Characterization and Antimicrobial Activity of Nigella sativa Extracts Encapsulated in Hydroxyapatite Sodium Silicate Glass Composite
Abstract
1. Introduction
2. Results
2.1. Extracts Yields
2.2. GC-MS Characterization
2.2.1. The Essential Oil (HS)
2.2.2. Hexane Extract (FH)
2.2.3. Acetone Extract (FA)
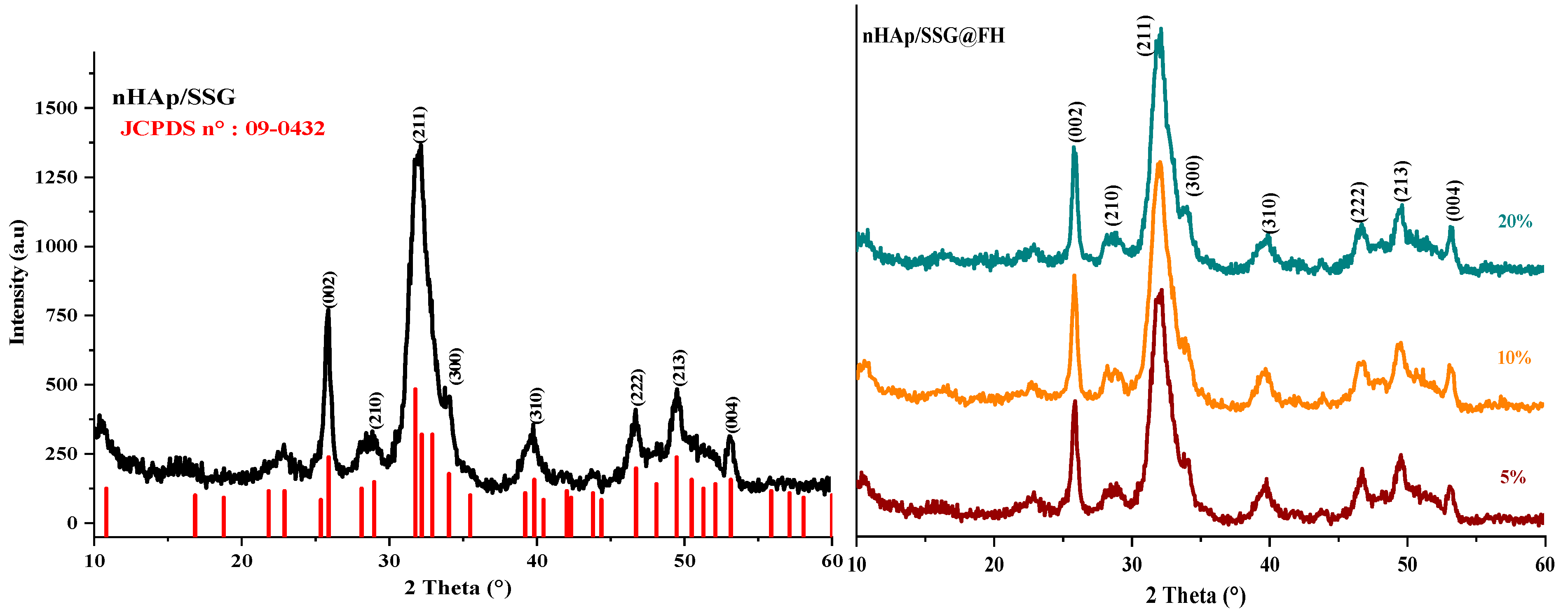
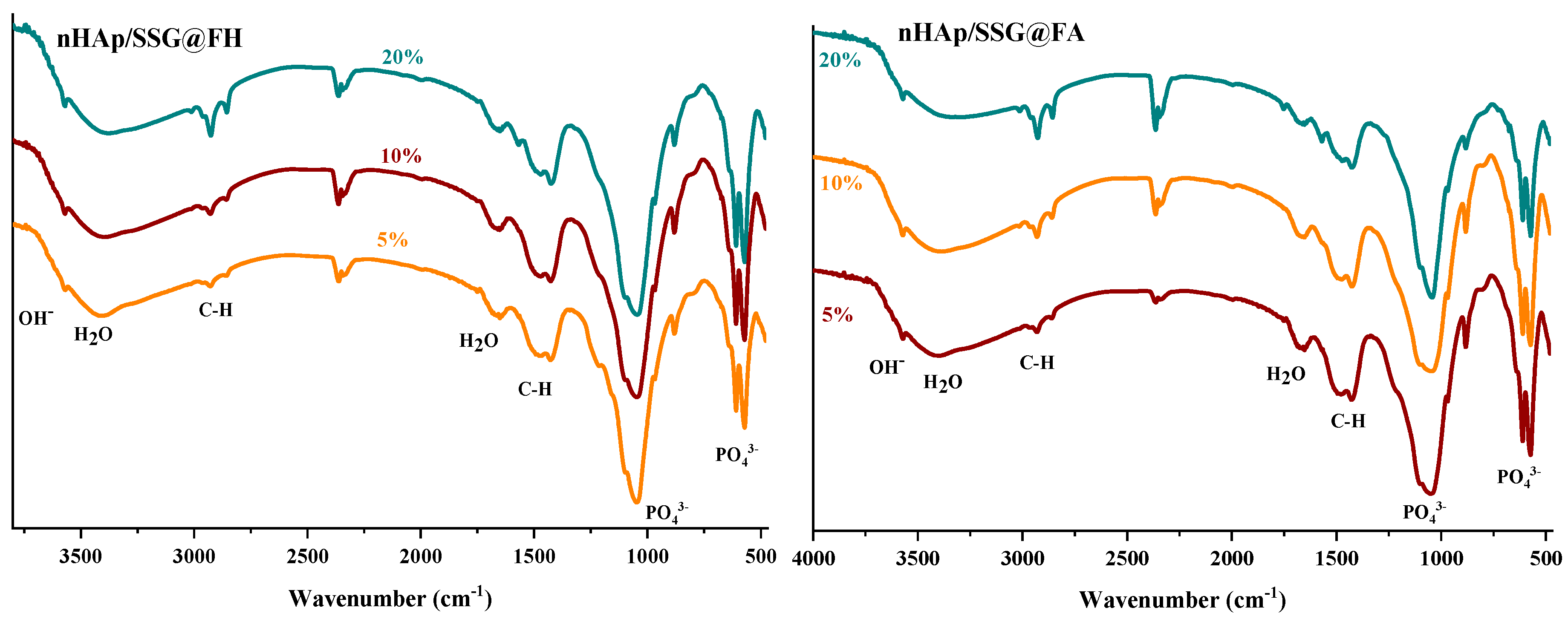
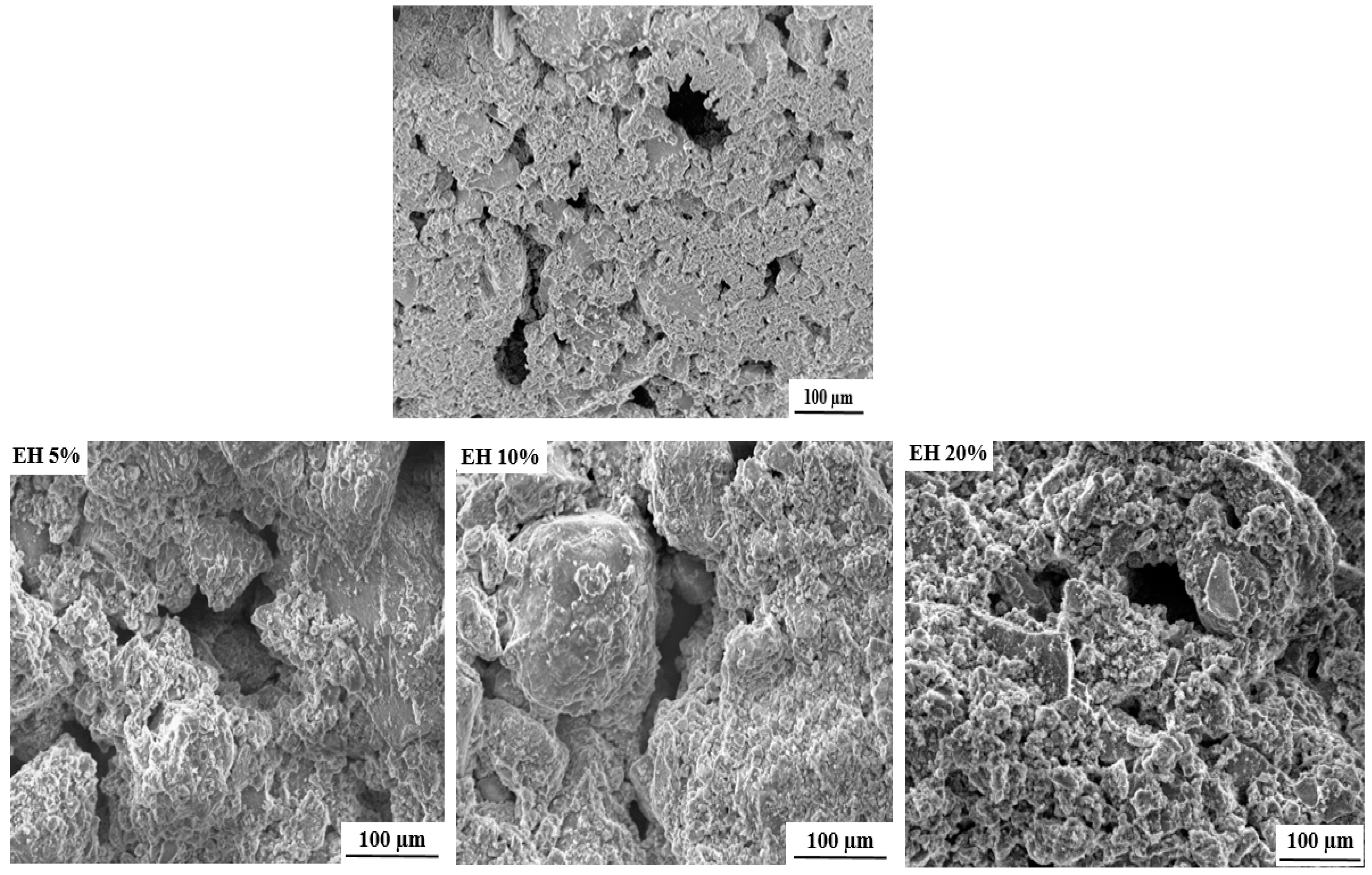
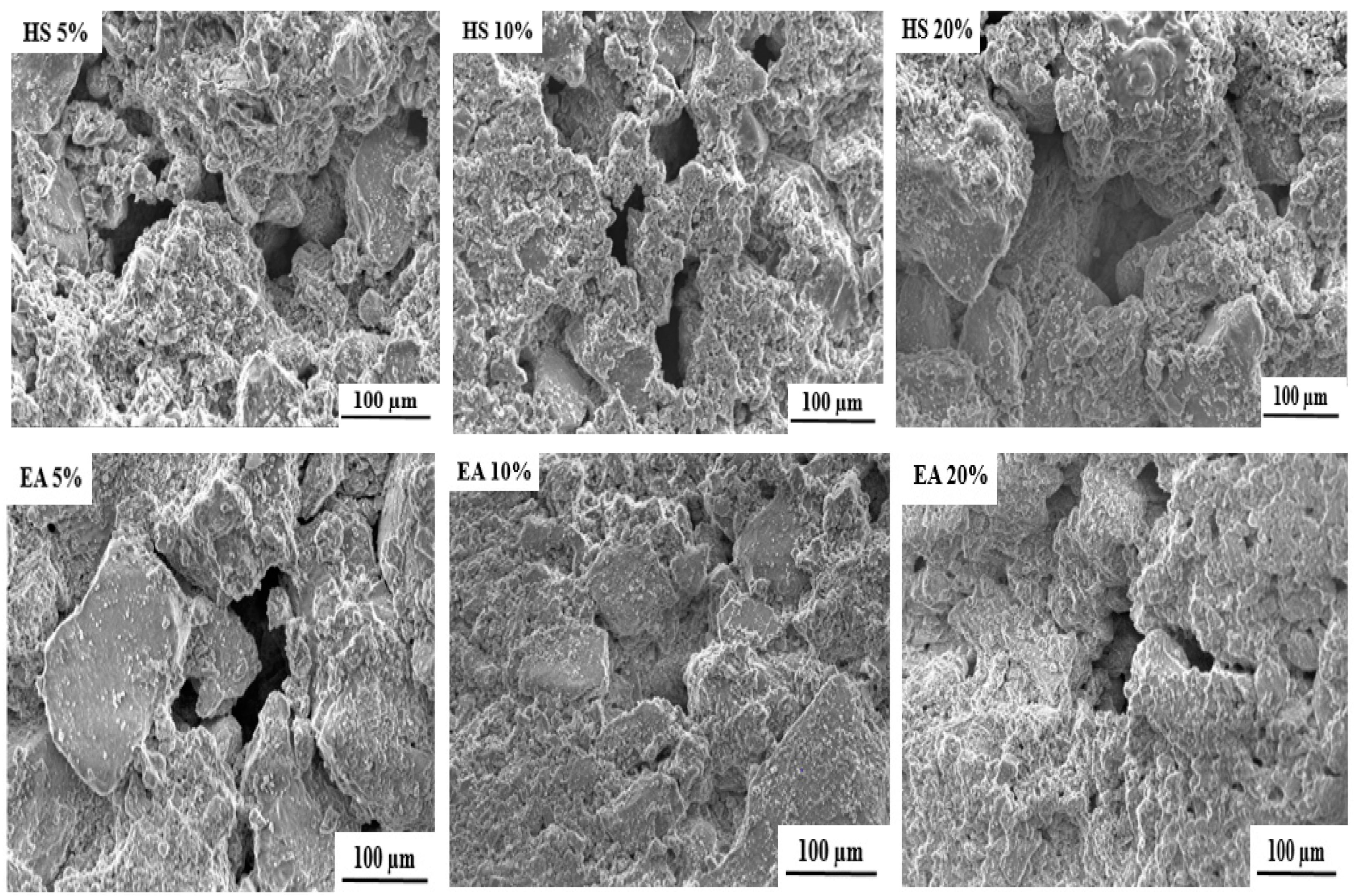
2.3. Scaffold Characterization
2.4. Antimicrobial Activity Determination
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Plant Material and Extracts
4.3. GC-MS Analysis
4.4. Hydroxyapatite Nanoparticles Preparation
4.5. Loaded nHap/SSG Scaffolds Preparation
4.6. Characterization of the Loaded Scaffolds
4.7. Evaluation of Antimicrobial Activity of Loaded Scaffolds
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tavakkoli, A.; Mahdian, V.; Razavi, B.M.; Hosseinzadeh, H. Review on Clinical Trials of Black Seed (Nigella sativa) and Its Active Constituent, Thymoquinone. J. Pharmacopunct. 2017, 20, 179. [Google Scholar] [CrossRef]
- Jin, Y.S. Recent advances in natural antifungal flavonoids and their derivatives. Bioorganic Med. Chem. Lett. 2019, 29, 126589. [Google Scholar] [CrossRef] [PubMed]
- Mechraoui, O.; Ladjel, S.; Nedjimi, M.S.; Belfar, M.L.; Moussaoui, Y. Determination of polyphenols content, antioxidant and antibacterial activity of Nigella sativa L. Seed phenolic extracts. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2018, 19, 411–421. [Google Scholar]
- Majdalawieh, A.F.; Fayyad, M.W. Recent advances on the anti-cancer properties of Nigella sativa, a widely used food additive. J. Ayurveda Integr. Med. 2016, 7, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Khan, M.R.; Mishra, S.K. An updated literature-based review: Phytochemistry, pharmacology and therapeutic promises of Nigella sativa L. Orient. Pharm. Exp. Med. 2019, 19, 115–129. [Google Scholar] [CrossRef]
- Houghton, P.; Zarka, R.; de las Heras, B.; Hoult, J. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995, 61, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Tiji, S.; Benayad, O.; Berrabah, M.; El Mounsi, I.; Mimouni, M. Phytochemical profile and antioxidant activity of Nigella sativa L growing in Morocco. Sci. World J. 2021, 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Majdalawieh, A.F.; Fayyad, M.W.; Nasrallah, G.K. Anti-cancer properties and mechanisms of action of thymoquinone, the major active ingredient of Nigella sativa. Crit. Rev. Food Sci. Nutr. 2017, 57, 3911–3928. [Google Scholar] [CrossRef]
