Evaluation of Bi-Lateral Co-Infections and Antibiotic Resistance Rates among COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting and Duration
2.2. Collection of Patient Data
2.3. Collection of Clinical Samples
2.4. Isolation and Identification of Bacterial Isolates
2.5. Antimicrobial Susceptibility Testing
2.6. Statistical Analysis
3. Results
3.1. Demographical Characteristics of Coronavirus Disease 2019 (COVID-19) Patients
3.2. Sample-Wise Positive Ratio of Bacterial Cultures
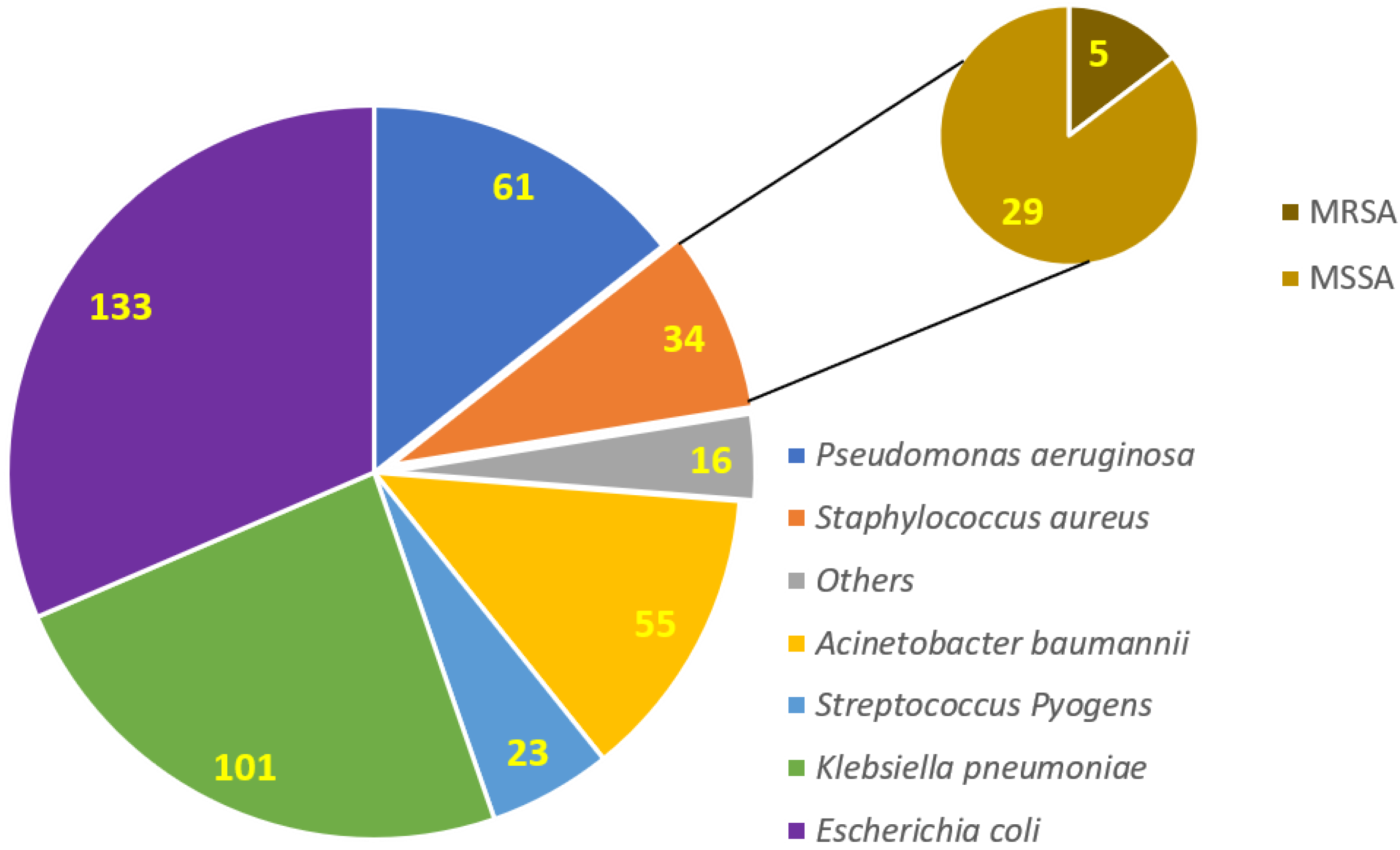
3.3. Isolation and Identification of Clinical Bacterial Isolates among COVID-19 Patients
3.4. Antibiotic Susceptibility Patterns of Individual Isolated Bacterial Isolates
3.5. Appearance of Symptoms in COVID-19 Patients
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bashir, M.F.; Jiang, B.; Komal, B.; Bashir, M.A.; Farooq, T.H.; Iqbal, N.; Bashir, M. Correlation between environmental pollution indicators and COVID-19 pandemic: A brief study in Californian context. Environ. Res. 2020, 187, 109652. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.; Lau, E.H.; Wong, J.Y.; et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Hendaus, M.A.; Jomha, F.A. COVID-19 induced superimposed bacterial infection. J. Biomol. Struct. Dyn. 2020, 39, 4185–4191. [Google Scholar] [CrossRef] [PubMed]
- Yeo, A.J.; Henningham, A.; Fantino, E.; Galbraith, S.; Krause, L.; Wainwright, C.E.; Sly, P.D.; Lavin, M.F. Increased susceptibility of airway epithelial cells from ataxia-telangiectasia to S. pneumoniae infection due to oxidative damage and impaired innate immunity. Sci. Rep. 2019, 9, 2627. [Google Scholar] [CrossRef]
- Baker, M.A.; Sands, K.E.; Huang, S.S.; Kleinman, K.; Septimus, E.J.; Varma, N.; Blanchard, J.; Poland, R.E.; Coady, M.H.; Yokoe, D.S.; et al. The Impact of Coronavirus Disease 2019 (COVID-19) on Healthcare-Associated Infections. Clin. Infect. Dis. 2021, 9, 1–7. [Google Scholar] [CrossRef]
- Knight, G.M.; Glover, R.E.; McQuaid, C.F.; Olaru, I.D.; Gallandat, K.; Leclerc, Q.J.; Fuller, N.M.; Willcocks, S.J.; Hasan, R.; van Kleef, E.; et al. Antimicrobial resistance and COVID-19: Intersections and implications. Elife 2021, 10, e64139. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Allawy, M.Y.; Al-Taie, B.S.; Al-Tikreeti, H.M. A Review Article: Resistant Bacterial Strains “Real Threat”. Int. J. Psychosoc. Rehabil. 2020, 24, 4206–4211. [Google Scholar] [CrossRef]
