Long-Term Impact of the COVID-19 Pandemic on In-Hospital Antibiotic Consumption and Antibiotic Resistance: A Time Series Analysis (2015–2021)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Data Collection and Outcomes
3. Results
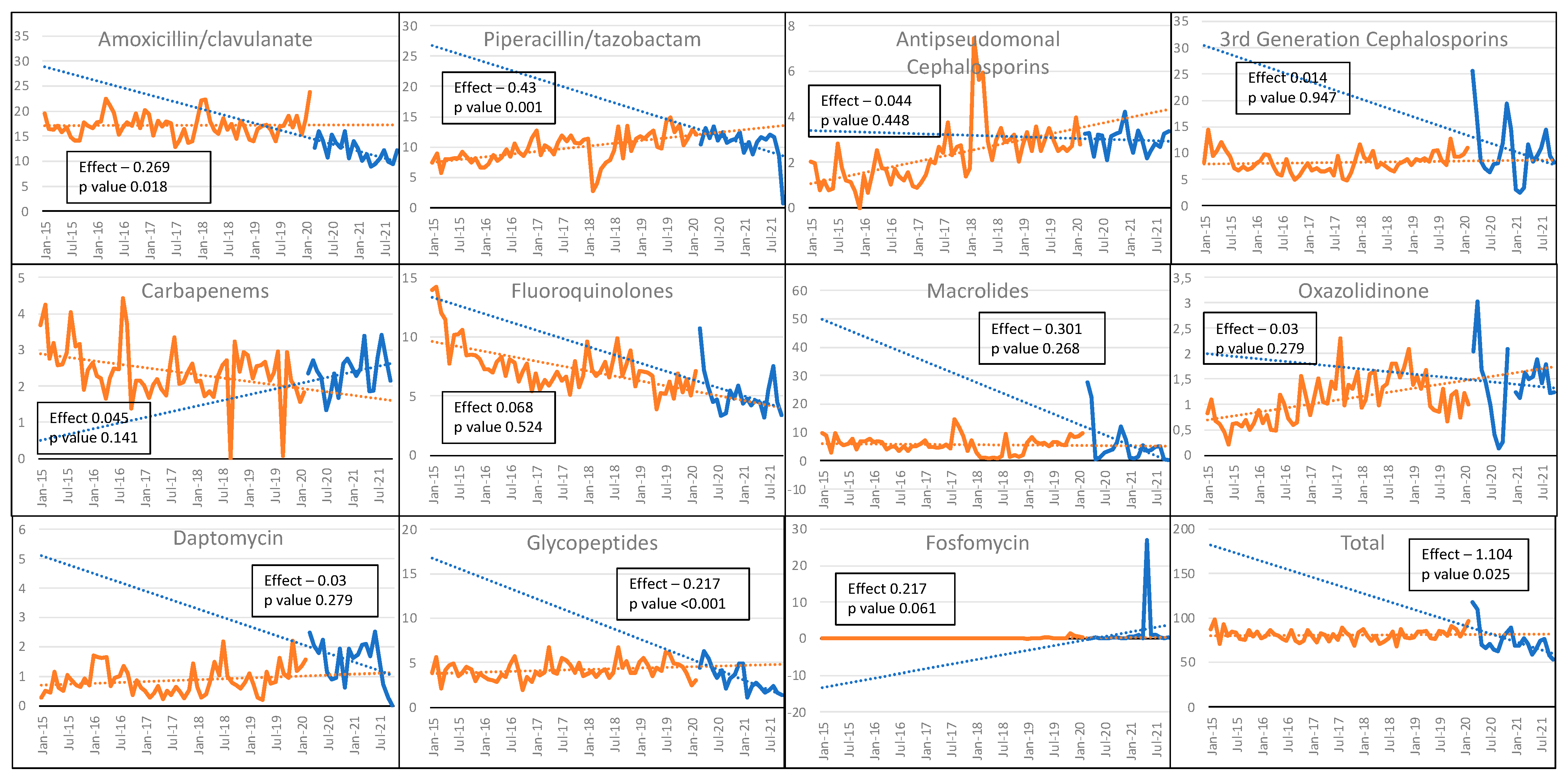
3.1. Antibiotics Consumption
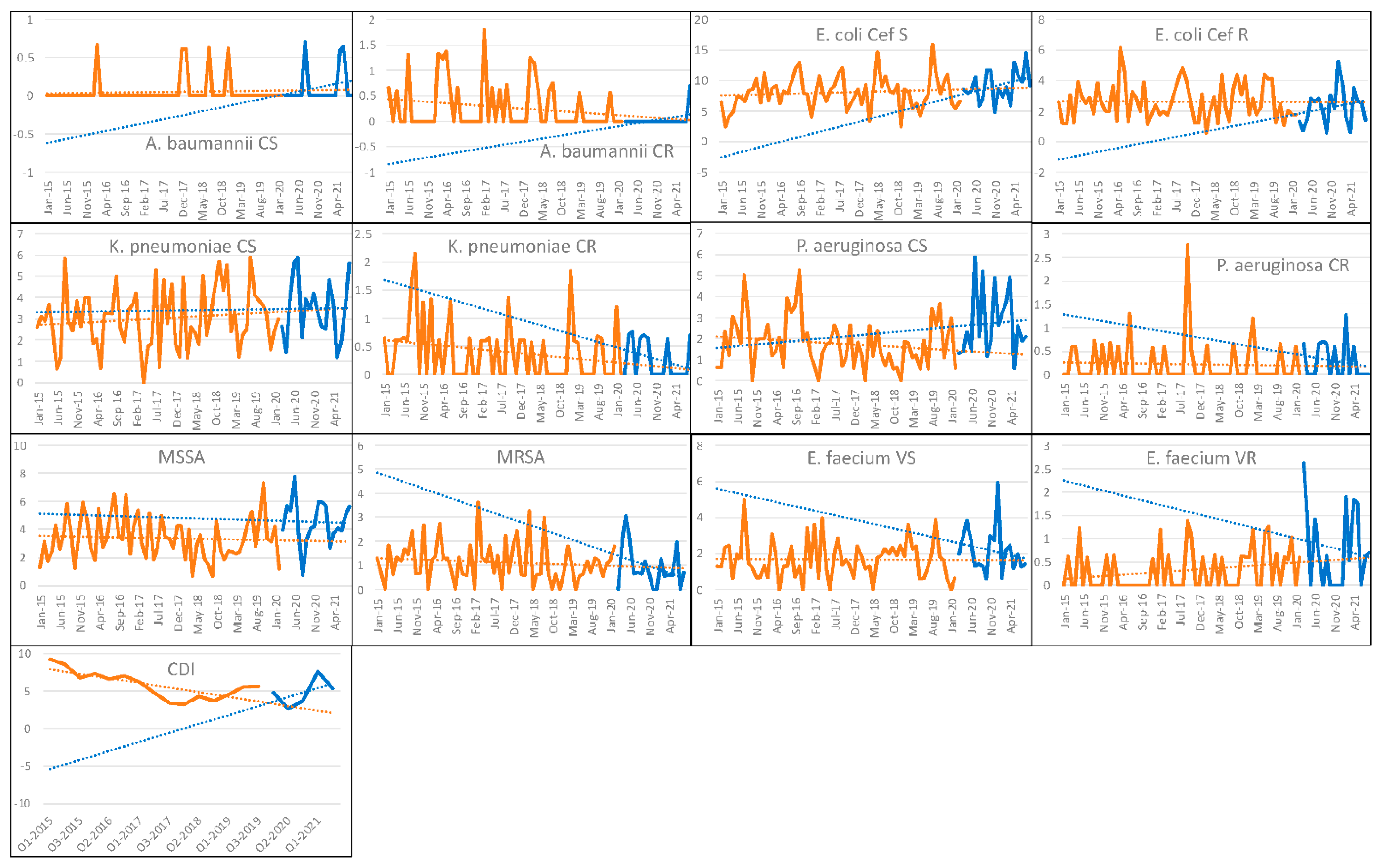
3.2. Bloodstream Infections and CDIs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3GC | Third-generation cephalosporin |
| AC | Antimicrobial consumption |
| AMR | Antimicrobial resistance |
| AS | Antimicrobial stewardship |
| BSI | Bloodstream infection |
| CAESAR | Central Asian and European Surveillance of Antimicrobial Resistance |
| CDI | Clostridioides difficile infection |
| Cef R | Third-generation cephalosporin-resistant |
| Cef S | Third-generation cephalosporin-susceptible |
| CL | Change in level |
| COVID-19 | Coronavirus disease 2019 |
| CR | Carbapenem-resistant |
| CRAB | Carbapenem-resistant Acinetobacter baumanii |
| CR-GNB | Carbapenem-resistant Gram-negative bacteria |
| CRO | Carbapenem-resistant organism |
| CS | Carbapenem-susceptible |
| CT | Change in trend |
| DDD | Defined daily dose |
| EARS-Net | European Antimicrobial Resistance Surveillance Network |
| HAI | Healthcare-associated infection |
| ICU | Intensive care unit |
| ID | Incidence density |
| IPC | Infection prevention and control |
| MDROs | Multidrug-resistant organisms |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MSSA | Methicillin-susceptible Staphylococcus aureus |
| PAF | Prospective audit and feedback |
| PD | Patient-days |
| PPE | Personal protective equipment |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| VAP | Ventilator-associated pneumonia |
| VR | Vancomycin-resistant |
| VS | Vancomycin-susceptible |
References
