A Review on the Antimicrobial Activity of Schiff Bases: Data Collection and Recent Studies
Abstract
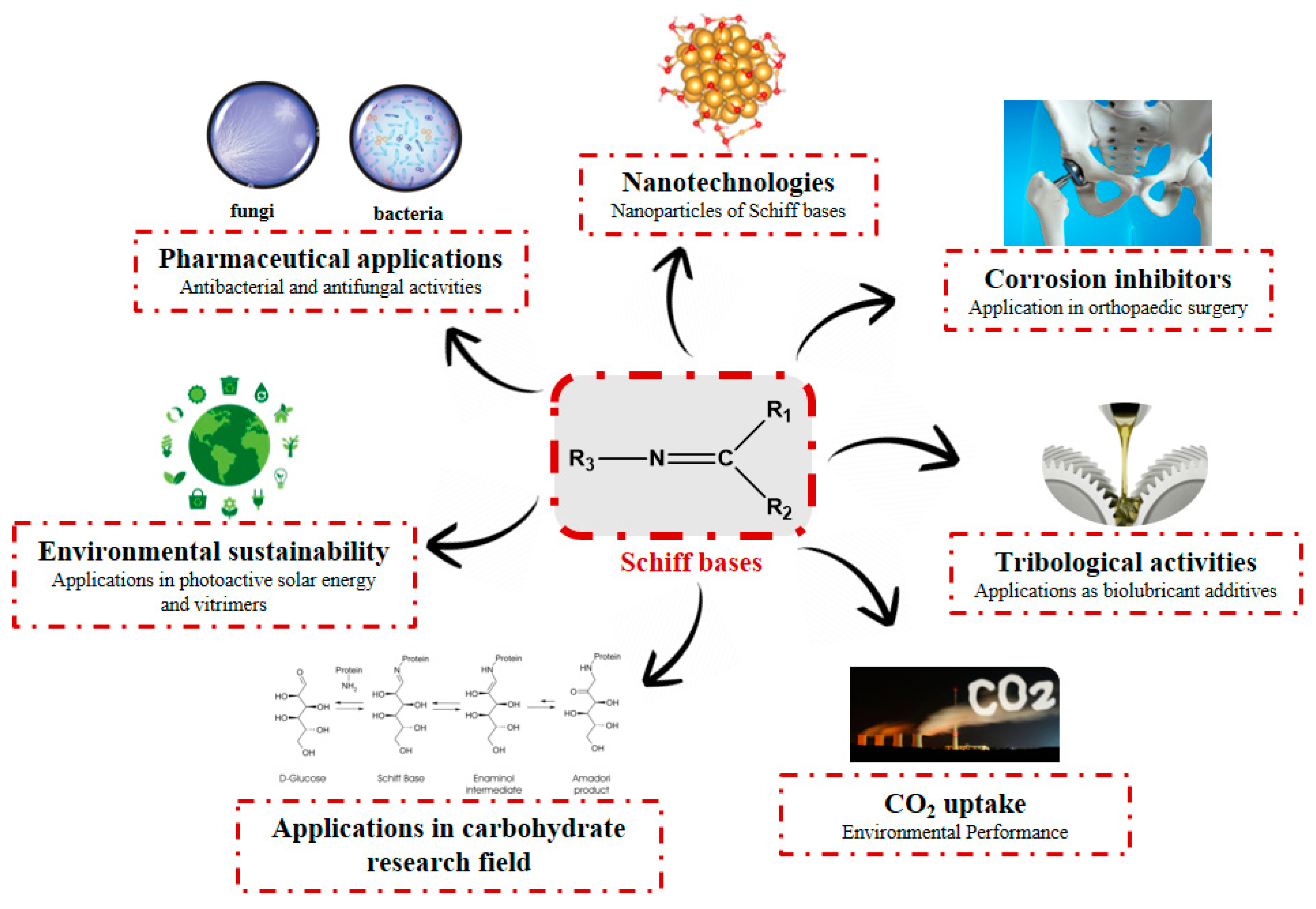
:1. Introduction
2. Schiff Bases as Antimicrobial Agents
2.1. Schiff Bases with Antibacterial Activity
| Structure | Compd | Antimicrobial Activity | Ref |
|---|---|---|---|
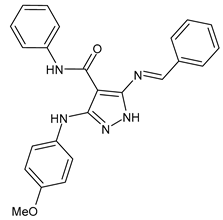 | 1 | MIC = 7.81 µg/mL (S. epidermidis) | [45] |
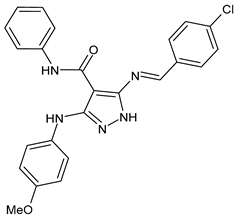 | 2 | MIC = 7.81 µg/mL (S. epidermidis) MIC = 15.62 µg/mL (A. baumanni) | [45] |
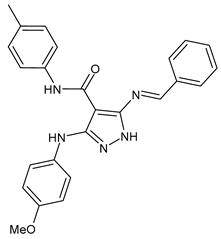 | 3 | MIC = 7.81 µg/mL (S. epidermidis) MIC = 15.62 µg/mL (A. baumanni) | [45] |
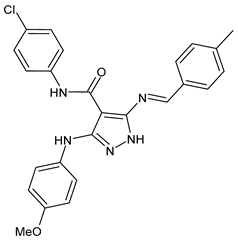 | 4 | MIC = 7.81 µg/mL (E. faecalis) | [45] |
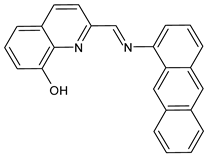 | 5 | IZD = 13 mm (B. cereus) | [47] |
 | 6 | IZD = 16 mm (B. cereus) IZD = 14 mm (E. coli 10536) | [47] |
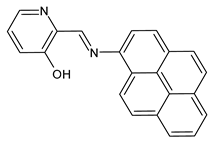
 | 7 | MIC = 25 µg/mL (M. luteus B1018) MIC = 12.5 µg/mL (S. aureus 23235) | [48] |
 | 8 | MIC = 25 µg/mL (M. luteus B1018) MIC = 12.5 µg/mL (S. aureus 23235) MIC = 12.5 µg/mL (A. niger 9642) | [48] |
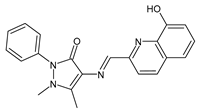
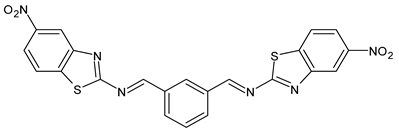 | 9 | IZD = 14 mm (S. aureus MTCC 1144) IZD = 15 mm (P. acnes MTCC 1951) | [49] |
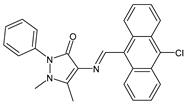
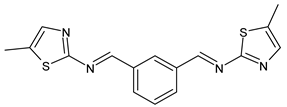 | 10 | IZD = 10 mm (S. aureus MTCC 1144) IZD = 14 mm (P. acnes MTCC 1951) | [49] |
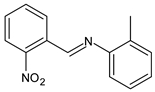 | 11 | MIC = 250 µg/mL (E. coli) MIC = 125 µg/mL (S. thyphymurium) | [50] |
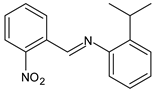 | 12 | MIC = 125 µg/mL (E. coli) MIC = 15.625 µg/mL (S. thyphymurium) MIC = 7.81 µg/mL (P. aeruginosa) | [50] |
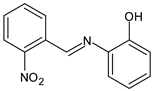 | 13 | MIC = 62.50 µg/mL (E. coli) MIC = 62.50 µg/mL (S. thyphymurium) | [50] |
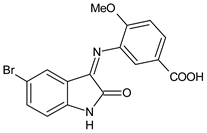 | 14 | MIC = 625 μg/mL (S. aureus 25923) MIC = 78 μg/mL (P. aeruginosa clinical strain) IZD = 15 mm (P. aeruginosa clinical strain) | [51] |
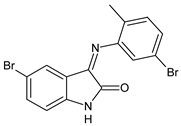 | 15 | MIC = 625 μg/mL (K. pneumoniae 700603) | [51] |
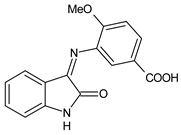 | 16 | MIC = 78 μg/mL (P. aeruginosa clinical strain) IZD = 24 mm (P. aeruginosa clinical strain) | [51] |
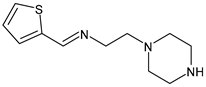 | 17 | IZD = 12 mm (S. aureus 25923) IZD = 11 mm (S. aureus MRSA) IZD = 9 mm (P. aeruginosa 27853) | [52] |
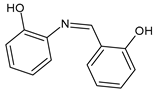 | 18 | IZD = 32 mm (S. epidermidis) IZD = 32 mm (P. aeruginosa) | [53] |
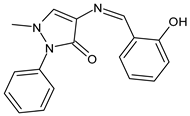 | 19 | IZD = 32.5 mm (S. aureus) | [53] |
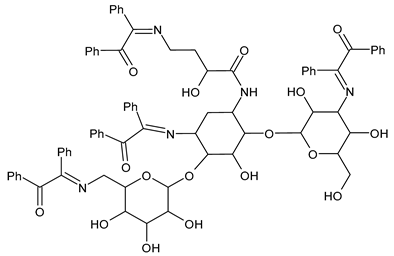 | 20 | IZD = 37 mm (S. aureus) IZD = 37 mm (E. coli) | [54] |
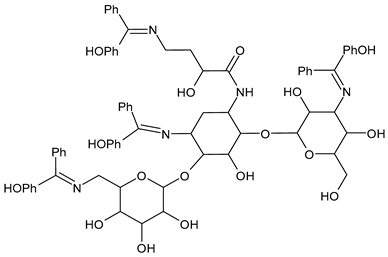 | 21 | IZD = 321 mm (S. aureus) IZD = 38 mm (E. coli) | [54] |