- Zeshan, B.; Karobari, M.I.; Afzal, N.; Siddiq, A.; Basha, S.; Basheer, S.N.; Peeran, S.W.; Mustafa, M.; Daud, N.H.A.; Ahmed, N.; et al. The Usage of Antibiotics by COVID-19 Patients with Comorbidities: The Risk of Increased Antimicrobial Resistance. Antibiotics 2022, 11, 35. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277. [Google Scholar]
- Bardi, T.; Pintado, V.; Gomez-Rojo, M.; Escudero-Sanchez, R.; Lopez, A.A.; Diez-Remesal, Y.; Castro, N.M.; Ruiz-Garbajosa, P.; Pestaña, D. Nosocomial infections associated to COVID-19 in the intensive care unit: Clinical characteristics and outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Liu, L.; Huang, T.; Zhu, Y. Nosocomial infections in psychiatric hospitals during the COVID-19 outbreak. Eur. J. Psychiatry 2020, 34, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.; Collins, J.; Barlow-Pay, F.; Rickard, F.; Bruce, E.; Verduri, A.; Quinn, T.J.; Mitchell, E.; Price, A.; Vilches-Moraga, A.; et al. Nosocomial COVID-19 infection: Examining the risk of mortality. The COPE-Nosocomial Study (COVID in Older PEople). J. Hosp. Infect. 2020, 106, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.; Baker, M.; Vaidya, V.; Tucker, R.; Resnick, A.; Morris, C.A.; Klompas, M.; Program, C.P.E. Incidence of nosocomial COVID-19 in patients hospitalized at a large US academic medical center. JAMA Netw. Open 2020, 3, e2020498. [Google Scholar] [CrossRef] [PubMed]
- Iyer, P.; Aziz, K.; Ojcius, D.M. Impact of COVID-19 on dental education in the United States. J. Dent. Educ. 2020, 84, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.C.H.; Farhanah, S.; Linn, K.Z.; Tang, Y.W.; Poon, C.Y.; Lim, A.Y.; Tan, H.R.; Binte Hamed, N.H.; Huan, X.; Puah, S.H.; et al. Nosocomial infections among COVID-19 patients: An analysis of intensive care unit surveillance data. Antimicrob. Resist. Infect. Control 2021, 10, 119. [Google Scholar] [CrossRef]
- Ahmed, N.; Rizvi, A.; Naeem, A.; Saleem, W.; Ahmed, A.; Parveen, S.; Ilyas, M. COVID-19 and public awareness. Prof. Med. J. 2020, 27, 1710–1716. [Google Scholar] [CrossRef]
- Yusof, W.; Irekeola, A.A.; Wada, Y.; Engku Abd Rahman, E.N.S.; Ahmed, N.; Musa, N.; Khalid, M.F.; Rahman, Z.A.; Hassan, R.; Yusof, N.Y.; et al. A Global Mutational Profile of SARS-CoV-2: A Systematic Review and Meta-Analysis of 368,316 COVID-19 Patients. Life 2021, 11, 1224. [Google Scholar] [CrossRef]
- Verroken, A.; Scohy, A.; Gérard, L.; Wittebole, X.; Collienne, C.; Laterre, P.-F. Co-infections in COVID-19 critically ill and antibiotic management: A prospective cohort analysis. Crit. Care 2020, 24, 410. [Google Scholar] [CrossRef]