- El Rabey, H.A.; Al-Seeni, M.N.; Bakhashwain, A.S. The antidiabetic activity of nigella sativa and propolis on streptozotocin-induced diabetes and diabetic nephropathy in male rats. Evid.-Based Complement. Altern. Med. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, G. Study on the effect of black cumin (Nigella sativa Linn.) on experimental renal ischemia-reperfusion injury in rats. Acta Cirúrgica Bras. 2015, 30, 542–550. [Google Scholar] [CrossRef][Green Version]
- Eatelaf, A. The percutaneous effect of black seed (Nigella sativa) oil as external topical treatment on bone healing in rabbits. QJVMS 2014, 13, 146–154. [Google Scholar]
- Arslan, A.H.; Tomruk, C.Ö.; Meydanlı, E.G.; Özdemir, İ.; Duygu Çapar, G.; Kütan, E.; Yılmaz, A.; Yalçın Ülker, G.M. Histopathological evaluation of the effect of systemic thymoquinone administration on healing of bone defects in rat tibia. Biotechnol. Biotechnol. Equip. 2016, 31, 175–181. [Google Scholar] [CrossRef]
- Molino, G.; Palmieri, M.C.; Montalbano, G.; Fiorilli, S.; Vitale-Brovarone, C. Biomimetic and mesoporous nano-hydroxyapatite for bone tissue application: A short review. Biomed. Mater. 2020, 15, 022001. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Gomes, P.; Duarte, J.A.; Almeida, M.M.; Costa, M.E.V.; Fernandes, M.H. Development of hydroxyapatite nanoparticles loaded with folic acid to induce osteoblastic differentiation. Int. J. Pharm. 2017, 516, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Kaya, Y.; Jodati, H.; Evis, Z. Effects of biomimetic synthesis route and sintering temperature on physicochemical, microstructural, and mechanical properties of hydroxyapatite. J. Aust. Ceram. Soc. 2021, 57, 1117–1129. [Google Scholar] [CrossRef]
- El-Bassyouni, G.T.; Eldera, S.S.; Kenawy, S.H.; Hamzawy, E.M. Hydroxyapatite nanoparticles derived from mussel shells for in vitro cytotoxicity test and cell viability. Heliyon 2020, 6, e04085. [Google Scholar] [CrossRef]
- Gomes, D.S.; Santos, A.M.C.; Neves, G.A.; Menezes, R.R. A brief review on hydroxyapatite production and use in biomedicine. Cerâmica 2019, 65, 282–302. [Google Scholar] [CrossRef]
- Głąb, M.; Kudłacik-Kramarczyk, S.; Drabczyk, A.; Walter, J.; Kordyka, A.; Godzierz, M.; Bogucki, R.; Tyliszczak, B.; Sobczak-Kupiec, A. Hydroxyapatite Obtained via the Wet Precipitation Method and PVP/PVA Matrix as Components of Polymer-Ceramic Composites for Biomedical Applications. Molecules 2021, 26, 4268. [Google Scholar] [CrossRef]
- Yusoff, M.F.M.; Kasim, N.H.A.; Himratul-Aznita, W.H.; Saidin, S.; Genasan, K.; Kamarul, T.; Radzi, Z. Physicochemical, antibacterial and biocompatibility assessments of silver incorporated nano-hydroxyapatite synthesized using a novel microwave-assisted wet precipitation technique. Mater. Charact. 2021, 178, 111169. [Google Scholar] [CrossRef]