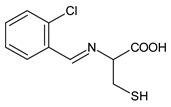 | 22 | MIC = 1.284 mM (S. aureus 6538; B. subtilis 6633; C. sporogenes 19404; M. luteus 4698; M. flavus 10240) MIC = 1.284 mM (E. coli 25922; S. aeruginosa 9027; Proteus hauseri 13315; K. Pneumoniae 10031; Salmonella enterica subsp. enterica serovar Enteritidis 13076) MIC = 1.284 mM (A. brasiliensis 16,404) | [55] |
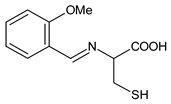 | 23 | MIC = 2.612 mM (S. aureus 6538; B. subtilis 6633; C. sporogenes 19404; M. luteus 4698; M. flavus 10240) MIC = 2.612 mM (E. coli 25922; S. aeruginosa 9027; Proteus hauseri 13315; K. Pneumoniae 10031; Salmonella enterica subsp. enterica serovar Enteritidis 13076) MIC = 1.306 mM (A. brasiliensis 16404) | [55] |
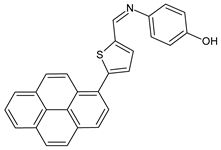 | 24 | MIC and/or IZD not given | [56] |
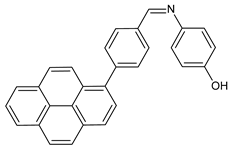 | 25 | MIC and/or IZD not given | [56] |
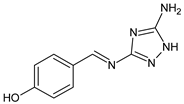 | 26 | IZD = 12 mm (C. salexigens and H. salina) IZD = 10 mm (H. halophila) | [57] |
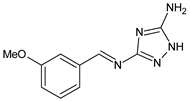 | 27 | IZD = 14 mm (C. salexigens) IZD = 12 mm (E. coli) IZD = 10 mm (C. israelensis) | [57] |
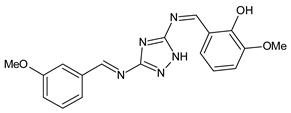 | 28 | IZD = 14 mm (C. salexigens) IZD = 13 mm (E. coli) | [57] |
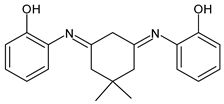 | 29 | IZD = 26 mm (at 500 μ/disc) | [58] |
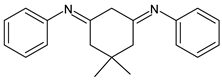 | 30 | IZD = 25 mm (at 500 μg/disc) | [58] |
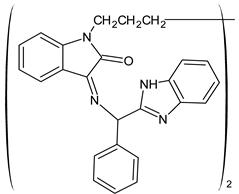 | 31 | IZD = 32 mm (E. coli) IZD = 22 mm (S. aureus) | [59] |
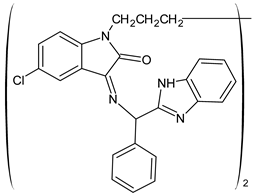 | 32 | IZD = 34 mm (E. coli) IZD = 28 mm (S. aureus) | [59] |
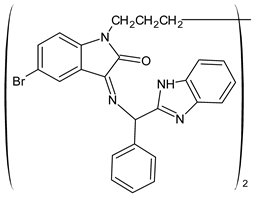 | 33 | IZD = 37 mm (E. coli) IZD = 34 mm (S. aureus) | [59] |
2.2. Schiff Bases with Antifungal Activity
| Structure | Compd | Antimicrobial Activity | Ref |
|---|---|---|---|
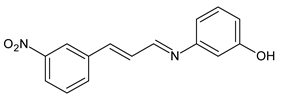 | 36 | MIC = 1.33 µg/mL (C. neoformans) MIC = 5.3 µg/mL (C. gatii) | [64] |
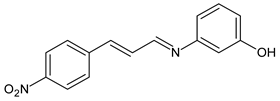 | 37 | MIC = 1.4 µg/mL (C. neoformans) MIC = 2.8 µg/mL (C. gatii) | [64] |
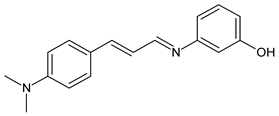 | 38 | MIC = 3.2 µg/mL (C. neoformans) MIC = 8.0 µg/mL (C. gatii) | [64] |
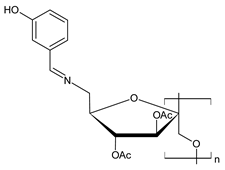 | 39 | II = 93% (B. cinerea) II = 83% (F. oxysporum f. sp. cucumerium Owen), II = 82% (P. asparagi) | [65] |
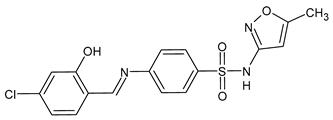 | 40 | MIC = 16 µg/mL (C. auris TDG1912; C. albicans NCPF3281) MIC = 16–32 µg/mL (C. albicans NCPF3179) MIC = 4–8 µg/mL (C. glabrata NCPF8018) MIC = 8–16 µg/mL (C. auris NCPF8971) MIC = 16 µg/mL (C. auris TDG2512, TDG2506, NCPF8984, NCPF8977) MIC = 16–32 µg/mL (C. auris TDG1102, TDG2211) | [67] |
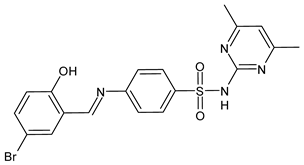 | 41 | MIC = 8–16 µg/mL (C. auris NCPF8971) MIC = 16 µg/mL (C. auris TDG2512, TDG2506, NCPF8984, NCPF8977) MIC = 16–32 µg/mL (C. auris TDG1102, TDG2211) | [67] |
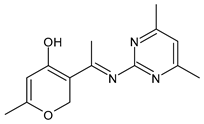 | 42 | IZD = 19 mm (A. niger) | [70] |
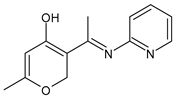 | 43 | IZD = 21 mm (C. albicans) | [70] |
