Synergistic Antibacterial Potential of 6-Pentyl-α-pyrone Lactone and Zinc Oxide Nanoparticles against Multidrug-Resistant Enterobacterales Isolated from Urinary Tract Infections in Humans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Urine Samples
2.2. Bacteriological Examination
2.3. Antimicrobial Susceptibility Testing
2.4. Plasmid Extraction and Detection of the Integrase Gene
2.5. Restriction Fragment Length Polymorphism (RFLP) for Integrons Categorization
2.6. Preparation of 6-Pentyl-α-Pyrone Lactone and Zinc Oxide Nanoparticles
2.7. Antimicrobial Activities of 6-Pentyl-α-Pyrone Lactone, Zinc Oxide Nanoparticles and Their Combination
2.8. Statistical Analysis
3. Results
3.1. Occurrence of Enterobacterales in Clinical Urine Samples
3.2. Antimicrobial Susceptibility Results
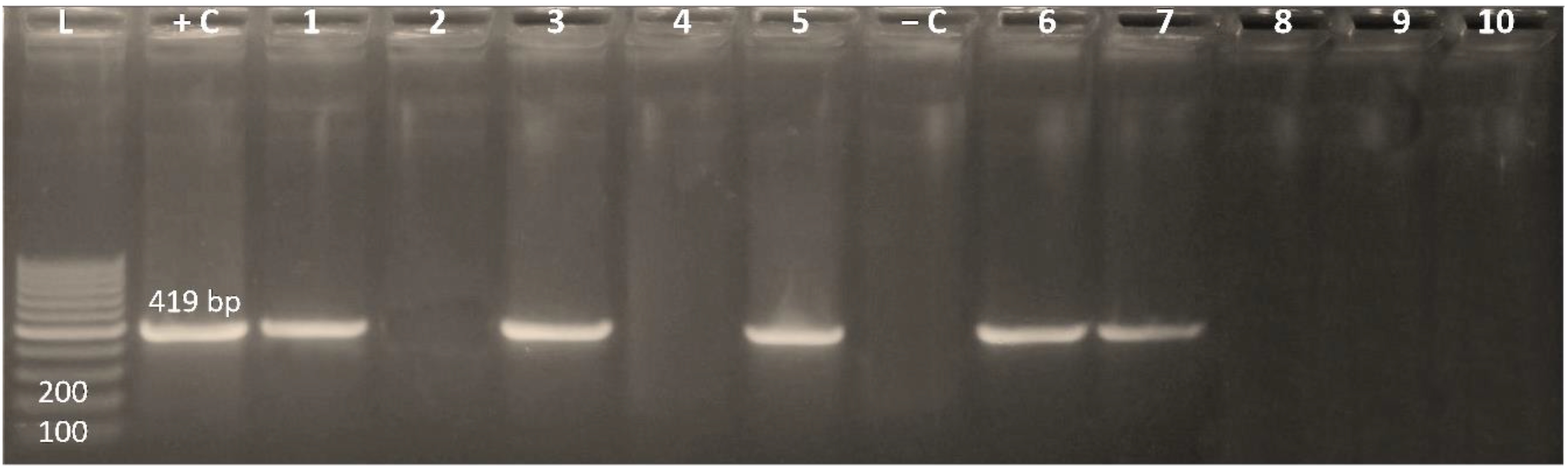
3.3. Existence of the Integrase Gene among MDR Enterobacterales Isolates
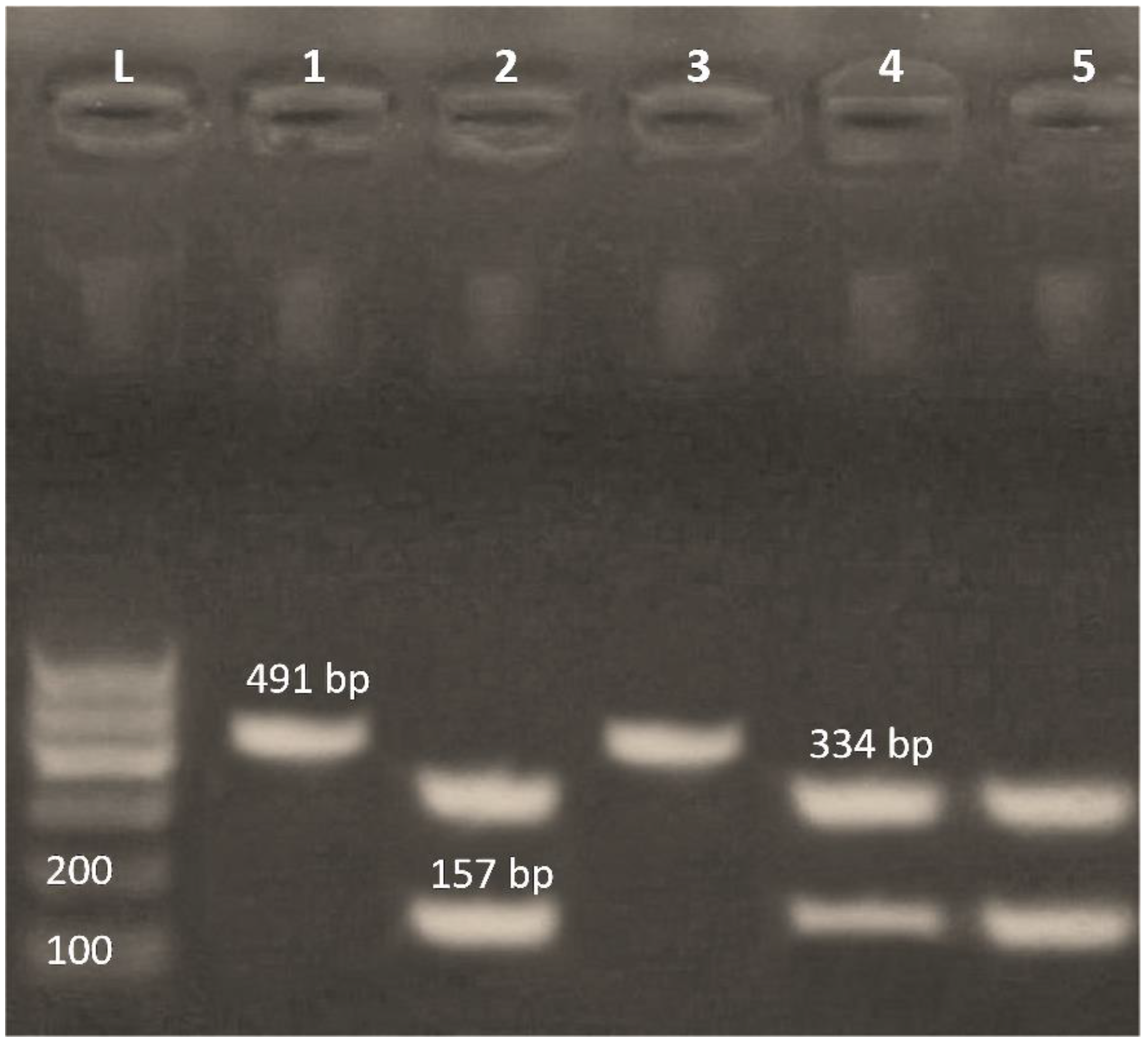
3.4. Detection of Class 1 and Class 2 Integrons by PCR-RFLP
3.5. Antimicrobial Activities of 6-Pentyl-α-Pyrone Lactone and Zinc Oxide Nanoparticles against MDR Enterobacterales Isolates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalra, O.P.; Raizada, A. Approach to a patient with urosepsis. J. Glob. Infec. Dis. 2009, 1, 57. [Google Scholar] [CrossRef] [PubMed]
- Walters, M.S.; Lane, M.C.; Vigil, P.D.; Smith, S.N.; Walk, S.T.; Mobley, H.L. Kinetics of uropathogenic Escherichia coli metapopulation movement during urinary tract infection. MBio 2012, 3, e00303-11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile genetic elements associated