- Antimicrobial resistance in the age of COVID-19. Nat. Microbiol. 2020, 5, 779. [CrossRef] [PubMed]
- Clancy, C.J.; Hong Nguyen, M. Coronavirus Disease 2019, Superinfections, and Antimicrobial Development: What Can We Expect? Clin. Infect. Dis. 2020, 71, 2736–2743. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.D.; Fairfield, C.J.; Drake, T.M.; Turtle, L.; Seaton, R.A.; Wootton, D.G.; Sigfrid, L.; Harrison, E.M.; Docherty, A.B.; de Silva, T.I.; et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: A multicentre, prospective cohort study. Lancet Microbe 2021, 2, e354–e365. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.P.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520. [Google Scholar] [CrossRef]
- Vaughn, V.M.; Gandhi, T.N.; Petty, L.A.; Patel, P.K.; Prescott, H.C.; Malani, A.N.; Ratz, D.; McLaughlin, E.; Chopra, V.; Flanders, S.A. Empiric Antibacterial Therapy and Community-onset Bacterial Coinfection in Patients Hospitalized with Coronavirus Disease 2019 (COVID-19): A Multi-hospital Cohort Study. Clin. Infect. Dis. 2021, 72, e533–e541. [Google Scholar] [CrossRef]
- Cantón, R.; Gijón, D.; Ruiz-Garbajosa, P. Antimicrobial resistance in ICUs: An update in the light of the COVID-19 pandemic. Curr. Opin. Crit. Care 2020, 26, 433–441. [Google Scholar] [CrossRef]
- Bassetti, M.; Giacobbe, D.R.; Aliberti, S.; Barisione, E.; Centanni, S.; De Rosa, F.G.; Di Marco, F.; Gori, A.; Granata, G.; Mikulska, M.; et al. Balancing evidence and frontline experience in the early phases of the COVID-19 pandemic: Current position of the Italian Society of Anti-infective Therapy (SITA) and the Italian Society of Pulmonology (SIP). Clin. Microbiol. Infect. 2020, 26, 880–894. [Google Scholar] [CrossRef]