- Wang, M.; Tang, T. Surface treatment strategies to combat implant-related infection from the beginning. J. Orthop. Transl. 2019, 17, 42–54. [Google Scholar] [CrossRef]
- Rameshbabu, N.; Kumar, T.S.S.; Prabhakar, T.G.; Sastry, V.S.; Murty, K.V.G.K.; Rao, K.P. Antibacterial nanosized silver substituted hydroxyapatite: Synthesis and characterization. J. Biomed. Mater. Res. Part A 2006, 80, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Tare, R.S.; Yang, L.Y.; Williams, D.F.; Ou, K.L.; Oreffo, R.O.C. Biofabrication of bone tissue: Approaches, challenges and translation for bone regeneration. Biomaterials 2016, 83, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, A.S.; Ribeiro, I.A.; Fernandes, M.H.; Cerdeira, A.C.; Vieira, B.J.; Waerenborgh, J.C.; Pereira, L.C.; Cláudio, R.; Carmezim, M.J.; Gomes, P.; et al. 3D-printed platform multi-loaded with bioactive, magnetic nanoparticles and an antibiotic for re-growing bone tissue. Int. J. Pharm. 2021, 593, 120097. [Google Scholar] [CrossRef] [PubMed]
- Drouet, C.; Bosc, F.; Banu, M.; Largeot, C. Nanocrystalline apatites: From powders to biomaterials. Powder Technol. 2009, 190, 118–122. [Google Scholar] [CrossRef]
- Lakrat, M.; Jabri, M.; Alves, M.; Fernandes, M.H.; Ansari, L.L.; Santos, C.; Mejdoubi, E.M. Three-dimensional Nano-Hydroxyapatite sodium silicate glass composite scaffold for bone tissue engineering-A new fabrication process at a near-room temperature. Mater. Chem. Phys. 2020, 260, 124185. [Google Scholar] [CrossRef]
- dos Santos, C.F.; Gomes, P.S.; Almeida, M.M.; Willinger, M.G.; Franke, R.P.; Fernandes, M.H.; Costa, M.E. Gold-dotted hydroxyapatite nanoparticles as multifunctional platforms for medical applications. RSC Adv. 2015, 5, 69184–69195. [Google Scholar] [CrossRef][Green Version]
- Scherrer, P. Bestimmung der inneren Struktur und der Größe von Kolloidteilchen mittels Röntgenstrahlen. In Kolloidchemie Ein Lehrbuch; Zsigmondy, R., Ed.; Springer: Berlin/Heidelberg, Germany, 1912; Volume 277, pp. 387–409. [Google Scholar] [CrossRef]
- Rodríguez-Lugo, V.; Karthik, T.V.K.; Mendoza-Anaya, D.; Rubio-Rosas, E.; Villaseñor Cerón, L.S.; Reyes-Valderrama, M.I.; Salinas-Rodríguez, E. Wet chemical synthesis of nanocrystalline hydroxyapatite flakes: Effect of pH and sintering temperature on structural and morphological properties. R. Soc. Open Sci. 2018, 5, 180962. [Google Scholar] [CrossRef]
- Lakrat, M.; Elansari, L.L.; Mejdoubi, E. Synthesis of B-type carbonated hydroxyapatite by a new dissolution-precipitation method. Mater. Today Proc. 2020, 31, S83–S88. [Google Scholar] [CrossRef]