with antimicrobial resistance. Clin. Microbiol. Rev. 2018, 31, e00088-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carattoli, A. Importance of integrons in the diffusion of resistance. Vet. Res. 2001, 32, 243–259. [Google Scholar] [CrossRef] [Green Version]
- Leverstein-van Hall, M.A.; Blok, H.E.M.; Donders, A.R.T.; Paauw, A.; Fluit, A.C.; Verhoef, J. Multidrug resistance among Enterobacteriaceae is strongly associated with the presence of integrons and is independent of species or isolate origin. J. Infec. Dis. 2003, 187, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Kaminska, P.S.; Yernazarova, A.; Murawska, E.; Swiecicki, J.; Fiedoruk, K.; Bideshi, D.K.; Swiecicka, I. Comparative analysis of quantitative reverse transcription real-time PCR and commercial enzyme imunoassays for detection of enterotoxigenic Bacillus thuringiensis isolates. FEMS Microbiol. Lett. 2014, 357, 34–39. [Google Scholar] [CrossRef] [Green Version]
- El-Rab, S.M.G.; Abo-Amer, A.E.; Asiri, A.M. Biogenic synthesis of ZnO nanoparticles and its potential use as antimicrobial agent against multidrug-resistant pathogens. Curr. Microbiol. 2020, 77, 1767–1779. [Google Scholar] [CrossRef]
- Ismaiel, A.A.; Ali, D.M. Antimicrobial properties of 6-pentyl-α-pyrone produced by endophytic strains of Trichoderma koningii and its effect on aflatoxin B1 production. Biologia 2017, 72, 1403–1415. [Google Scholar] [CrossRef]
- Hall, H.E.; Brown, D.F.; Lewis, K.H. Examination of market foods for coliform organisms. Appl. Microbiol. 1967, 15, 1062–1069. [Google Scholar] [CrossRef]