- Patel, P.R.; Weiner-Lastinger, L.M.; Dudeck, M.A.; Fike, L.V.; Kuhar, D.T.; Edwards, J.R.; Pollock, D.; Benin, A. Impact of COVID-19 pandemic on central-line–associated bloodstream infections during the early months of 2020, National Healthcare Safety Network. Infect. Control Hosp. Epidemiol. 2021, 43, 790–793. [Google Scholar] [CrossRef]
- Khatri, A.; Malhotra, P.; Izard, S.; Kim, A.; Oppenheim, M.; Gautam-Goyal, P.; Chen, T.; Doan, T.L.; Berlinrut, I.; Niknam, N.; et al. Hospital-Acquired Bloodstream Infections in Patients Hospitalized with Severe Acute Respiratory Syndrome Coronavirus 2 Infection (Coronavirus Disease 2019): Association with Immunosuppressive Therapies. Open Forum Infect. Dis. 2021, 8, ofab339. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Scaravilli, V.; Mangioni, D.; Scudeller, L.; Alagna, L.; Bartoletti, M.; Bellani, G.; Biagioni, E.; Bonfanti, P.; Bottino, N.; et al. Hospital-Acquired Infections in Critically Ill Patients with COVID-19. Chest 2021, 160, 454–465. [Google Scholar] [CrossRef]
- Pérez-Granda, M.J.; Carrillo, C.S.; Rabadán, P.M.; Valerio, M.; Olmedo, M.; Muñoz, P.; Bouza, E. Increase in the frequency of catheter-related bloodstream infections during the COVID-19 pandemic: A plea for control. J. Hosp. Infect. 2021, 119, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Rouzé, A.; Martin-Loeches, I.; Povoa, P.; Makris, D.; Artigas, A.; Bouchereau, M.; Lambiotte, F.; Metzelard, M.; Cuchet, P.; Boulle Geronimi, C.; et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: A European multicenter cohort study. Intensive Care Med. 2021, 47, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Weiner-Lastinger, L.M.; Pattabiraman, V.; Konnor, R.Y.; Patel, P.R.; Wong, E.; Xu, S.Y.; Smith, B.; Edwards, J.R.; Dudeck, M.A. The impact of coronavirus disease 2019 (COVID-19) on healthcare-associated infections in 2020: A summary of data reported to the National Healthcare Safety Network. Infect. Control Hosp. Epidemiol. 2022, 43, 12–25. [Google Scholar] [CrossRef]