3. Chitosan-Based Schiff Bases (CBSs)
4. Nanoformulations and Nanomedicines
4.1. Nanoparticles of Schiff Bases
4.2. Nanoparticles of Schiff Bases with Chitosan
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A. baumanni | Acinetobacter baumanni |
| ADMET | Absorption, distribution, metabolism, excretion and toxicity |
| A. niger | Aspergillus niger |
| B. cereus | Bacillus cereus |
| B. cinerea | Botrytis cinerea |
| B. subtilis | Bacillus subtilis |
| C. albicanss | Candida albicans |
| C. auris | Candida auris |
| C. glabrata | Candida glabrata |
| CBSs | Chitosan-based Schiff bases |
| ClfA | Clumping factor A |
| CrtM | Dehydrosqualene synthase |
| DFT | Density functional theory |
| DHFR | Dihydrofolate reductase |
| E. coli | Escherichia coli |
| E. faecalis | Enterococcus faecalis |
| FDA | Food and Drug Administration |
| F. graminearum | Fusarium graminearum |
| F. oxysporum | Fusarium oxysporum |
| IC50 | Concentration which kills or inhibits cell viability by 50% |
| II | Inhibitory Index |
| IZD | Inhibition zone diameter |
| K. pneumoniae | Klebsiella pneumoniae |
| MEPS | Molecular electrostatic potential surface |
| M. luteus | Micrococcus luteus |
| MTCC | Microbial Type Culture Collection |
| N. gonorrhoeae | Neisseria gonorrhoeae |
| P. acnes | Propionic bacteria acnes |
| P. aeruginosa | Pseudomonas aeruginosa |
| P. asparagi | Phomopsis asparagi |
| P. syringae | Pseudomonas syringae |
| PEG | Polyethylene glycol |
| QSAR | Quantitative structure activity relationship |
| SBs | Schiff bases |
| S. aureus | Staphylococcus aureus |
| S. epidermidis | Staphylococcus epidermidis |
| S. faecalis | Streptococcus faecalis |
| UPPS | Undecaprenyl diphosphate synthase |
References
- Wang, X.; Ding, G.; Duan, Y.; Zhu, Y.; Zhu, G.; Wang, M.; Li, X.; Zhang, Y.; Qin, X.; Hung, C.H. A novel triphenylamine-based bis-Schiff bases fluorophores with AIE-Activity as the hydrazine fluorescence turn-off probes and cell imaging in live cells. Talanta 2020, 217, 121029. [Google Scholar] [CrossRef] [PubMed]
- Berrones-Reyes, J.C.; Muñoz-Flores, B.M.; Cantón-Diáz, A.M.; Treto-Suárez, M.A.; Páez-Hernández, D.; Schott, E.; Zarate, X.; Jiménez-Pérez, V.M. Quantum chemical elucidation of the turn-on luminescence mechanism in two new Schiff bases as selective chemosensors of Zn2+: Synthesis, theory and bioimaging applications. RSC Adv. 2019, 9, 30778–30789. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, Y.; Sawamura, J.; Murata, Y.; Nishikata, T.; Yazaki, R.; Ohshima, T. Amino acid schiff base bearing benzophenone imine as a platform for highly congested unnatural α-amino acid synthesis. J. Am. Chem. Soc. 2020, 142, 8498–8505. [Google Scholar] [PubMed]