- Finegold, S.M.; Martin, W.J.; Scott, E.G. Bailey and Scott’s Diagnostic Microbiology, 5th ed.; The C.V. Mosby Company: Taipey, Taiwan, 1978; pp. 445–481. [Google Scholar]
- Heijnen, L.; Medema, G. Quantitative Detection of E. Coli, E. Coli O157 and Other Shiga Toxin Producing E. Coli in Water Samples Using a Culture Method Combined with Real-Time PCR. J. Water Health 2006, 4, 487–498. [Google Scholar] [CrossRef] [Green Version]
- Brisse, S.; Verhoef, J. Phylogenetic diversity of Klebsiella pneumoniae and Klebsiella oxytoca clinical isolates revealed by randomly amplified polymorphic DNA, gyrA and parC genes sequencing and automated ribotyping. Int. J. Syst. Evol. Microbiol. 2001, 51, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Anbazhagan, D.; Kathirvalu, G.G.; Mansor, M.; Yan, G.O.S.; Yusof, Y.M.; Sekaran, S.D. Multiplex Polymerase Chain Reaction (PCR) Assays for the Detection of Enterobacteriaceae in Clinical Samples. Afr. J. Microbiol. Res. 2010, 4, 1186–1191. [Google Scholar]
- Bi, S.; Tang, S.; Wu, X.; Chen, S. Quantitative detection of Proteus species by real-time polymerase chain reaction using SYBR Green. Ann. Microbiol. 2013, 63, 1205–1208. [Google Scholar] [CrossRef]
- Bauer, A.W. Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol. 1966, 45, 149–158. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Supplement M100 Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- EUCAST: The European Committee on Antimicrobial Susceptibility Testing (2021). Breakpoint Tables for Interpretation of MICs and Zone Diameters Version 11.0. Available online: http://www.eucast.org (accessed on 20 December 2021).
