Prescribing Patterns and Variations of Antibiotic Use for Children in Ambulatory Care: A Nationwide Study
Abstract
:1. Introduction
2. Results
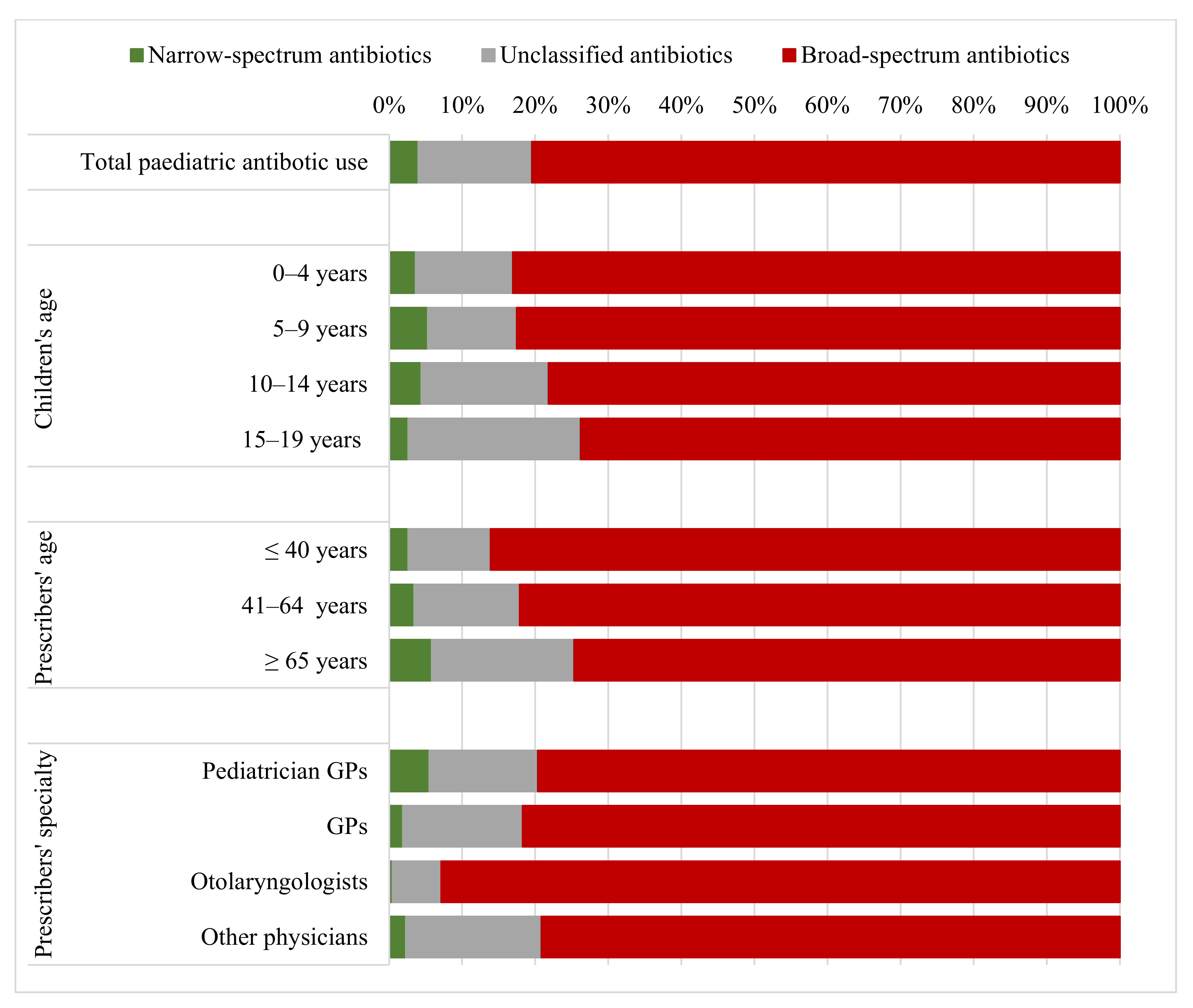
2.1. Scale and Patterns of Antibiotic Use
2.2. Antibiotic Use According to Prescribers’ Age and Specialty
2.3. Regional Variation of Paediatric Antibiotic Use
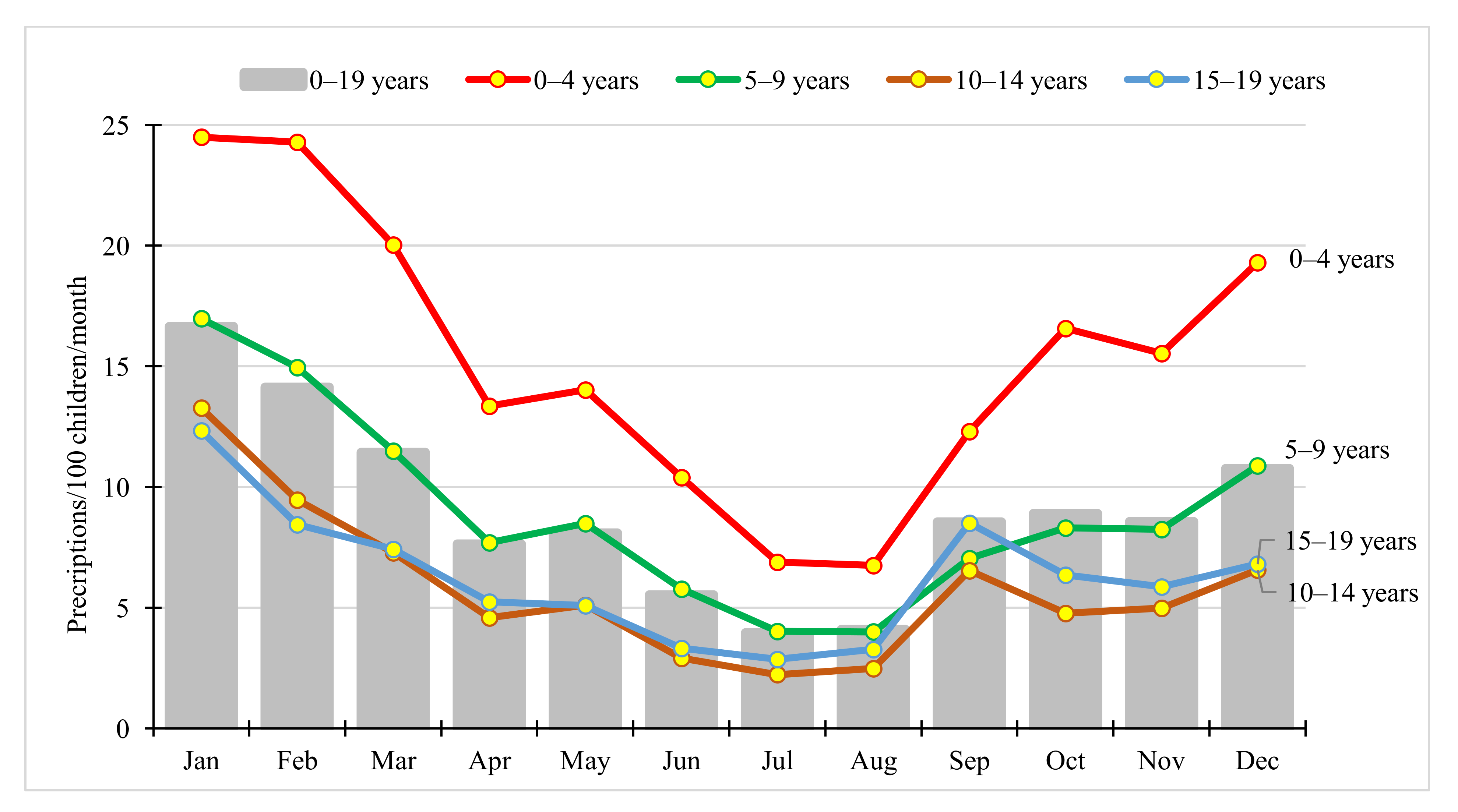
2.4. Seasonal Variation in Paediatric Antibiotic Use
3. Discussion
3.1. Scale and Patterns of Antibiotic Use
3.2. Antibiotic Prescription According to Prescribers’ Age and Specialty
3.3. Regional Variation in Paediatric Antibiotic Use
3.4. Seasonal Variation in Paediatric Antibiotic Use
3.5. Strengths and Limitations
4. Materials and Methods
Study Design, Study Period and Data Source
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Antimicrobial Resistance. n.d. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 27 July 2019).