- Satpati, S.; Saha, S.K.; Suhasaria, A.; Banerjee, P.; Sukul, D. Adsorption and anti-corrosion characteristics of vanillin Schiff bases on mild steel in 1 M HCl: Experimental and theoretical study. RSC Adv. 2020, 10, 9258–9273. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Li, W.; Zhu, S.; Wang, L.; Guan, S. Corrosion inhibition of Schiff bases for Mg-Zn-Y-Nd alloy in normal saline: Experimental and theoretical investigations. Corros. Sci. 2021, 184, 109268. [Google Scholar] [CrossRef]
- Madani, A.; Sibous, L.; Hellal, A.; Kaabi, I.; Bentouhami, E. Synthesis, density functional theory study, molecular dynamics simulation and anti-corrosion performance of two benzidine Schiff bases. J. Mol. Struct. 2021, 1235, 130224. [Google Scholar] [CrossRef]
- Kontham, V.; Ansari, K.R.; Padmaja, K.V. Tribological properties of 10-undecenoic acid-derived schiff base lubricant additives. Arab. J. Sci. Eng. 2021, 46, 5593–5603. [Google Scholar] [CrossRef]
- Kontham, V.; Ansari, K.R.; Padmaja, K.V.; Madhu, D. Synthesis and evaluation of stearic acid based heterocyclic Schiff bases as biolubricant additives in epoxy karanja fatty acid 2-ethyl hexyl esters base oil. Industr. Crops Prod. 2021, 159, 113061. [Google Scholar] [CrossRef]
- Murmu, M.; Sengupta, S.; Pal, R.; Mandal, S.; Murmu, N.C.; Banerjee, P. Efficient tribological properties of azomethine-functionalized chitosan as a bio-lubricant additive in paraffin oil: Experimental and theoretical analysis. RSC Adv. 2020, 10, 33401–33416. [Google Scholar] [CrossRef]
- Kumar, B.; Kuntail, J.; Verma, D.K.; Rastogi, R.B.; Sinha, I. Mechanism of triboactivity of Schiff bases: Experimental and molecular dynamics simulations studies. J. Mol. Liq. 2019, 289, 111171. [Google Scholar] [CrossRef]
- Maity, D. Recent studies on applications of Schiff bases and their complexes in atmospheric carbon dioxide capture. Russ. J. Gen. Chem. 2020, 90, 2473–2483. [Google Scholar] [CrossRef]
- Xing, H.; Yaylayan, V. Mechanochemical generation of Schiff bases and Amadori products and utilization of diagnostic MS/MS fragmentation patterns in negative ionization mode for their analysis. Carbohydr. Res. 2020, 495, 108091. [Google Scholar] [CrossRef] [PubMed]
- Alamro, F.S.; Gomha, S.M.; Shaban, M.; Altowyan, A.S.; Abolibda, T.Z.; Ahmed, H.A. Optical investigations and photoactive solar energy applications of new synthesized Schiff base liquid crystal derivatives. Sci. Rep. 2021, 11, 1–11. [Google Scholar]
- Gomha, S.M.; Ahmed, H.A.; Shaban, M.; Abolibda, T.Z.; Khushaim, M.S.; Alharbi, K.A. Synthesis, optical characterizations and solar energy applications of new Schiff base materials. Materials 2021, 14, 3718. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Tian, Y.; Cheng, J.; Zhang, J. A biomass-based Schiff base vitrimer with both excellent performance and multiple degradability. Pol. Chem. 2021, 12, 6527–6537. [Google Scholar] [CrossRef]