- Tambekar, D.H.; Dhanorkar, D.V.; Gulhane, S.R.; Khandelwal, V.K.; Dudhane, M.N. Antibacterial susceptibility of some urinary tract pathogens to commonly used antibiotics. Afr. J. Biotechnol. 2006, 5, 1562–1565. [Google Scholar]
- White, P.A.; McIver, C.J.; Deng, Y.M.; Rawlinson, W.D. Characterization of two new gene cassettes, aadA5 and dfrA17. FEMS Microbiol. Lett. 2000, 182, 265–269. [Google Scholar] [CrossRef] [Green Version]
- Suntako, R. Effect of zinc oxide nanoparticles synthesized by a precipitation method on mechanical and morphological properties of the CR foam. Bull. Mat. Sci. 2015, 38, 1033–1038. [Google Scholar] [CrossRef] [Green Version]
- Valgas, C.; Machado de Souza, S.; Smânia, E.F.A.; Smânia, A.J.R. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Rankin, I.D. MIC testing. Manual of antimicrobial susceptibility testing. In American Society for Microbiology; Pan American Health Organization: Washington, DC, USA, 2005; pp. 53–62. [Google Scholar]
- Yadav, M.K.; Park, S.W.; Chae, S.W.; Song, J.J.; Kim, H.C. Antimicrobial activities of Eugenia caryophyllata extract and its major chemical constituent eugenol against Streptococcus pneumonia. Acta Pathol. Microbiol. Immunol. Scand. 2013, 121, 1198–1206. [Google Scholar] [CrossRef]
- Hsieh, M.H.; Chen, M.Y.; Victor, L.Y.; Chow, J.W. Synergy assessed by checkerboard a critical analysis. Diagn. Microbiol. Infect.Dis. 1993, 16, 343–349. [Google Scholar] [CrossRef]
- Hamilton-Miller, J.M.T. Calculating MIC 50. J. Antimicrob. Chemother. 1991, 27, 863–864. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S. Sampling, 3rd ed.; JohnWiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- SAS Institute Inc. SAS/STAT Statistics user’s guide. In Statistical Analytical System, 5th ed.; SAS Institute Inc.: Cary, NC, USA, 2012. [Google Scholar]
- Podschun, R.; Ullmann, U. Klebsiella spp. as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998, 11, 589–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navon-Venezia, S.; Kondratyeva, K.; Carattoli, A. Klebsiella pneumoniae: A major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Rev. 2017, 41, 252–275. [Google Scholar] [CrossRef]
- Abdelwahab, R.; Yasir, M.; Godfrey, R.E.; Christie, G.S.; Element, S.J.; Saville, F.; Browning, D.F. Antimicrobial resistance and gene regulation in Enteroaggregative Escherichia coli from Egyptian children with diarrhoea: Similarities and differences. Virulence 2021, 12, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Kishk, R. Bacterial Pattern of Community acquired Urinary Tract Infections: A Challenge for Antimicrobial Resistance. Egypt. J. Med. Microbiol. 2021, 30, 153–162. [Google Scholar]
- Elshamy, A.A.; Saleh, S.E.; Alshahrani, M.Y.; Aboshanab, K.M.; Aboulwafa, M.M.; Hassouna, N.A. OXA-48 Carbapenemase-Encoding Transferable Plasmids of Klebsiella pneumoniae Recovered from Egyptian Patients Suffering from Complicated Urinary Tract Infections. Biology 2021, 10, 889. [Google Scholar] [CrossRef] [PubMed]
- Kotb, D.N.; Mahmoud, M.S.; Mahdi, W.K.; Khairy, R.M. Prevalence and Antimicrobial Resistance of Urinary Tract Infections in Upper Egypt. MJMR 2019, 30, 78–85. [Google Scholar]