- World Health Organization (WHO). Guidelines on prudent use of antimicrobials. n.d. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/antimicrobial-resistance/publications/guidelines-on-prudent-use-of-antimicrobials (accessed on 27 July 2019).
- Posfay-Barbe, K.M. Infections in paediatrics: Old and new diseases. Swiss Med. Wkly. 2012, 142, 1–10. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Antimicrobial consumption. In Annual Epidemiological Report for 2017; ECDC: Stockholm, Sweden, 2018. [Google Scholar]
- World Health Organization (WHO). WHO Collaborationg Centre for Drug Statistics Methodology. Definition and general considerations. n.d. Available online: https://www.whocc.no/ddd/definition_and_general_considera (accessed on 27 July 2019).
- Borck, B.; Korsgaard, H.; Sönksen, U.W.; Hammerum, A.M.; Bager, F.; Birk, T.T.; Jensen, L.B.; Jensen, A.N.; de Knegt, L.V.; Dalby, T.; et al. DANMAP 2017—Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark; Technical University of Denmark: Kongens Lyngby, Denmark, 2017; ISSN 1600-2032. [Google Scholar]
- Parviainen, S.; Saastamoinen, L.; Lauhio, A.; Sepponen, K. Outpatient antibacterial use and costs in children and adolescents: A nationwide register-based study in Finland, 2008–2016. J. Antimicrob. Chemother. 2019, 74, 2426–2433. [Google Scholar] [CrossRef] [Green Version]
- Holstiege, J.; Garbe, E. Systemic antibiotic use among children and adolescents in Germany: A population-based study. Eur. J. Pediatr. 2013, 172, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Kourlaba, G.; Kourkouni, E.; Spyridis, N.; Gerber, J.S.; Kopsidas, J.; Mougkou, K.; Lourida, A.; Zaoutis, T.E. Antibiotic prescribing and expenditures in outpatient paediatrics in Greece, 2010–2013. J. Antimicrob. Chemother. 2015, 70, 2405–2408. [Google Scholar] [CrossRef] [Green Version]
- Holstiege, J.; Schink, T.; Molokhia, M.; Mazzaglia, G.; Innocenti, F.; Oteri, A.; Bezemer, I.; Poluzzi, E.; Puccini, A.; Ulrichsen, S.P.; et al. Systemic antibiotic prescribing to paediatric outpatients in 5 European countries: A population-based cohort study. BMC Pediatr. 2014, 14, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blix, H.S.; Engeland, A.; Litleskare, I.; Rønning, M. Age- and gender-specific antibacterial prescribing in Norway. J. Antimicrob. Chemother. 2007, 59, 971–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozic, B.; Bajcetic, M. Use of antibiotics in paediatric primary care settings in Serbia. Arch. Dis. Child 2015, 100, 966–969. [Google Scholar] [CrossRef] [PubMed]
- Public Health Agency of Sweden. Swedress-Svarm 2017. Consumption of Antibiotics and Occurrence of Resistance in Sweden; Solna/Uppsala; Public Health Agency of Sweden: Solna, Sweden, 2017; ISSN 1650-6332. [Google Scholar]