- Bouhaouss, A.; Bensaoud, A.; El Moussaouiti, M.; Ferhat, M. Analyse fine de l’apatite analogue aux biomateriaux par la spectroscopie infrarouge. Phys. Chem. News 2001, 1, 125–129. [Google Scholar]
- Lakrat, M.; Azzaoui, K.; Jodeh, S.; Akartasse, N.; Mejdoubi, E.; Lamhamdi, A. The removal of methyl orange by nanohydroxyapatite from aqueous solution: Isotherm, kinetics and thermodynamics studies. Desalin. Water Treat. 2017, 85, 237–249. [Google Scholar] [CrossRef]
- Amna, T.; Alghamdi, A.A.A.; Shang, K.; Hassan, M.S. Nigella sativa-Coated Hydroxyapatite Scaffolds: Synergetic Cues to Stimulate Myoblasts Differentiation and Offset Infections. Tissue Eng. Regen. Med. 2021, 18, 787–795. [Google Scholar] [CrossRef]
- Gerige, S.J.; Gerige, M.K.Y.; Rao, M.; Ramanjaneyulu. GC-MS analysis of Nigella sativa seeds and antimicrobial activity of its volatile oil. Braz. Arch. Biol. Technol. 2009, 52, 1189–1192. [Google Scholar] [CrossRef]
- Rohman, A.; Ariani, R. Authentication of Nigella sativa seed oil in binary and ternary mixtures with corn oil and soybean oil using FTIR spectroscopy coupled with partial least square. Sci. World J. 2013, 2013, 740142. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, G.; Basu, B. A porous hydroxyapatite scaffold for bone tissue engineering: Physico-mechanical and biological evaluations. Ceram. Int. 2012, 38, 341–349. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef]
- Dalli, M.; Azizi, S.E.; Benouda, H.; Azghar, A.; Tahri, M.; Bouammali, B.; Maleb, A.; Gseyra, N. Molecular Composition and Antibacterial Effect of Five Essential Oils Extracted from Nigella sativa L. Seeds against Multidrug-Resistant Bacteria: A Comparative Study. Evid.-Based Complement. Altern. Med. 2021, 2021, 6643765. [Google Scholar] [CrossRef]
- Mohammed, N.K.; Manap, M.Y.A.; Tan, C.P.; Muhialdin, B.J.; Alhelli, A.M.; Hussin, A.S.M. The Effects of Different Extraction Methods on Antioxidant Properties, Chemical Composition, and Thermal Behavior of Black Seed (Nigella sativa L.) Oil. Evid.-Based Complement. Altern. Med. 2016, 2016, 10. [Google Scholar] [CrossRef]
- Khoddami, A.; Ghazali, H.M.; Yassoralipour, A.; Ramakrishnan, Y.; Ganjloo, A. Physicochemical Characteristics of Nigella Seed (Nigella sativa L.) Oil as Affected by Different Extraction Methods. J. Am. Oil Chem. Soc. 2011, 88, 533–540. [Google Scholar] [CrossRef]
- Matthäus, B. Antioxidant activity of extracts obtained from residues of different oilseeds. J. Agric. Food Chem. 2002, 50, 3444–3452. [Google Scholar] [CrossRef]