- WHO Regional Office for Europe and European Centre for Disease Prevention and Control. Surveillance of Antimicrobial Resistance in Europe, 2020 Data; Executive Summary; WHO Regional Office for Europe: Copenhagen, Denmark, 2021. [Google Scholar]
- Baker, M.A.; Sands, K.E.; Huang, S.S.; Kleinman, K.; Septimus, E.J.; Varma, N.; Blanchard, J.; Poland, R.E.; Coady, M.H.; Yokoe, D.S.; et al. The Impact of COVID-19 on Healthcare-Associated Infections. Clin. Infect. Dis. 2022, 74, 1748–1754. [Google Scholar] [CrossRef]
- Russo, A.; Gavaruzzi, F.; Ceccarelli, G.; Borrazzo, C.; Oliva, A.; Alessandri, F.; Magnanimi, E.; Pugliese, F.; Venditti, M. Multidrug-resistant Acinetobacter baumannii infections in COVID-19 patients hospitalized in intensive care unit. Infection 2021, 50, 83–92. [Google Scholar] [CrossRef]
- Kampmeier, S.; Tönnies, H.; Correa-Martinez, C.L.; Mellmann, A.; Schwierzeck, V. A nosocomial cluster of vancomycin resistant enterococci among COVID-19 patients in an intensive care unit. Antimicrob. Resist. Infect. Control 2020, 9, 1–6. [Google Scholar] [CrossRef]
- Coşkun, A.S.; Durmaz, Ş.Ö. Fungal Infections in COVID-19 Intensive Care Patients. Pol. J. Microbiol. 2021, 70, 395–400. [Google Scholar] [CrossRef]
- Arastehfar, A.; Daneshnia, F.; Hilmioglu-Polat, S.; Fang, W.; Yaşar, M.; Polat, F.; Metin, D.Y.; Rigole, P.; Coenye, T.; Ilkit, M.; et al. First Report of Candidemia Clonal Outbreak Caused by Emerging Fluconazole-Resistant Candida parapsilosis Isolates Harboring Y132F and/or Y132F+K143R in Turkey. Antimicrob. Agents Chemother. 2020, 64, e01001-20. [Google Scholar] [CrossRef]
- WHO Guidelines on Hand Hygiene in Health Care: A Summary. Available online: https://apps.who.int/iris/handle/10665/70126 (accessed on 21 February 2022).