- Desouky, D.E.; Gabr, H.M.; El-Helbawy, M.; Hathout, H.M. Urinary Tract Infection: Prevalence, Risk Factors, Bacterial Etiologies and Antimicrobial Resistance Profile among Egyptian Diabetic Patients: Urinary Tract Infection: Prevalence, Risk Factors, Bacterial Etiologies and Antimicrobial Resistance Profile among Egyptians. Eur. J. Med. Health Sci. 2020, 2, 1–6. [Google Scholar]
- Öztürk, R.; Tazegul, G. Bacteria Causing Community-Acquired Urinary Tract Infections and Their Antibiotic Susceptibility Patterns in Outpatients Attending at a State Hospital in Turkey. Cureus 2021, 13, e17753. [Google Scholar] [CrossRef]
- Said, A.; El-Gamal, M.S.; Abu-Elghait, M.; Salem, S.S. Isolation, Identification and Antibiotic Susceptibility Pattern of Urinary Tract Infection Bacterial Isolates. Lett. Appl. NanoBioSci. 2021, 10, 2820–2830. [Google Scholar]
- Tartor, Y.H.; Abd El-Aziz, N.K.; Gharieb, R.M.A.; El Damaty, H.M.; Enany, S.; Soliman, E.A.; Abdellatif, S.S.; Attia, A.S.A.; Bahnass, M.M.; El-Shazly, Y.A.; et al. Whole-Genome Sequencing of Gram-Negative Bacteria Isolated From Bovine Mastitis and Raw Milk: The First Emergence of Colistin mcr-10 and Fosfomycin fosA5 Resistance Genes in Klebsiella pneumoniae in Middle East. Front. Microbiol. 2021, 12, 770813. [Google Scholar] [CrossRef] [PubMed]
- Shash, R.Y.; Elshimy, A.A.; Soliman, M.Y.; Mosharafa, A.A. Molecular Characterization of Extended-Spectrum β-Lactamase Enterobacteriaceae Isolated from Egyptian Patients with Community- and Hospital-Acquired Urinary Tract Infection. Am. J. Trop. Med. Hyg. 2019, 100, 522–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legese, M.H.; Weldearegay, G.M.; Asrat, D. Extended-spectrum beta-lactamase-and carbapenemase-producing Enterobacteriaceae among Ethiopian children. Infec. Drug Resis. 2017, 10, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmowalid, G.A.; Ahmad, A.A.M.; Hassan, M.N.; Abd El-Aziz, N.K.; Abdelwahab, A.M.; Elwan, S.I. Molecular detection of new SHV β-lactamase variants in clinical Escherichia coli and Klebsiella pneumoniae isolates from Egypt. Comp. Immunol. Microbiol. Infect. Dis. 2018, 60, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Elmonir, W.; El-Aziz, A.; Norhan, K.; Tartor, Y.H.; Moustafa, S.M.; Abo Remela, E.M.; Eissa, R.; Saad, H.A.; Abdel Tawab, H. Emergence of Colistin and Carbapenem Resistance in Extended-Spectrum β-Lactamase Producing Klebsiella pneumoniae Isolated from Chickens and Humans in Egypt. Biology 2021, 10, 373. [Google Scholar] [CrossRef]
- Tartor, Y.H.; Gharieb, R.M.A.; Abd El-Aziz, N.K.; El Damaty, H.M.; Enany, S.; Khalifa, E.; Attia, A.S.A.; Abdellatif, S.S.; Ramadan, H. Virulence determinants and plasmid-mediated colistin resistance mcr genes in Gram-negative bacteria isolated from bovine milk. Front. Cell. Infect. Microbiol. 2021, 11, 761417. [Google Scholar] [CrossRef]
- Lazm, A.M.; Al-Allak, M.H.; Al-Asskar, J.A.; Al-Dahmoshi, H.O.; Al-Khafaji, N.S. Antibiotics resistance patterns among Enterobacteriaceae isolated from different clinical samples. Drug Invent. Today 2019, 12, 938–942. [Google Scholar]
- Abd El-Aziz, N.K.; Ammar, A.M.; Hamdy, M.M.; Gobouri, A.A.; Azab, E.; Sewid, A.H. First Report of aacC5-aadA7Δ4 Gene Cassette Array and Phage Tail Tape Measure Protein on Class 1 Integrons of Campylobacter Species Isolated from Animal and Human Sources in Egypt. Animals 2020, 10, 2067. [Google Scholar] [CrossRef]