- Baccolini, V.; Migliara, G.; Isonne, C.; Dorelli, B.; Barone, L.; Giannini, D.; Marotta, D.; Marte, M.; Mazzalai, E.; Alessandri, F.; et al. The impact of the COVID-19 pandemic on healthcare-associated infections in intensive care unit patients: A retrospective cohort study. Antimicrob. Resist. Infect. Control 2021, 10, 87. [Google Scholar] [CrossRef]
- Rothe, K.; Feihl, S.; Schneider, J.; Wallnöfer, F.; Wurst, M.; Lukas, M.; Treiber, M.; Lahmer, T.; Heim, M.; Dommasch, M.; et al. Rates of bacterial co-infections and antimicrobial use in COVID-19 patients: A retrospective cohort study in light of antibiotic stewardship. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, S.; Taylor, A.; Brown, A.; De Kraker, M.E.; El-Saed, A.; Alshamrani, M.; Hendriksen, R.S.; Jacob, M.; Löfmark, S.; Perovic, O.; et al. Impact of the COVID-19 pandemic on the surveillance, prevention and control of antimicrobial resistance: A global survey. J. Antimicrob. Chemother. 2021, 76, 3045–3058. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.G.; Ahammad, S.Z. COVID-19 and antimicrobial resistance: A cross-study. Sci. Total Environ. 2022, 807, 150873. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.; Chen, S.-Y.; Ko, W.-C.; Hsueh, P.-R. Increased antimicrobial resistance during the COVID-19 pandemic. Int. J. Antimicrob. Agents 2021, 57, 106324. [Google Scholar] [CrossRef]
- Tiri, B.; Sensi, E.; Marsiliani, V.; Cantarini, M.; Priante, G.; Vernelli, C.; Martella, L.A.; Costantini, M.; Mariottini, A.; Andreani, P.; et al. Antimicrobial stewardship program, COVID-19, and infection control: Spread of carbapenem-resistant Klebsiella pneumoniae colonization in ICU COVID-19 patients. What did not work? J. Clin. Med. 2020, 9, 2744. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.; Yang, Y.; Cai, P.; Cao, J.; Cai, X.; Zhang, Y. Etiology and antimicrobial resistance of secondary bacterial infections in patients hospitalized with COVID-19 in Wuhan, China: A retrospective analysis. Antimicrob. Resist. Infect. Control 2020, 9, 153. [Google Scholar] [CrossRef] [PubMed]
- Monnet, D.L.; Harbarth, S. Will coronavirus disease (COVID-19) have an impact on antimicrobial resistance? Eurosurveillance 2020, 25, 2001886. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.A.; Sands, K.; Huang, S.S.; Kleinman, K.; Septimus, E.; Varma, N.; Blanchard, E.J.; Poland, R.; Coady, M.H.; Yokoe, D.S.; et al. 171. The Impact of COVID-19 on Healthcare-Associated Infections. In Open Forum Infectious Diseases; Oxford University: Oxford, UK, 2021; pp. S102–S103. [Google Scholar]