- D’Antuono, L.F.; Moretti, A.; Lovato, A.F.S. Seed yield, yield components, oil content and essential oil content and composition of Nigella sativa L. and Nigella damascena L. Ind. Crops Prod. 2002, 15, 59–69. [Google Scholar] [CrossRef]
- Sun, T.; Ho, C.T. Antioxidant activities of buckwheat extracts. Food Chem. 2005, 90, 743–749. [Google Scholar] [CrossRef]
- Singh, G.; Marimuthu, P.; de Heluani, C.S.; Catalan, C. Chemical constituents and antimicrobial and antioxidant potentials of essential oil and acetone extract of Nigella sativa seeds. J. Sci. Food Agric. 2005, 85, 2297–2306. [Google Scholar] [CrossRef]
- Burits, M.; Bucar, F. Antioxidant Activity of Nigella sativa Essential Oil. Phyther. Res. 2000, 328, 323–328. [Google Scholar] [CrossRef]
- Piras, A.; Rosa, A.; Marongiu, B.; Porcedda, S.; Falconieri, D.; Dessi, M.A.; Ozcelik, B.; Koca, U. Chemical composition and in vitro bioactivity of the volatile and fixed oils of Nigella sativa L. extracted by supercritical carbon dioxide. Ind. Crops Prod. 2013, 46, 317–323. [Google Scholar] [CrossRef]
- Hasanzadeh, K.M.; Ramazanie, M.; Golmohammadzadeh, S. Volatile Constituents of Nigella sativa L. Seeds. Orient. J. Chem. 2000, 16, 461–462. [Google Scholar]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Cicchetti, E.; Merle, P.; Chaintreau, A. Quantitation in gas chromatography: Usual practices. Flavour Fragr. J. 2008, 23, 450–459. [Google Scholar] [CrossRef]
- Gharby, S.; Harhar, H.; Guillaume, D.; Roudani, A.; Boulbaroud, S.; Ibrahimi, M.; Ahmad, M.; Sultana, S.; Hadda, T.B.; Chafchaouni-Moussaoui, I.; et al. Chemical investigation of Nigella sativa L. seed oil produced in Morocco. J. Saudi Soc. Agric. Sci. 2015, 14, 172–177. [Google Scholar] [CrossRef]
- Ajita, J.; Saravanan, S.; Selvamurugan, N. Effect of size of bioactive glass nanoparticles on mesenchymal stem cell proliferation for dental and orthopedic applications. Mater. Sci. Eng. C 2015, 53, 142–149. [Google Scholar] [CrossRef]
- Shuid, A.N.; Mohamed, N.; Mohamed, I.N.; Othman, F.; Suhaimi, F.; Mohd Ramli, E.S.; Muhammad, N.; Soelaiman, I.N. Nigella sativa: A Potential Antiosteoporotic Agent. Evid.-Based Complement. Altern. Med. 2012, 2012, 696230. [Google Scholar] [CrossRef]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef]
- Cobourne-Duval, M.K.; Taka, E.; Mendonca, P.; Bauer, D.; Soliman, K.F.A. The Antioxidant Effects of Thymoquinone in Activated BV-2 Murine Microglial Cells. Neurochem. Res. 2016, 41, 3227–3238. [Google Scholar] [CrossRef] [PubMed]