- Beovic, B.; Dousak, M.; Ferreira-Coimbra, J.; Nadrah, K.; Rubulotta, F.; Belliato, M.; Berger-Estilita, J.; Ayoade, F.; Rello, J.; Erdem, H. Antibiotic use in patients with COVID-19: A ‘snapshot’ Infectious Diseases International Research Initiative (ID-IRI) survey. J. Antimicrob. Chemother. 2020, 75, 3386–3390. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and Fungal Coinfection in Individuals with Coronavirus: A Rapid Review To Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Grau, S.; Echeverria-Esnal, D.; Gómez-Zorrilla, S.; Navarrete-Rouco, M.E.; Masclans, J.R.; Espona, M.; Gracia-Arnillas, M.P.; Duran, X.; Comas, M.; Horcajada, J.P.; et al. Evolution of Antimicrobial Consumption During the First Wave of COVID-19 Pandemic. Antibiotics 2021, 10, 132. [Google Scholar] [CrossRef]
- Grau, S.; Hernández, S.; Echeverría-Esnal, D.; Almendral, A.; Ferrer, R.; Limón, E.; Horcajada, J.P.; Grau, C.; Hernández, S.; Echeverría-Esnal, S.; et al. Antimicrobial Consumption among 66 Acute Care Hospitals in Catalonia: Impact of the COVID-19 Pandemic. Antibiotics 2021, 10, 943. [Google Scholar] [CrossRef] [PubMed]
- Kamara, I.F.; Kumar, A.M.V.; Maruta, A.; Fofanah, B.D.; Njuguna, C.K.; Shongwe, S.; Moses, F.; Tengbe, S.M.; Kanu, J.S.; Lakoh, S.; et al. Antibiotic Use in Suspected and Confirmed COVID-19 Patients Admitted to Health Facilities in Sierra Leone in 2020–2021: Practice Does Not Follow Policy. Int. J. Environ. Res. Public Health 2022, 19, 4005. [Google Scholar] [CrossRef]
- The Medicines Utilisation Monitoring Centre. National Report on Antibiotics Use in Italy. Year 2020; Italian Medicines Agency: Rome, Italy, 2022. [Google Scholar]
- Rawson, T.M.; Ming, D.; Ahmad, R.; Moore, L.S.P.; Holmes, A.H. Antimicrobial use, drug-resistant infections and COVID-19. Nat. Rev. Microbiol. 2020, 18, 409–410. [Google Scholar] [CrossRef] [PubMed]
- Livermore, D.M. Antibiotic resistance during and beyond COVID-19. JAC-Antimicrob. Resist. 2021, 3, i5–i16. [Google Scholar] [CrossRef]
- Abaleke, E.; Abbas, M.; Abbasi, S.; Abbott, A.; Abdelaziz, A.; Abdelbadiee, S.; Abdelfattah, M.; Abdul, B.; Abdul Rasheed, A.; Abdul-Kadir, R.; et al. Azithromycin in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 605–612. [Google Scholar] [CrossRef]
- Llor, C. Antibiotics Use in Primary Care during COVID-19. Antibiotics 2022, 11, 744. [Google Scholar] [CrossRef]
- Bara, W.; Brun-Buisson, C.; Coignard, B.; Watier, L. Outpatient Antibiotic Prescriptions in France: Patients and Providers Characteristics and Impact of the COVID-19 Pandemic. Antibiotics 2022, 11, 643. [Google Scholar] [CrossRef]
- Hek, K.; Ramerman, L.; Weesie, Y.M.; Lambooij, A.C.; Lambert, M.; Heins, M.J.; Hendriksen, J.M.T.; Verheij, R.A.; Cals, J.W.L.; van Dijk, L. Antibiotic Prescribing in Dutch Daytime and Out-of-Hours General Practice during the COVID-19 Pandemic: A Retrospective Database Study. Antibiotics 2022, 11, 309. [Google Scholar] [CrossRef]