| SR Number. | Test Name | Description |
|---|---|---|
| 1 | Catalase | Used to differentiate Staphylococcus spp. and Streptococcus spp. |
| 2 | Coagulase | Used to differentiate Staphylococcus aureus from others |
| 3 | Oxidase | Used to identify Pseudomonas spp. |
| 4 | Indole | Used to differentiate E. coli from other lactose fermenters |
| 5 | API | A strip of biochemical tests with reference database, different types were used to identify almost all kinds of organisms |
| 6 | DNAse | Used to identify Staphylococcus aureus |
| Characteristics | Number (n) | Percentage (%) | |
|---|---|---|---|
| Gender | Male | 652 | 55.96 |
| Female | 513 | 44.03 | |
| Age (Years) | <30 | 152 | 13.04 |
| 30–50 | 581 | 49.87 | |
| >50 | 432 | 37.08 | |
| Comorbidities | Kidney diseases | 152 | 13.04 |
| Hypertension | 126 | 10.81 | |
| Liver disease | 114 | 9.78 | |
| Hepatitis B | 42 | 3.60 | |
| Hepatitis C | 86 | 7.38 | |
| Meningoencephalitis | 24 | 2.06 | |
| Diabetes mellitus | 97 | 8.32 | |
| Gastrointestinal disorders | 201 | 17.25 | |
| None | 323 | 27.72 | |
| Smokers | Yes | 556 | 47.72 |
| No | 609 | 52.27 | |
| Admission ward (COVID-19 unit) | Intensive care unit | 197 | 16.90 |
| Gastro ward | 186 | 15.96 | |
| Nephrology ward | 146 | 12.53 | |
| Hepatology ward | 236 | 20.25 | |
| General medical ward | 284 | 24.37 | |
| Emergency | 116 | 9.95 | |
| Serial Number | Specimen | Frequency (n = 1165) | Positive for Bacterial Cultures (n = 423) |
|---|---|---|---|
| 1 | Blood | 391 | 146 |
| 2 | Urine | 273 | 114 |
| 3 | Sputum | 123 | 56 |
| 4 | Throat Swab | 87 | 11 |
| 5 | Tracheal Aspirate | 113 | 41 |
| 6 | Broncho alveolar lavage | 63 | 23 |
| 7 | Pus/Wound swab | 115 | 32 |
| Antibiotics | Resistance Percentage (%) | |
|---|---|---|
| Staphylococcus aureus (n = 34) | Streptococcus pyogenes (n = 23) | |
| Amikacin | 11.76 | NT |
| Chloramphenicol * | 17.64 | 82.60 |
| Cefoxitin | 14.70 | NT |
| Ciprofloxacin | 47.05 | NT |
| Co-trimoxazole | 35.29 | NT |
| Clindamycin | 47.05 | NT |
| Erythromycin * | 47.05 | 60.86 |
| Fusidic acid * | 35.29 | NT |
| Gentamicin | 50.00 | NT |
| Linezolid | 0 | NT |
| Penicillin | NT | 100 |
| Tetracycline | 61.76 | 82.60 |
| Teicoplanin | 0 | NT |
| Tobramycin | 44.11 | NT |
| Ceftriaxone | NT | 82.60 |
| Levofloxacin | 47.05 | 82.60 |
| Vancomycin | 0 | 0 |
| Antibiotics | Resistance Percentage (%) | |||
|---|---|---|---|---|