- Mouwakeh, A.; Kincses, A.; Nové, M.; Mosolygó, T.; Mohácsi-Farkas, C.; Kiskó, G.; Spengler, G. Nigella sativa essential oil and its bioactive compounds as resistance modifiers against Staphylococcus aureus. Phyther. Res. 2019, 33, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, C.M.B.; Thys, R.C.S.; Brandelli, A. Antifungal properties of phosphatidylcholine-oleic acid liposomes encapsulating garlic against environmental fungal in wheat bread. Int. J. Food Microbiol. 2019, 293, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ashby, R.; Solaiman, D.K.Y.; Uknalis, J.; Fan, X. Inactivation of Salmonella spp. and Listeria spp. by palmitic, stearic, and oleic acid sophorolipids and thiamine dilauryl sulfate. Front. Microbiol. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tajkarimi, M.; Ibrahim, S.A. Antimicrobial activity of ascorbic acid alone or in combination with lactic acid on Escherichia coli O157:H7 in laboratory medium and carrot juice. Food Control 2011, 22, 801–804. [Google Scholar] [CrossRef]
- Kouassi, Y.A.O.; Shelef, L.A. Inhibition of Listeria monocytogenes by cinnamic acid: Possible interaction of the acid with cysteinyl residues. J. Food Saf. 1998, 18, 231–242. [Google Scholar] [CrossRef]
- Tiji, S.; Rokni, Y.; Asehraou, A.; Mimouni, M. Chemical composition related to Antimicrobial activity of Moroccan Nigella sativa extracts and isolated fractions. Evid.-Based Complement. Altern. Med. 2021, 2021, 1–14. [Google Scholar] [CrossRef]
- Ravindran, J.; Nair, H.B.; Sung, B.; Prasad, S.; Tekmal, R.R.; Aggarwal, B.B. Thymoquinone Poly (lactideco-glycolide) Nanoparticles Exhibit Enhanced Antiproliferative, Anti-inflammatory and Chemosensitization Potential. Biochem. Pharmacol. 2010, 79, 7. [Google Scholar] [CrossRef]
- Kazmi, A.; Khan, M.A.; Ali, H. Biotechnological approaches for production of bioactive secondary metabolites in Nigella sativa: An up-to-date review. Int. J. Second. Metab. 2019, 6, 172–195. [Google Scholar] [CrossRef]
- Mallakpour, S.; Okhovat, M. Hydroxyapatite mineralization of chitosan-tragacanth blend/ZnO/Ag nanocomposite films with enhanced antibacterial activity. Int. J. Biol. Macromol. 2021, 175, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, H.; Weir, M.D.; Schneider, A.; Ren, K.; Homayounfar, N.; Oates, T.W.; Zhang, K.; Liu, J.; Hu, T.; et al. An antibacterial and injectable calcium phosphate scaffold delivering human periodontal ligament stem cells for bone tissue engineering. RSC Adv. 2020, 10, 40157–40170. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Haider, S.; Haider, A.; Abd Razak, S.I.; Kadir, M.R.A.; Shah, S.A.; Javed, A.; Shakir, I.; Al-Zahrani, A.A. Development of porous, antibacterial and biocompatible GO/n-HAp/bacterial cellulose/β-glucan biocomposite scaffold for bone tissue engineering. Arab. J. Chem. 2021, 14, 102924. [Google Scholar] [CrossRef]