- Benko, R.; Matuz, M.; Silva, A.; Ferreira, J.; Machado, M.C.; Furtado, C.; Fungie Galistiani, G.; Bordas, R.; Blix, H.S. Cross-national comparison of paediatric antibiotic use in Norway, Portugal and Hungary. Basic Clin. Pharmacol. Toxicol. 2019, 124, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Coenen, S.; Ferech, M.; Haaijer-Ruskamp, F.M.; Butler, C.C.; Vander Stichele, R.H.; Verheij, T.J.M.; Monnet, D.L.; Little, P.; Goossens, P.L.H. European Surveillance of Antimicrobial Consumption (ESAC): Quality indicators for outpatient antibiotic use in Europe. Qual. Saf. Health Care 2007, 16, 440–445. [Google Scholar] [CrossRef] [Green Version]
- Bergman, U.; Popa, C.; Tomson, Y.; Wettermark, B.; Einarson, T.R.; Åberg, H.; Sjöqvist, F. Drug utilization 90%—A simple method for assessing the quality of drug prescribing. Eur. J. Clin. Pharmacol. 1998, 54, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Holstiege, J.; Schulz, M.; Akmatov, M.K.; Steffen, A.; Bätzing, J. Marked reductions in outpatient antibiotic prescriptions for children and adolescents—A population-based study covering 83% of the paediatric population, Germany, 2010 to 2018. Eurosurveillance 2020, 25, 1900599. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.K.; Hollander, G.A.; Mcmichael, A.; Mcmichael, A. Evolution of the immune system in humans from infancy to old age. Proc. Biol. Sci. 2015, 282, 1–12. [Google Scholar] [CrossRef]
- Bouman, A.; Jan Heineman, M.; Faas, M.M. Sex hormones and the immune response in humans. Hum. Reprod. Update 2005, 11, 411–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Addressing Sex and Gender in Epidemic-Prone Infectious Diseases; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Muenchhoff, M.; Goulder, P.J.R. Sex differences in pediatric infectious diseases. J. Infect. Dis. 2014, 209, S120–S126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Zazara, D.E.; Arck, P.C. Developmental origin and sex-specific risk for infections and immune diseases later in life. Semin. Immunopathol. 2019, 41, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Pottegård, A.; Broe, A.; Aabenhus, R.; Bjerrum, L.; Hallas, J.; Damkier, P. Use of antibiotics in children: A Danish nationwide drug utilization study. Pediatr. Infect. Dis. J. 2015, 34, e16–e22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrajolo, C.; Sultana, J.; Ientile, V.; Scavone, C.; Scondotto, G.; Tari, M.; Trifirò, G.; Rossi, F.; Capuano, A. Gender differences in outpatient pediatric drug utilization: A cohort study from southern Italy. Front. Pharmacol. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Monasta, L.; Ronfani, L.; Marchetti, F.; Montico, M.; Brumatti, L.; Bavcar, A.; Grasso, D.; Barbiero, C.; Tamburlini, G. Burden of disease caused by otitis media: Systematic review and global estimates. PLoS ONE 2012, 7, e36226. [Google Scholar] [CrossRef]
- Karinauske, E.; Kasciuskeviciute, S.; Morkuniene, V.; Garuoliene, K.; Kadusevicius, E. Antibiotic prescribing trends in a pediatric population in Lithuania in 2003-2012: Observational study. Medicine 2019, 98, e17220. [Google Scholar] [CrossRef] [PubMed]