- Borek, A.J.; Maitland, K.; McLeod, M.; Campbell, A.; Hayhoe, B.; Butler, C.C.; Morrell, L.; Roope, L.S.J.; Holmes, A.; Walker, A.S.; et al. Impact of the COVID-19 Pandemic on Community Antibiotic Prescribing and Stewardship: A Qualitative Interview Study with General Practitioners in England. Antibiotics 2021, 10, 1531. [Google Scholar] [CrossRef]
- Macera, M.; Onorato, L.; Calò, F.; Monari, C.; Annibale, R.; Signoriello, G.; Donnarumma, G.; Montemurro, M.V.; Galdiero, M.; Coppola, N. The Impact of the SARS-CoV-2 Pandemic on a Persuasive Educational Antimicrobial Stewardship Program in a University Hospital in Southern Italy: A Pre-Post Study. Antibiotics 2021, 10, 1405. [Google Scholar] [CrossRef]
- Polemis, M.; Mandilara, G.; Pappa, O.; Argyropoulou, A.; Perivolioti, E.; Koudoumnakis, N.; Pournaras, S.; Vasilakopoulou, A.; Vourli, S.; Katsifa, H.; et al. COVID-19 and antimicrobial resistance: Data from the greek electronic system for the surveillance of antimicrobial resistance—WHONET-Greece (January 2018–March 2021). Life 2021, 11, 996. [Google Scholar] [CrossRef]
- Gomez-Simmonds, A.; Annavajhala, M.K.; McConville, T.H.; Dietz, D.E.; Shoucri, S.M.; Laracy, J.C.; Rozenberg, F.D.; Nelson, B.; Greendyke, W.G.; Furuya, E.Y.; et al. Carbapenemase-producing Enterobacterales causing secondary infections during the COVID-19 crisis at a New York City hospital. J. Antimicrob. Chemother. 2021, 76, 380–384. [Google Scholar] [CrossRef]
- Pasquini, Z.; Barocci, I.; Brescini, L.; Candelaresi, B.; Castelletti, S.; Iencinella, V.; Mazzanti, S.; Procaccini, G.; Orsetti, E.; Pallotta, F.; et al. Bloodstream infections in the COVID-19 era: Results from an Italian multi-centre study. Int. J. Infect. Dis. 2021, 111, 31–36. [Google Scholar] [CrossRef]
- O’Toole, R.F. The interface between COVID-19 and bacterial healthcare-associated infections. Clin. Microbiol. Infect. 2021, 27, 1772–1776. [Google Scholar] [CrossRef]
- Bagattini, M.; Shbaklo, N.; Corcione, S.; Vicentini, C.; Giordano, S.; Fiorentino, D.; Bianco, G.; Cattel, F.; Cavallo, R.; Zotti, C.M.; et al. An Observational Study of MDR Hospital-Acquired Infections and Antibiotic Use during COVID-19 Pandemic: A Call for Antimicrobial Stewardship Programs. Antibiotics 2022, 11, 695. [Google Scholar] [CrossRef]
- Perez, S.; Innes, G.K.; Walters, M.S.; Mehr, J.; Arias, J.; Greeley, R.; Chew, D. Increase in Hospital-Acquired Carbapenem-Resistant Acinetobacter baumannii Infection and Colonization in an Acute Care Hospital During a Surge in COVID-19 Admissions—New Jersey, February–July 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 1827–1831. [Google Scholar] [CrossRef]
- Bonazzetti, C.; Morena, V.; Giacomelli, A.; Oreni, L.; Casalini, G.; Galimberti, L.R.; Bolis, M.; Rimoldi, M.; Ballone, E.; Colombo, R.; et al. Unexpectedly High Frequency of Enterococcal Bloodstream Infections in Coronavirus Disease 2019 Patients Admitted to an Italian ICU: An Observational Study. Crit. Care Med. 2021, 49, E31–E40. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Battaglini, D.; Ball, L.; Brunetti, I.; Bruzzone, B.; Codda, G.; Crea, F.; De Maria, A.; Dentone, C.; Di Biagio, A.; et al. Bloodstream infections in critically ill patients with COVID-19. Eur. J. Clin. Investig. 2020, 50, e13319. [Google Scholar] [CrossRef]