- Oiye, É.N.; Ribeiro, M.F.M.; Katayama, J.M.T.; Tadini, M.C.; Balbino, M.A.; Eleotério, I.C.; Magalhães, J.; Castro, A.S.; Silva, R.S.M.; da Cruz Júnior, J.W.; et al. Electrochemical sensors containing schiff bases and their transition metal complexes to detect analytes of forensic, pharmaceutical and environmental interest. A review. Crit. Rev. Anal. Chem. 2019, 49, 488–509. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Yadav, R.; Kumar, K.; Pandey, H.; Pandey, R. Conventional vs. Microwave assisted SiO2/P2O5 catalyzed synthesis of Schiff bases. J. Phys. Conf. Ser. 2020, 1504, 012002. [Google Scholar] [CrossRef]
- Wady, A.F.; Hussein, M.B.; Mohammed, M.M. Synthesis, characterization of Schiff bases derived from salicylaldehyde with some amino acids by a new developed method. Sch. Int. J. Chem. Mater. Sci. 2021, 4, 46–53. [Google Scholar]
- Sk, I.; Khan, M.A.; Haque, A.; Ghosh, S.; Roy, D.; Homechuadhuri, S.; Alam, M.A. Synthesis of gold and silver nanoparticles using Malva verticillata leaves extract: Study of gold nanoparticles catalysed reduction of nitro-Schiff bases and antibacterial activities of silver nanoparticles. Curr. Res. Green Sustain. Chem. 2020, 3, 100006. [Google Scholar] [CrossRef]
- Munawar, K.S.; Haroon, S.M.; Hussain, S.A.; Raza, H. Schiff bases: Multipurpose pharmacophores with extensive biological applications. J. Basic Appl. Sci. 2018, 14, 217–229. [Google Scholar] [CrossRef] [Green Version]
- Iacopetta, D.; Lappano, R.; Mariconda, A.; Ceramella, J.; Sinicropi, M.S.; Saturnino, C.; Talia, M.; Cirillo, F.; Martinelli, F.; Puoci, F. Newly synthesized imino-derivatives analogues of resveratrol exert inhibitory effects in breast tumor cells. Int. J. Mol. Sci. 2020, 21, 7797. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.; Pandurangan, A.; Singh, N.; Tiwari, A.K. A systemic review of Schiff bases as an analgesic, anti-inflammatory. Int. J. Curr. Pharm. Res. 2012, 4, 5–11. [Google Scholar]
- Hameed, A.; Al-Rashida, M.; Uroos, M.; Abid Ali, S.; Khan, K.M. Schiff bases in medicinal chemistry: A patent review (2010-2015). Exp. Opin. Ther. Pat. 2017, 27, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Martins, C.V.B.; de Fátima, Â. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Saturnino, C.; Bonomo, M.G.; Franchini, C.; Sinicropi, M.S. Schiff bases: Interesting scaffolds with promising antitumoral properties. Appl. Sci. 2021, 11, 1877. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Burmistrova, D.A.; Arsenyev, M.V.; Almyasheva, N.R.; Ivanova, E.S.; Smolyaninova, S.A.; Pashchenko, K.P.; Poddel’sky, A.I.; Berberova, N.T. Catechol-and phenol-containing thio-Schiff bases: Synthesis, electrochemical properties and biological evaluation. ChemistrySelect 2021, 6, 10609–10618. [Google Scholar] [CrossRef]
- Howsaui, H.B.; Basaleh, A.S.; Abdellattif, M.H.; Hassan, W.M.; Hussien, M.A. Synthesis, structural investigations, molecular docking, and anticancer activity of some novel Schiff bases and their uranyl complexes. Biomolecules 2021, 11, 1138. [Google Scholar] [CrossRef]
