4.6 | −5.4 |
| Camphor | −5.5 | −5.9 | −5.6 | −5.7 | −4.6 | −5.2 | −5.9 |
| α-Terpineol Acetate | −5.9 | −6.7 | −6.4 | −6.5 | −6.1 | −6.0 | −5.9 |
| Carvenone Oxide | −5.9 | −5.7 | −5.9 | −6.1 | −4.7 | −5.3 | −5.7 |
| Carvacrol | −6.2 | −6.2 | −6.0 | −7.3 | −6.0 | −5.4 | −5.3 |
| trans-β-Terpinyl Butanoate | −6.1 | −6.6 | −6.1 | −6.9 | −6.4 | −5.2 | −6.2 |
| Ascaridole | −6.8 | −5.8 | −6.1 | −6.5 | −5.2 | −5.4 | −5.6 |
| Bacteria | Inhibition Zones (IZ) of D. ambrosioides Essential Oils | ||
|---|---|---|---|
| SEO IZ (mm) | LEO IZ (mm) | FEO IZ (mm) | |
| E. coli | 16 ± 0.23 | 9 ± 0.20 | 24 ± 0.10 |
| S. aureus | 15 ± 0.11 | 10 ± 0.31 | 20 ± 0.00 |
| E. faecalis1 | 8.0 ± 0.11 | 9 ± 0.31 | 16 ± 0.00 |
| E. faecalis2 | 18 ± 0.40 | 13 ± 0.21 | 14 ± 0.20 |
| Bacteria | Essential Oils of D. ambrosioides | |||||
|---|---|---|---|---|---|---|
| SEO | LEO | FEO | ||||
| MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | |
| E. coli | 18 | 18 | 105 | 110 | 6 | 12 |
| S. aureus | 18 | 18 | 110 | 110 | 12 | 18 |
| E. faecalis1 | ≥110 | ≥110 | ≥110 | ≥110 | 105 | 110 |
| E. faecalis2 | 18 | 18 | 105 | ≥110 | 105 | 105 |
| Compound N. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug-Likeness | Lipinski’s rule of five | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Bioavailability Score (%) | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | |
| Absorption | Water Solubility | −2.74 | −3.89 | −3.50 | −3.45 | −1.73 | −2.57 | −3.19 | −2.35 | −2.40 | −2.16 | −3.35 | −2.04 | −3.31 | −3.92 | −2.23 |
| Caco2 Permeability | 1.39 | 1.52 | 1.40 | 1.41 | 1.51 | 1.63 | 1.60 | 1.35 | 1.47 | 1.49 | 1.62 | 1.51 | 1.60 | 1.65 | 1.61 | |
| Intestinal Absorption (Human) (%) | 96.3 | 93.6 | 95.8 | 96.2 | 96.3 | 96.6 | 90.8 | 93.1 | 94.2 | 95.9 | 96.2 | 98.2 | 90.8 | 95.3 | 96.3 | |
| Skin Permeability | −4.82 | −3.92 | −3.89 | −3.94 | −5.60 | −5.57 | −4.87 | −6.24 | −5.43 | −5.67 | −4.69 | −5.95 | −4.74 | −4.27 | −5.73 | |
| P-glycoprotein Substrate | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | |
| P-glycoprotein I Inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | |
| P-glycoprotein II Inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | |
| Distribution | VDss (human) | 0.51 | 0.72 | 0.39 | 0.41 | 0.06 | 0.15 | 0.51 | 0.10 | 0.47 | 0.33 | 0.13 | 0.20 | 0.51 | 0.26 | 0.35 |
| BBB permeability | 0.76 | 0.47 | 0.73 | 0.75 | 0.49 | 0.51 | 0.40 | 0.58 | 0.75 | 0.61 | 0.42 | 0.55 | 0.40 | 0.53 | 0.63 | |
| CNS permeability | −2.25 | −1.39 | −2.37 | −2.04 | −2.17 | −2.67 | −1.66 | −2.61 | −2.45 | −2.15 | −2.84 | −2.51 | −1.66 | −2.72 | −2.74 | |
| Metabolism | CYP2D6 Substrate | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No |
| CYP3A4 Substrate | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | |
| CYP2D6 Inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | |
| CYP3A4 Inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | |
| Excretion | Total Clearance | 0.02 | 0.24 | 0.21 | 0.21 | 0.33 | 0.37 | 0.21 | 0.28 | 0.01 | 0.10 | 1.24 | 1.14 | 0.20 | 1.31 | 1.33 |
| Renal OCT2 Substrate | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | |
| Toxicity | AMES Toxicity | No | No | No | No | No | No | No | Yes | No | No | No | Yes | No | No | No |
| Hepatotoxicity | No | No | No | No | No | No | Yes | No | No | No | No | No | Yes | No | No | |
| hERG I Inhibitor | No | No | No | No | No | No | No | No | No | No | No | No | No | No | No | |
| Skin Sensitization | No | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | |
| Proteins | PDB ID | Grid Box Size | Grid Box Center | Native Ligand | Reference |
|---|---|---|---|---|---|
| Lipoxygenase | 1N8Q | size_x = 40 | center_x = 22.455 | Protocatechuic Acid | [71] |
| size_y = 40 | center_y = 1.2930 | ||||
| size_z = 40 | center_z = 20.362 | ||||
| CYP2C9 | 1OG5 | size_x = 12.387 | center_x = −19.823 | Warfarin | [71] |
| size_y = 11.653 | center_y = 86.686 | ||||
| size_z = 11.654 | center_z = 38.275 | ||||
| NADPH Oxidase | 2CDU | size_x = 14.007 | center_x = 18.997 | Adenosine-5′-Diphosphate | [71,72] |
| size_y = 14.997 | center_y = −5.777 | ||||
| size_z = 18.795 | center_z = −1.808 | ||||
| Bovine Serum Albumin (BSA) | 4JK4 | size_x = 40 | center_x = 95.873 | 3,5-Diiodosalicylic Acid | [73] |
| size_y = 40 | center_y = 16.048 | ||||
| size_z = 40 | center_z = 13.494 | ||||
| DNA Gyrase Topoisomerase II (E. coli) | 1KZN | size_x = 40 | center_x = 19.528 | Clorobiocin | [40] |
| size_y = 40 | center_y = 19.500 | ||||
| size_z = 40 | center_z = 43.031 | ||||
| Enoyl-Acyl Carrier Protein Reductase (S. aureus) | 3GNS | size_x = 40 | center_x = −14.280 | Triclosan | [42,43] |
| size_y = 40 | center_y = 0.56200 | ||||
| size_z = 40 | center_z = −21.462 | ||||
| Glucosamine-6-Phosphate Synthase | 2VF5 | size_x = 70 | center_x = 30.590 | Glucosamine-6-Phosphate | [41] |
| size_y = 64 | center_y = 15.822 | ||||
| size_z = 56 | center_z = 3.4970 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandsi, F.; Elbouzidi, A.; Lafdil, F.Z.; Meskali, N.; Azghar, A.; Addi, M.; Hano, C.; Maleb, A.; Gseyra, N. Antibacterial and Antioxidant Activity of Dysphania ambrosioides (L.) Mosyakin and Clemants Essential Oils: Experimental and Computational Approaches. Antibiotics 2022, 11, 482. https://doi.org/10.3390/antibiotics11040482
Kandsi F, Elbouzidi A, Lafdil FZ, Meskali N, Azghar A, Addi M, Hano C, Maleb A, Gseyra N. Antibacterial and Antioxidant Activity of Dysphania ambrosioides (L.) Mosyakin and Clemants Essential Oils: Experimental and Computational Approaches. Antibiotics. 2022; 11(4):482. https://doi.org/10.3390/antibiotics11040482
Chicago/Turabian StyleKandsi, Fahd, Amine Elbouzidi, Fatima Zahra Lafdil, Nada Meskali, Ali Azghar, Mohamed Addi, Christophe Hano, Adil Maleb, and Nadia Gseyra. 2022. "Antibacterial and Antioxidant Activity of Dysphania ambrosioides (L.) Mosyakin and Clemants Essential Oils: Experimental and Computational Approaches" Antibiotics 11, no. 4: 482. https://doi.org/10.3390/antibiotics11040482
APA StyleKandsi, F., Elbouzidi, A., Lafdil, F. Z., Meskali, N., Azghar, A., Addi, M., Hano, C., Maleb, A., & Gseyra, N. (2022). Antibacterial and Antioxidant Activity of Dysphania ambrosioides (L.) Mosyakin and Clemants Essential Oils: Experimental and Computational Approaches. Antibiotics, 11(4), 482. https://doi.org/10.3390/antibiotics11040482