- Garrido-Molina, J.M.; Márquez-Hernández, V.V.; Alcayde-García, A.; Ferreras-Morales, C.A.; García-Viola, A.; Aguilera-Manrique, G.; Gutiérrez-Puertas, L. Disinfection of gloved hands during the COVID-19 pandemic. J. Hosp. Infect. 2021, 107, 5–11. [Google Scholar] [CrossRef]
- Tiri, B.; Sensi, E.; Marsiliani, V.; Cantarini, M.; Priante, G.; Vernelli, C.; Martella, L.A.; Costantini, M.; Mariottini, A.; Andreani, P.; et al. Antimicrobial Stewardship Program, COVID-19, and Infection Control: Spread of Carbapenem-Resistant Klebsiella Pneumoniae Colonization in ICU COVID-19 Patients. What Did Not Work? J. Clin. Med. 2020, 9, 2744. [Google Scholar] [CrossRef]
- García-Meniño, I.; Forcelledo, L.; Rosete, Y.; García-Prieto, E.; Escudero, D.; Fernández, J. Spread of OXA-48-producing Klebsiella pneumoniae among COVID-19-infected patients: The storm after the storm. J. Infect. Public Health 2021, 14, 50–52. [Google Scholar] [CrossRef]
- Granata, G.; Petrosillo, N.; Al Moghazi, S.; Caraffa, E.; Puro, V.; Tillotson, G.; Cataldo, M.A. The burden of Clostridioides difficile infection in COVID-19 patients: A systematic review and meta-analysis. Anaerobe 2021, 74, 102484. [Google Scholar] [CrossRef]
- Granata, G.; Bartoloni, A.; Codeluppi, M.; Contadini, I.; Cristini, F.; Fantoni, M.; Ferraresi, A.; Fornabaio, C.; Grasselli, S.; Lagi, F.; et al. The Burden of Clostridioides Difficile Infection during the COVID-19 Pandemic: A Retrospective Case-Control Study in Italian Hospitals (CloVid). J. Clin. Med. 2020, 9, 3855. [Google Scholar] [CrossRef]
- Sandhu, A.; Tillotson, G.; Polistico, J.; Salimnia, H.; Cranis, M.; Moshos, J.; Cullen, L.; Jabbo, L.; Diebel, L.; Chopra, T. Clostridioides difficile in COVID-19 Patients, Detroit, Michigan, USA, March–April 2020-Volume 26, Number 9—September 2020-Emerging Infectious Diseases journal-CDC. Emerg. Infect. Dis. 2020, 26, 2272–2274. [Google Scholar] [CrossRef]
- Linares-García, L.; Cárdenas-Barragán, M.E.; Hernández-Ceballos, W.; Pérez-Solano, C.S.; Morales-Guzmán, A.S.; Miller, D.S.; Schmulson, M. Bacterial and Fungal Gut Dysbiosis and Clostridium difficile in COVID-19: A Review. J. Clin. Gastroenterol. 2022, 56, 285. [Google Scholar] [CrossRef]
- Spigaglia, P. Clostridioides difficile infection (CDI) during the COVID-19 pandemic. Anaerobe 2022, 74, 102518. [Google Scholar] [CrossRef]
- Meschiari, M.; Lòpez-Lozano, J.M.; Di Pilato, V.; Gimenez-Esparza, C.; Vecchi, E.; Bacca, E.; Orlando, G.; Franceschini, E.; Sarti, M.; Pecorari, M.; et al. A five-component infection control bundle to permanently eliminate a carbapenem-resistant Acinetobacter baumannii spreading in an intensive care unit. Antimicrob. Resist. Infect. Control 2021, 10, 123. [Google Scholar] [CrossRef]