- Lusini, G.; Lapi, F.; Sara, B.; Vannacci, A.; Mugelli, A.; Kragstrup, J.; Bjerrum, L. Antibiotic prescribing in paediatric populations: A comparison between Viareggio, Italy and Funen, Denmark. Eur. J. Public Health 2009, 19, 434–438. [Google Scholar] [CrossRef] [Green Version]
- Dumpis, U.; Dimina, E.; Akermanis, M.; Tirans, E.; Veide, S. Assessment of antibiotic prescribing in Latvian general practitioners. BMC Fam. Pract. 2013, 14, 9. [Google Scholar] [CrossRef] [Green Version]
- Jackson, C.; Hsia, Y.; Bielicki, J.A.; Ellis, S.; Stephens, P.; Wong, I.C.K.; Sharland, M. Estimating global trends in total and childhood antibiotic consumption, 2011–2015. BMJ Glob. Health 2019, 4, 2011–2015. [Google Scholar] [CrossRef] [Green Version]
- Matuz, M.; Benkő, R.; Hajdú, E.; Viola, R.; Soós, G. Evaluation of ambulatory antibiotic use in Hungary using drug-specific quality indicators. Orv. Hetil. 2013, 154, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.E.; Staub, M.; Banerjee, R. Population-based assessment of patient and provider characteristics influencing pediatric outpatient antibiotic use in a high antibiotic-prescribing state. Infect. Control. Hosp. Epidemiol. 2020, 41, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Rezal, R.S.; Hassali, M.A.; Alrasheedy, A.A.; Saleem, F.; Yusof, F.A.; Godman, B. Physicians’ knowledge, perceptions and behaviour towards antibiotic prescribing: A systematic review of the literature. Expert. Rev. Anti. Infect. Ther. 2015, 13, 665–680. [Google Scholar] [CrossRef] [Green Version]
- Benko, R.; Matuz, M.; Hajdú, E.; Bor, A.; Doró, P.; Viola, R.; Soós, G. Hazai kórházi antibiotikum-alkalmazás az elmúlt két évtizedben (1996–2015). Orv. Hetil. 2016, 157, 1839–1846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matuz, M.; Benko, R.; Doro, P.; Hajdu, E.; Nagy, G.; Nagy, E.; Monnet, D.L.; Soos, G. Regional variations in community consumption of antibiotics in Hungary, 1996–2003. Br. J. Clin. Pharmacol. 2006, 61, 96–100. [Google Scholar] [CrossRef] [Green Version]
- Központi Statisztikai Hivatal. Yearbook of Health Statistics; Központi Statisztikai Hivatal: Budapest, Hungary, 2017. [Google Scholar]
- Papp, M.; Körösi, L.; Sándor, J.; Nagy, C.; Juhász, A.; Ádány, R. Workforce crisis in primary healthcare worldwide: Hungarian example in a longitudinal follow-up study. BMJ Open 2019, 9, e024957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elseviers, M.M.; Ferech, M.; Vander Stichele, R.H.; Goossens, H. Antibiotic use in ambulatory care in Europe (ESAC data 1997–2002): Trends, regional differences and seasonal fluctuations. Pharmacoepidemiol. Drug Saf. 2007, 16, 115–123. [Google Scholar] [CrossRef]
- Goossens, H.; Ferech, M.; Vander Stichele, R.; Elseviers, M. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet 2005, 365, 579–587. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Antimicrobial consumption database. Quality indicators for antibiotic consumption in the community. n.d. Available online: https://www.ecdc.europa.eu/en/antimicrobial-consumption/database/quality-indicators (accessed on 21 December 2021).