- Kaczmarek, M.T.; Zabiszak, M.; Nowak, M.; Jastrzab, R. Lanthanides: Schiff base complexes, applications in cancer diagnosis, therapy, and antibacterial activity. Coord. Chem. Rev. 2018, 370, 42–54. [Google Scholar] [CrossRef]
- Catalano, A.; Sinicropi, M.S.; Iacopetta, D.; Ceramella, J.; Mariconda, A.; Rosano, C.; Scali, E.; Saturnino, C.; Longo, P. A review on the advancements in the field of metal complexes with Schiff bases as antiproliferative agents. Appl. Sci. 2021, 11, 6027. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug resistance (MDR): A widespread phenomenon in pharmacological therapies. Molecules, 2021; submitted. [Google Scholar]
- Iacopetta, D.; Catalano, A.; Ceramella, J.; Saturnino, C.; Salvagno, L.; Ielo, I.; Drommi, D.; Scali, E.; Plutino, M.R.; Rosace, G.; et al. The different facets of triclocarban: A review. Molecules 2021, 26, 2811. [Google Scholar] [CrossRef]
- Catalano, A.; Iacopetta, D.; Pellegrino, M.; Aquaro, S.; Franchini, C.; Sinicropi, M.S. Diarylureas: Repositioning from antitumor to antimicrobials or multi-target agents against new pandemics. Antibiotics 2021, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, M.; Sadiq, S.; Sadiq, A.; Younas, U.; Ashraf, A.; Saeed, Z.; Zuber, M.; Adnan, A. Azo-Schiff base derivatives of transition metal complexes as antimicrobial agents. Coord. Chem. Rev. 2021, 447, 214128. [Google Scholar] [CrossRef]
- Mohan, C.; Kumar, V.; Kumari, N.; Kumari, S.; Yadav, J.; Gandass, T.; Yadav, S. Synthesis, characterization and antibacterial activity of semicarbazide based Schiff bases and their Pb(II), Zr(IV) and U(VI) complexes. Adv. J. Chem. Sect. B 2020, 2, 187–196. [Google Scholar]
- Khalid, S.; Sumrra, S.H.; Chohan, Z.H. Isatin endowed metal chelates as antibacterial and antifungal agents. Sains Malays. 2020, 49, 1891–1904. [Google Scholar] [CrossRef]
- Xua, Y.; Shia, Y.; Leia, F.; Dai, L. A novel and green cellulose-based Schiff base-Cu (II) complex and its excellent antibacterial activity. Carbohydr. Polym. 2020, 230, 115671. [Google Scholar] [CrossRef]
- Pour, S.R.; Abdolmaleki, A.; Dinari, M. Immobilization of new macrocyclic Schiff base copper complex on graphene oxide nanosheets and its catalytic activity for olefins epoxidation. J. Mater. Sci. 2019, 54, 2885–2896. [Google Scholar] [CrossRef]
- Fernández-G. J.M.; del Rio-Portilla, F.; Quiroz-García, B.; Toscano, R.A.; Salcedo, R. The structures of some ortho-hydroxy Schiff base ligands. J. Mol. Struct. 2001, 561, 197–207. [Google Scholar] [CrossRef]
- Antony, R.; Arun, T.; Manickam, S.T.D. A review on applications of chitosan-based Schiff bases. Int. J. Biol. Macromol. 2019, 129, 615–633. [Google Scholar] [CrossRef]
- Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Zafar, A.; Alsaidan, O.A.; Alruwaili, N.K.; Gilani, S.J.; Rizwanullah, M. Recent advancement in chitosan-based nanoparticles for improved oral bioavailability and bioactivity of phytochemicals: Challenges and perspectives. Polymers 2021, 13, 4036. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M.; Cheng, Q.; Wang, C.; Li, H.; Han, X.; Fan, Z.; Su, G.; Pan, D.; Li, Z. Research progress of adsorption and removal of heavy metals by chitosan and its derivatives: A review. Chemosphere 2021, 279, 130927. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Confederat, L.G.; Tuchilus, C.G.; Dragan, M.; Sha’at, M.; Dragostin, O.M. Preparation and antimicrobial activity of chitosan and its derivatives: A concise review. Molecules 2021, 26, 3694. [Google Scholar] [CrossRef] [PubMed]
- Amourizi, F.; Dashtian, K.; Ghaedi, M.; Hosseinzadeh, B. An asymmetric Schiff base-functionalized gold nanoparticle-based colorimetric sensor for Hg2+ ion determination: Experimental and DFT studies. Anal. Methods. 2021, 13, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Whitty-Léveillé, L.; Tremblay-Cantin, J.C.; Picard-Lafond, A.; Boudreau, D.; Reynier, N.; Larivière, D. Core-shell nanoparticles bearing Schiff base ligand for the selective extraction of uranium from REE leach liquors. Hydrometal. 2021, 208, 105780. [Google Scholar] [CrossRef]
- Rosato, A.; Catalano, A.; Carocci, A.; Carrieri, A.; Carone, A.; Caggiano, G.; Franchini, C.; Corbo, F.; Montagna, M.T. In vitro interactions between anidulafungin and nonsteroidal anti-inflammatory drugs on biofilms of Candida spp. Bioorg. Med. Chem. 2016, 24, 1002–1005. [Google Scholar] [CrossRef]
- Hassan, A.S.; Askar, A.A.; Nossier, E.S.; Naglah, A.M.; Moustafa, G.O.; Al-Omar, M.A. Antibacterial evaluation, in silico characters and molecular docking of Schiff bases derived from 5-aminopyrazoles. Molecules 2019, 24, 3130. [Google Scholar] [CrossRef] [Green Version]
- Pozzi, C.; Ferrari, S.; Cortesi, D.; Luciani, R.; Stroud, R.M.; Catalano, A.; Costi, M.P.; Mangani, S. The structure of Enterococcus faecalis thymidylate synthase provides clues about folate bacterial metabolism. Acta Cryst. D 2012, 68, 1232–1241. [Google Scholar] [CrossRef]
- Gümüş, A.; Okumuş, V.; Gümüş, S. Synthesis, biological evaluation of antioxidant-antibacterial activities and computational studies of novel anthracene-and pyrene-based Schiff base derivatives. Turk. J. Chem. 2020, 44, 1200–1215. [Google Scholar] [CrossRef]
- Erturk, A.G. Synthesis, structural identifications of bioactive two novel Schiff bases. J. Mol. Struct. 2020, 1202, 27299. [Google Scholar] [CrossRef]
- Mishra, N.; Kumar, K.; Pandey, H.; Anand, S.R.; Yadav, R.; Srivastava, S.P.; Pandey, R. Synthesis, characterization, optical and anti-bacterial properties of benzothiazole Schiff bases and their lanthanide(III)complexes. J. Saudi Chem. Soc. 2020, 24, 925–933. [Google Scholar] [CrossRef]
- Yusuf, T.L.; Oladipo, S.D.; Olagboye, S.A.; Zamisa, S.J.; Tolufashe, G.F. Solvent-free synthesis of nitrobenzyl Schiff bases: Characterization, antibacterial studies, density functional theory and molecular docking studies. J. Mol. Struct. 2020, 1222, 128857. [Google Scholar] [CrossRef]