- Tiji, S.; Bouhrim, M.; Addi, M.; Drouet, S.; Lorenzo, J.M.; Hano, C.; Bnouham, M.; Mimouni, M. Linking the Phytochemicals and the α-Glucosidase and α-Amylase Enzyme Inhibitory Effects of Nigella sativa Seed Extracts. Foods 2021, 10, 1818. [Google Scholar] [CrossRef]
- Lucero, M.; Estell, R.; Tellez, M.; Fredrickson, E. A retention index calculator simplifies identification of plant volatile organic compounds. Phytochem. Anal. 2009, 20, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Dobaria, J.; Raval, S. Separation of Phytochemicals from Peucedanum Nagpurense by Using Separation of Phytochemicals from Peucedanum. J. Cell Tissue Res. 2016, 15, 5275–5281. [Google Scholar]
- Guérin-Faublée, V.; Carret, G. L’antibiogramme: Principe, méthodologie intérêt et limites. Journées Natl. GTV-INRA 1999, 5–12. [Google Scholar]



| Elution Order | Component | RT 1 | % Area 2 |
|---|---|---|---|
| 1 | Alpha-ThujeneOriganene (C10H16) | 5.000 | 13.70 |
| 2 | Alpha-Pinene (C10H16) | 5.133 | 2.21 |
| 3 | Beta-Pinene (C10H16) | 5.842 | 2.19 |
| 4 | 1,2,4, trimethylbenzene, (C9H12). | 6.100 | 1.30 |
| 5 | Beta-Cymene (C10H14) | 6.600 | 38.05 |
| 6 | Gamma-Terpinene (C10H16) | 7.158 | 0.69 |
| 7 | Aldehyde lilac (C10H16O2) | 7.792 | 0.55 |
| 8 | Carvacrol (C10H14O) | 8.175 | 2.19 |
| 9 | Thymoquinone | 10.233 | 5.69 |
| Elution Order | Component | RT 1 | % Area 2 |
|---|---|---|---|
| 1 | 2.4-Decadienal | 15.100 | 1.79 |
| 2 | 2-oxo-methyl ester Hexadecanoic acid | 15.592 | 1.06 |
| 3 | Phenol, 4-methoxy-2,3,6-trimethyl- | 18.417 | 1.56 |
| 4 | Palmitic acid, methyl ester | 22.600 | 1.32 |
| 5 | L(+)Ascorbic acid 2.6-dihexadecanoate | 23.108 | 4.39 |
| 6 | Oleic acid methyl ester | 24.358 | 2.96 |
| 7 | Linoleic acid | 25.117 | 80.65 |
| 8 | E/Z-1,3,12-Nonadecatriene | 25.608 | 6.24 |
| Elution Order | Component | RT 1 | % Area 2 |
|---|---|---|---|
| 1 | Pentanoic acid, heptyl (C12H24O2) | 4.47 | 2.72 |
| 2 | 1-Hepten-5-yne, 2-methyl-3-methylene (C9H12) | 4.92 | 4.56 |
| 3 | (R)-(2.2-dimethyl-1,3-dioxolane-4)methanol (C6H12O3) | 5.14 | 3.28 |
| 4 | Cumol (C9H12) | 5.46 | 3.84 |
| 5 | Psi-cumene (C9H12). | 5.73 | 3.23 |
| 6 | Benzene (1,3,3-trimethylnonyl) (C18H30) | 5.95 | 21.62 |
| 7 | beta.-Cymene (C10H14) | 6.41 | 15.76 |
| 8 | Decane, 2.9-dimethyl (C12H26) | 7.50 | 17.31 |
| 9 | 1.3-Dioxolane-4-methanol,2,2-dimethyl,acetate (C8H14O4) | 7.66 | 2.94 |
| 10 | Dodecane (C12H26) | 8.98 | 3.56 |
| 11 | p-Cymen-3-ol (C4H14O) | 10.53 | 1.84 |
| 12 | Glycerine diacetate (C7H12O5) | 11.13 | 1.88 |
| 13 | Stearic acid (C18H36O2) | 18.12 | 0.73 |
| 14 | Palmitic acid (C16H32O2) | 18.43 | 7.29 |