- Laszlo, S.; Katalin, S.; Júlia, T.; Gy, V.; Peter, A.; Gy, P.; Andrea, V.; Zsófia, M. Child healthcare in hungary. Turk. Pediatr. Ars. 2020, 55, S41–S56. [Google Scholar] [CrossRef] [PubMed]

| Age Groups | Number of Population | Number of Antibiotic Prescriptions | Percentage of Total Prescriptions Redeemed (%) |
|---|---|---|---|
| 0–4 years | 461,739 | 849,139 | 12.50 |
| 5–9 years | 474,702 | 511,965 | 7.54 |
| 10–14 years | 486,424 | 341,209 | 5.02 |
| 15–19 years | 493,069 | 372,213 | 5.48 |
| All children and adolescents | 1,915,934 | 2,074,526 | 30.54 |
| All inhabitants | 9,797,561 | 6,792,714 | 100.00 |
| Sex | Age Groups (years) | Prescription/100 Children/Year | B/N | N % | |||
|---|---|---|---|---|---|---|---|
| All Antibiotics | B | N | Unclassified | ||||
| All Children | 0–19 | 108.28 | 87.11 | 4.35 | 16.82 | 20.04 | 4.01 |
| Girls | 0–19 | 109.88 | 87.06 | 4.38 | 18.44 | 19.88 | 3.99 |
| Boys | 0–19 | 106.76 | 87.16 | 4.32 | 15.28 | 20.19 | 4.04 |
| All children | 0–4 | 183.90 | 152.71 | 6.74 | 24.45 | 22.67 | 3.66 |
| 5–9 | 107.85 | 89.01 | 5.74 | 13.10 | 15.50 | 5.32 | |
| 10–14 | 70.15 | 54.84 | 3.10 | 12.20 | 17.69 | 4.42 | |
| 15–19 | 75.49 | 55.70 | 2.00 | 17.79 | 27.86 | 2.65 | |
| Girls | 0–4 | 177.13 | 146.30 | 6.54 | 24.29 | 22.35 | 3.69 |
| 5–9 | 107.26 | 87.81 | 5.73 | 13.71 | 15.32 | 5.34 | |
| 10–14 | 71.03 | 54.97 | 3.22 | 12.85 | 17.10 | 4.53 | |
| 15–19 | 87.78 | 62.53 | 2.19 | 23.06 | 28.54 | 2.50 | |
| Boys | 0–4 | 190.31 | 158.78 | 6.92 | 24.61 | 22.96 | 3.63 |
| 5–9 | 108.41 | 90.14 | 5.75 | 12.52 | 15.68 | 5.30 | |
| 10–14 | 69.31 | 54.73 | 2.99 | 11.59 | 18.30 | 4.32 | |
| 15–19 | 63.89 | 49.25 | 1.82 | 12.83 | 27.09 | 2.84 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galistiani, G.F.; Benkő, R.; Babarczy, B.; Papp, R.; Hajdu, Á.; Szabó, É.H.; Viola, R.; Papfalvi, E.; Visnyovszki, Á.; Matuz, M. Prescribing Patterns and Variations of Antibiotic Use for Children in Ambulatory Care: A Nationwide Study. Antibiotics 2022, 11, 189. https://doi.org/10.3390/antibiotics11020189
Galistiani GF, Benkő R, Babarczy B, Papp R, Hajdu Á, Szabó ÉH, Viola R, Papfalvi E, Visnyovszki Á, Matuz M. Prescribing Patterns and Variations of Antibiotic Use for Children in Ambulatory Care: A Nationwide Study. Antibiotics. 2022; 11(2):189. https://doi.org/10.3390/antibiotics11020189
Chicago/Turabian StyleGalistiani, Githa Fungie, Ria Benkő, Balázs Babarczy, Renáta Papp, Ágnes Hajdu, Éva Henrietta Szabó, Réka Viola, Erika Papfalvi, Ádám Visnyovszki, and Mária Matuz. 2022. "Prescribing Patterns and Variations of Antibiotic Use for Children in Ambulatory Care: A Nationwide Study" Antibiotics 11, no. 2: 189. https://doi.org/10.3390/antibiotics11020189
APA StyleGalistiani, G. F., Benkő, R., Babarczy, B., Papp, R., Hajdu, Á., Szabó, É. H., Viola, R., Papfalvi, E., Visnyovszki, Á., & Matuz, M. (2022). Prescribing Patterns and Variations of Antibiotic Use for Children in Ambulatory Care: A Nationwide Study. Antibiotics, 11(2), 189. https://doi.org/10.3390/antibiotics11020189