- Shinohara, D.R.; Dos Santos Saalfeld, S.M.; Martinez, H.V.; Altafini, D.D.; Costa, B.B.; Fedrigo, N.H.; Tognim, M.C.B. Outbreak of endemic carbapenem-resistant Acinetobacter baumannii in a coronavirus disease 2019 (COVID-19)–specific intensive care unit. Infect. Control Hosp. Epidemiol. 2022, 43, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, T.; Fedorowsky, R.; Yerushalmi, R.; Lellouche, J.; Nutman, A. An outbreak of carbapenem-resistant Acinetobacter baumannii in a COVID-19 dedicated hospital. Infect. Prev. Pract. 2021, 3, 100113. [Google Scholar] [CrossRef] [PubMed]
- Mullié, C.; Lemonnier, D.; Adjidé, C.C.; Maizel, J.; Mismacque, G.; Cappe, A.; Carles, T.; Pierson-Marchandise, M.; Zerbib, Y. Nosocomial outbreak of monoclonal VIM carbapenemase-producing Enterobacter cloacae complex in an intensive care unit during the COVID-19 pandemic: An integrated approach. J. Hosp. Infect. 2022, 120, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Montrucchio, G.; Corcione, S.; Sales, G.; Curtoni, A.; De Rosa, F.G.; Brazzi, L. Carbapenem-resistant Klebsiella pneumoniae in ICU-admitted COVID-19 patients: Keep an eye on the ball. J. Glob. Antimicrob. Resist. 2020, 23, 398–400. [Google Scholar] [CrossRef]
- Porretta, A.D.; Baggiani, A.; Arzilli, G.; Casigliani, V.; Mariotti, T.; Mariottini, F.; Scardina, G.; Sironi, D.; Totaro, M.; Barnini, S.; et al. Increased Risk of Acquisition of New Delhi Metallo-Beta-Lactamase-Producing Carbapenem-Resistant Enterobacterales (NDM-CRE) among a Cohort of COVID-19 Patients in a Teaching Hospital in Tuscany, Italy. Pathogens 2020, 9, 635. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meschiari, M.; Onorato, L.; Bacca, E.; Orlando, G.; Menozzi, M.; Franceschini, E.; Bedini, A.; Cervo, A.; Santoro, A.; Sarti, M.; et al. Long-Term Impact of the COVID-19 Pandemic on In-Hospital Antibiotic Consumption and Antibiotic Resistance: A Time Series Analysis (2015–2021). Antibiotics 2022, 11, 826. https://doi.org/10.3390/antibiotics11060826
Meschiari M, Onorato L, Bacca E, Orlando G, Menozzi M, Franceschini E, Bedini A, Cervo A, Santoro A, Sarti M, et al. Long-Term Impact of the COVID-19 Pandemic on In-Hospital Antibiotic Consumption and Antibiotic Resistance: A Time Series Analysis (2015–2021). Antibiotics. 2022; 11(6):826. https://doi.org/10.3390/antibiotics11060826
Chicago/Turabian StyleMeschiari, Marianna, Lorenzo Onorato, Erica Bacca, Gabriella Orlando, Marianna Menozzi, Erica Franceschini, Andrea Bedini, Adriana Cervo, Antonella Santoro, Mario Sarti, and et al. 2022. "Long-Term Impact of the COVID-19 Pandemic on In-Hospital Antibiotic Consumption and Antibiotic Resistance: A Time Series Analysis (2015–2021)" Antibiotics 11, no. 6: 826. https://doi.org/10.3390/antibiotics11060826
APA StyleMeschiari, M., Onorato, L., Bacca, E., Orlando, G., Menozzi, M., Franceschini, E., Bedini, A., Cervo, A., Santoro, A., Sarti, M., Venturelli, C., Biagioni, E., Coloretti, I., Busani, S., Girardis, M., Lòpez-Lozano, J.-M., & Mussini, C. (2022). Long-Term Impact of the COVID-19 Pandemic on In-Hospital Antibiotic Consumption and Antibiotic Resistance: A Time Series Analysis (2015–2021). Antibiotics, 11(6), 826. https://doi.org/10.3390/antibiotics11060826