- Abdel-Rhman, S.H.; Elbargisy, R.M.; Rizk, D.E. Characterization of Integrons and Quinolone Resistance in Clinical Escherichia coli Isolates in Mansoura City, Egypt. Int. J. Microbiol. 2021, 2021, 6468942. [Google Scholar] [CrossRef]
- Khalifa, H.O.; Soliman, A.M.; Ahmed, A.M.; Shimamoto, T.; Nariya, H.; Matsumoto, T.; Shimamoto, T. High prevalence of antimicrobial resistance in Gram-negative bacteria isolated from clinical settings in Egypt: Recalling for judicious use of conventional antimicrobials in developing nations. Microb. Drug Resis. 2019, 25, 371–385. [Google Scholar] [CrossRef]
- Barns, J.N.; Ezeamagu, C.O.; Nkemjika, M.E.; Akindele, T.S. Prevalence of integrons in Enterobacteriaceae obtained from clinical samples. J. Microbiol. Antimicrob. 2021, 13, 1–10. [Google Scholar]
- Jiang, J.; Pi J and Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef] [PubMed]
| Enterobacterales Species | Age | Sex | |||||
|---|---|---|---|---|---|---|---|
| C (n = 9) | EA (n = 7) | YA (n = 53) | MA (n = 28) | OA (n = 13) | F (n = 70) | M (n = 40) | |
| E. coli | 2 (22.22) | 1 (14.29) | 23 (43.39) * | 11 (39.28) | 5 (38.46) | 33 (47.14) * | 9 (30) |
| Klebsiella | 1 (11.11) | - | 16 (30.18) * | - | 1 (7.69) | 17 (24.28) * | 1 (3.33) |
| Citrobacter | - | - | 1 (1.88) | 1 (3.57) | - | 2 (2.85) NE | - |
| Proteus | - | - | - | - | 1 (7.69) | - | 1 (3.33) NE |
| Total | 3 (33.33) (Ref.) | 1 (14.29) (0.33 ¶) * | 40 (75.47) (0.952 ¶) ns | 12 (42.86) (0.266 ¶) * | 7 (53.85) (0.153 ¶) * | 52 (74.29) (Ref.) | 11 (27.50) (0.200 ¶) * |
| Antimicrobial Agent | Susceptibility * | MAR Index | p-Value | ||
|---|---|---|---|---|---|
| Sensitive | Intermediate | Resistant | |||
| Amoxicillin clavulanic acid (AMC) | 7 (11.11) | 6 (9.52) | 50 (79.36) | 0.050 | 0.001 |
| Ampicillin sulbactam (SAM) | 6 (9.52) | 11 (17.46) | 46 (73.01) | 0.045 | 0.001 |
| Piperacillin tazobactam (TPZ) | 7 (11.11) | 4 (6.34) | 52 (82.53) | 0.051 | 0.001 |
| Amikacin (AK) | 17 (26.98) | 4 (6.34) | 42 (66.67) | 0.042 | 0.001 |
| Gentamycin (CN) | 12 (19.04) | 6 (9.52) | 45 (71.42) | 0.044 | 0.001 |
| Imipenem (IMP) | 25 (39.68) | 9 (14.28) | 29 (46.03) | 0.028 | 0.004 |
| Meropenem (MEM) | 23 (36.50) | 16 (25.39) | 24 (38.09) | 0.023 | 0.404 |
| Doxycycline (DO) | 27 (42.85) | 3 (4.76) | 33 (52.38) | 0.033 | 0.001 |
| Ciprofloxacin (CIP) | 7 (11.11) | 3 (4.76) | 53 (84.12) | 0.052 | 0.001 |
| Levofloxacin (LEV) | 10 (15.87) | 4 (6.34) | 49 (77.77) | 0.047 | 0.001 |
| Trimethoprime + sulfamethaxazole (SXT) | 11 (17.46) | 6 (9.52) | 46 (73.01) | 0.047 | 0.001 |
| Nitrofurantoin (F) | 22 (34.92) | 15 (23.80) | 26 (41.26) | 0.027 | 0.228 |
| Cefuroxime (CXM) | 1 (52.17) | 2 (00.00) | 60 (47.82) | 0.059 | 0.001 |
| Cefepime (FEB) | 0 (00.00) | 2 (3.17) | 61 (96.82) | 0.060 | 0.001 |
| Cefotaxime (CTX) | 0 (00.00) | 0 (00.00) | 63 (100.00) | 0.062 | NE |
| Erythromycin (E) | 4 (6.34) | 1 (1.58) | 58 (92.06) | 0.057 | 0.001 |
| Isolate No. | Antimicrobial Resistant Pattern | Bacterial Species | MIC (μg/mL) | Interactive Category | |||
|---|---|---|---|---|---|---|---|
| Fungal Extract | ZnONPs | Fungal Extract/ZnONPs | ΣFIC | ||||