| 15 | Linoleic acid (C18H32O2) | 19.40 | 1.12 |
| 16 | alpha.-Glyceryl linoleate (C21H38O4) | 20.03 | 6.85 |
| 17 | Oleic acid (C18H34O2) | 20.07 | 0.56 |
| 18 | Nonadecanoic acid (C21H42O2) | 20.26 | 0.98 |
| Extracts | Inhibition Zones Diameter (in mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| nHAp/SSG@ | ||||||||||
| Strain | Control | FH | FA | HS | ||||||
| 1.5% | 3% | 6% | 1.5% | 3% | 6% | 1.5% | 3% | 6% | ||
| Yeast strain | ||||||||||
| C. albicans | 0 ± 0.23 | 19 ± 0.21 | 17 ± 0.25 | 12 ± 0.70 | 15 ± 0.56 | 13 ± 0.35 | 12 ± 0.42 | 11.5 ± 0.42 | 12 ± 0.70 | 11 ± 0.28 |
| Gram negative | ||||||||||
| P. aeruginosa | 0 ± 0.28 | 11 ± 0.57 | 10 ± 0.21 | 07 ± 0.28 | 11 ± 0.85 | 10 ± 0.42 | 7.6 ± 0.28 | 11.7 ± 0.14 | 11 ± 0.57 | 8.9 ± 0.35 |
| E. coli | 0 ± 0.14 | 11 ± 0.35 | 11 ± 0.42 | 09 ± 0.14 | 11 ± 0.85 | 11 ± 0.85 | 9.5 ± 0.35 | 11 ± 0.14 | 12 ± 0.14 | 11 ± 0.28 |
| Gram positive | ||||||||||
| M. luteus | 0 ± 0.45 | 18 ± 0.70 | 20 ± 0.85 | 15 ± 0.14 | 16 ± 0.42 | 18.9 ± 0.21 | 13.2 ± 0.70 | 11.9 ± 0.70 | 12.2 ± 0.21 | 11.3 ± 0.85 |
| S. aureus | 0 ± 0.31 | 12 ± 0.49 | 20 ± 0.35 | 11 ± 0.49 | 11 ± 0.28 | 19 ± 0.28 | 10 ± 0.70 | 12 ± 0.14 | 13 ± 0.07 | 11 ± 0.07 |
| L. innocua | 0 ± 0.65 | 12 ± 0.35 | 10 ± 0.85 | 09 ± 0.28 | 12 ± 0.28 | 11 ± 0.07 | 10.2 ± 0.78 | 12 ± 0.70 | 10.6 ± 0.14 | 9.3 ± 0.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiji, S.; Lakrat, M.; Rokni, Y.; Mejdoubi, E.M.; Hano, C.; Addi, M.; Asehraou, A.; Mimouni, M. Characterization and Antimicrobial Activity of Nigella sativa Extracts Encapsulated in Hydroxyapatite Sodium Silicate Glass Composite. Antibiotics 2022, 11, 170. https://doi.org/10.3390/antibiotics11020170
Tiji S, Lakrat M, Rokni Y, Mejdoubi EM, Hano C, Addi M, Asehraou A, Mimouni M. Characterization and Antimicrobial Activity of Nigella sativa Extracts Encapsulated in Hydroxyapatite Sodium Silicate Glass Composite. Antibiotics. 2022; 11(2):170. https://doi.org/10.3390/antibiotics11020170
Chicago/Turabian StyleTiji, Salima, Mohammed Lakrat, Yahya Rokni, El Miloud Mejdoubi, Christophe Hano, Mohamed Addi, Abdeslam Asehraou, and Mostafa Mimouni. 2022. "Characterization and Antimicrobial Activity of Nigella sativa Extracts Encapsulated in Hydroxyapatite Sodium Silicate Glass Composite" Antibiotics 11, no. 2: 170. https://doi.org/10.3390/antibiotics11020170
APA StyleTiji, S., Lakrat, M., Rokni, Y., Mejdoubi, E. M., Hano, C., Addi, M., Asehraou, A., & Mimouni, M. (2022). Characterization and Antimicrobial Activity of Nigella sativa Extracts Encapsulated in Hydroxyapatite Sodium Silicate Glass Composite. Antibiotics, 11(2), 170. https://doi.org/10.3390/antibiotics11020170