| E. coli (n = 133) | Klebsiella spp. (n = 101) | Acinetobacter baumannii (n = 55) | P. aeruginosa (n = 61) | |
| Ampicillin | 84.21 | 100.00 | NT | NT |
| Amp-clavulanic acid | 88.72 | 90.09 | NT | NT |
| Amikacin | 12.03 | 16.83 | 100.00 | 13.11 |
| Ceftriaxone | 42.10 | 84.15 | NT | NT |
| Cefuroxime | 56.39 | 89.10 | NT | NT |
| Cefixime | 56.39 | 87.12 | NT | NT |
| Ceftazidime | NT | NT | 100 | 24.59 |
| Chloramphenicol * | 58.64 | 87.12 | NT | NT |
| Ciprofloxacin | 72.18 | 87.12 | 100 | 75.40 |
| Levofloxacin | NT | NT | 100 | NT |
| Co-trimoxazole | 81.20 | 73.26 | 100 | NT |
| Gentamicin | 38.34 | 27.72 | 96.36 | 19.67 |
| Imipenem | 6.01 | 16.83 | 92.72 | 27.86 |
| Meropenem | 6.76 | 16.83 | 92.72 | 29.50 |
| Piperacillin-tazobactam | 18.04 | 17.82 | 100.00 | 9.83 |
| Tetracycline | 85.71 | 70.29 | 100.00 | NT |
| Tigecycline | 0 | 0 | 74.54 | NT |
| Tobramycin | 51.87 | 0 | 90.90 | 29.50 |
| Colistin | 0 | 0 | 0 | NT |
| Polymyxin B | 0 | 0 | 0 | NT |
| Cefepime | 0 | 50.49 | 100.00 | 52.45 |
| Nitrofurantoin ** | 13.53 | 42.57 | NT | NT |
| Fosfomycin ** | 11.27 | NT | NT | NT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, N.; Khan, M.; Saleem, W.; Karobari, M.I.; Mohamed, R.N.; Heboyan, A.; Rabaan, A.A.; Mutair, A.A.; Alhumaid, S.; Alsadiq, S.A.; et al. Evaluation of Bi-Lateral Co-Infections and Antibiotic Resistance Rates among COVID-19 Patients. Antibiotics 2022, 11, 276. https://doi.org/10.3390/antibiotics11020276
Ahmed N, Khan M, Saleem W, Karobari MI, Mohamed RN, Heboyan A, Rabaan AA, Mutair AA, Alhumaid S, Alsadiq SA, et al. Evaluation of Bi-Lateral Co-Infections and Antibiotic Resistance Rates among COVID-19 Patients. Antibiotics. 2022; 11(2):276. https://doi.org/10.3390/antibiotics11020276
Chicago/Turabian StyleAhmed, Naveed, Madiha Khan, Waqas Saleem, Mohmed Isaqali Karobari, Roshan Noor Mohamed, Artak Heboyan, Ali A. Rabaan, Abbas Al Mutair, Saad Alhumaid, Salman A. Alsadiq, and et al. 2022. "Evaluation of Bi-Lateral Co-Infections and Antibiotic Resistance Rates among COVID-19 Patients" Antibiotics 11, no. 2: 276. https://doi.org/10.3390/antibiotics11020276
APA StyleAhmed, N., Khan, M., Saleem, W., Karobari, M. I., Mohamed, R. N., Heboyan, A., Rabaan, A. A., Mutair, A. A., Alhumaid, S., Alsadiq, S. A., Bueid, A. S., Santali, E. Y., & Alestad, J. H. (2022). Evaluation of Bi-Lateral Co-Infections and Antibiotic Resistance Rates among COVID-19 Patients. Antibiotics, 11(2), 276. https://doi.org/10.3390/antibiotics11020276