| 1 | SAM, TPZ, AK, CN, IPM, DO, CIP, LEV, SXT, CXM, FEP, CTX, E | E. coli | 32 | 32 | ½ | 0.0937 | Synergism |
| 2 | AMC, SAM, TPZ, AK, CN, IPM, MEM, DO, CIP, LEV, SXT, CXM, FEP, CTX, E | E. coli | 32 | 0.015 | 0.0075/0.015 | 0.5004 | Synergism |
| 3 | AMC, SAM, TPZ, AK, CN, IPM, MEM, DO, CIP, LEV, SXT, F, CXM, FEP, CTX, E | Klebsiella | 16 | 0.015 | 0.0075/0.015 | 0.5009 | Synergism |
| 4 | AMC, SAM, TPZ, CN, DO, CIP, LEV, SXT, F, CXM, FEP, CTX, E | Klebsiella | 32 | 0.062 | 0.0075/0.015 | 0.1214 | Synergism |
| 5 | AMC, SAM, CN, IPM, DO, CIP, LEV, SXT, CXM, FEP, CTX, E | Klebsiella | 32 | 1 | 0.0075/0.015 | 0.0079 | Synergism |
| 6 | AMC, SAM, TPZ, AK, CN, IPM, MEM, DO, CIP, LEV, SXT, F, CXM, FEP, CTX, E | Klebsiella | 16 | 8 | 0.031/0.062 | 0.0077 | Synergism |
| 7 | AMC, SAM, TPZ, AK, CN, IPM, MEM, DO, CIP, LEV, SXT, F, CXM, FEP, CTX, E | Klebsiella | 32 | 1 | 0.0075/0.015 | 0.0079 | Synergism |
| 8 | AMC, SAM, TPZ, AK, CN, IPM, MEM, CIP, LEV, SXT, F, CXM, FEP, CTX, E | E. coli | 32 | 16 | 0.0075/0.015 | 0.00093 | Synergism |
| 9 | AMC, SAM, TPZ, AK, CN, IPM, MEM, DO, CIP, LEV, SXT, F, CXM, FEP, CTX, E | E. coli | 32 | 0.015 | 0.015/0.031 | 1.0009 | Indifference |
| 10 | AMC, SAM, TPZ, AK, CN, IPM, DO, CIP, LEV, SXT, CXM, FEP, CTX, E | Klebsiella | 16 | 32 | 0.0075/0.015 | 0.0032 | Synergism |
| Means ± SE | 27.2 ± 2.44 | 9.01 ± 4.16 * | 0.109 ± 0.09/0.219 ± 0.197 *,‡ | ||||
| MIC | Antibacterial Agents (μg/mL) | ||
|---|---|---|---|
| Fungal Metabolite | ZnONPs | Combinations of ZnONPs and Fungal Metabolite | |
| MIC range | 16–64 | 0.015–32 | 0.0075/0.015-½ |
| MIC 50 a | 32 | 1 | 0.0075/0.015 |
| MIC 90 b | 16 | 0.015 | 0.0075/0.015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotb, A.M.E.; Abd El-Aziz, N.K.; Elariny, E.Y.T.; Yahya, R.; Alkhalifah, D.H.M.; Ahmed, R.M. Synergistic Antibacterial Potential of 6-Pentyl-α-pyrone Lactone and Zinc Oxide Nanoparticles against Multidrug-Resistant Enterobacterales Isolated from Urinary Tract Infections in Humans. Antibiotics 2022, 11, 440. https://doi.org/10.3390/antibiotics11040440
Kotb AME, Abd El-Aziz NK, Elariny EYT, Yahya R, Alkhalifah DHM, Ahmed RM. Synergistic Antibacterial Potential of 6-Pentyl-α-pyrone Lactone and Zinc Oxide Nanoparticles against Multidrug-Resistant Enterobacterales Isolated from Urinary Tract Infections in Humans. Antibiotics. 2022; 11(4):440. https://doi.org/10.3390/antibiotics11040440
Chicago/Turabian StyleKotb, Ahmed M. E., Norhan K. Abd El-Aziz, Eman Y. T. Elariny, Reham Yahya, Dalal Hussien M. Alkhalifah, and Rania M. Ahmed. 2022. "Synergistic Antibacterial Potential of 6-Pentyl-α-pyrone Lactone and Zinc Oxide Nanoparticles against Multidrug-Resistant Enterobacterales Isolated from Urinary Tract Infections in Humans" Antibiotics 11, no. 4: 440. https://doi.org/10.3390/antibiotics11040440
APA StyleKotb, A. M. E., Abd El-Aziz, N. K., Elariny, E. Y. T., Yahya, R., Alkhalifah, D. H. M., & Ahmed, R. M. (2022). Synergistic Antibacterial Potential of 6-Pentyl-α-pyrone Lactone and Zinc Oxide Nanoparticles against Multidrug-Resistant Enterobacterales Isolated from Urinary Tract Infections in Humans. Antibiotics, 11(4), 440. https://doi.org/10.3390/antibiotics11040440