- Chemchem, M.; Menacer, R.; Merabet, N.; Bouridane, H.; Yahiaoui, S.; Moussaoui, S.; Belkhiri, L. Green synthesis, antibacterial evaluation and QSAR analysis of some isatin Schiff bases. J. Mol. Struct. 2020, 1208, 127853. [Google Scholar] [CrossRef]
- Warad, I.; Ali, O.; Al Ali, A.; Jaradat, N.A.; Hussein, F.; Abdallah, L.; Al-Zaqri, N.; Alsalme, A.; Alharthi, F.A. Synthesis and spectral Identification of three Schiff bases with a 2-(piperazin-1-yl)-N-(thiophen-2-yl methylene) ethanamine moiety acting as novel pancreatic lipase inhibitors: Thermal, DFT, antioxidant, antibacterial, and molecular docking investigations. Molecules 2020, 25, 2253. [Google Scholar] [CrossRef] [PubMed]
- Bayeh, Y.; Mohammed, F.; Gebrezgiabher, M.; Elemo, F.; Getachew, M.; Thomas, M. Synthesis, characterization and antibacterial activities of polydentate Schiff bases, based on salicylaldehyde. Adv. Biol. Chem. 2020, 10, 127–139. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, M.A.; Ahmed, I.; Pervaiz, I.; Shah, H.S. Development of Schiff bases from amikacin: Synthesis, antibacterial, anti-urease activities and molecular docking studies. Lett. Drug Des. Discov. 2020, 17, 1579–1588. [Google Scholar] [CrossRef]
- Salihović, M.; Pazalja, M.; Halilović, S.Š.; Veljović, E.; Mahmutović-Dizdarević, I.; Roca, S.; Novaković, I.; Trifunović, S. Synthesis, characterization, antimicrobial activity and DFT study of some novel Schiff bases. J. Mol Struct. 2021, 1241, 130670. [Google Scholar] [CrossRef]
- Srinivasan, V.; Khamrang, T.; Ponraj, C.; Saravanan, D.; Yamini, R.; Bera, S.; Jhonsi, M.A. Pyrene based Schiff bases: Synthesis, crystal structure, antibacterial and BSA binding studies. J. Mol. Struct. 2021, 1225, 129153. [Google Scholar] [CrossRef]
- Sumrra, S.H.; Zafar, W.; Asghar, M.L.; Mushtaq, F.; Raza, M.A.; Nazar, M.F.; Nadeem, M.A.; Imran, M.; Mumtaz, S. Computational investigation of molecular structures, spectroscopic properties, cholinesterase inhibition and antibacterial activities of triazole Schiff bases endowed metal chelates. J. Mol. Struct. 2021, 1238, 130382. [Google Scholar] [CrossRef]
- Ragi, K.; Kakkassery, J.T.; Raphael, V.P.; Johnson, R. In vitro antibacterial and in silico docking studies of two Schiff bases on Staphylococcus aureus and its target proteins. Fut. J. Pharm. Sci. 2021, 7, 1–9. [Google Scholar]
- Singhal, S.; Khanna, P.; Khanna, L. Synthesis, comparative in vitro antibacterial, antioxidant & UV fluorescence studies of bis indole Schiff bases and molecular docking with ct-DNA & SARS-CoV-2 Mpro. Luminescence 2021, 36, 1531–1543. [Google Scholar]
- Aragón-Muriel, A.; Liscano, Y.; Upegui, Y.; Robledo, S.M.; Ramírez-Apan, M.T.; Morales-Morales, D.; Oñate-Garzón, J.; Polo-Cerón, D. In vitro evaluation of the potential pharmacological activity and molecular targets of new benzimidazole-based schiff base metal complexes. Antibiotics 2021, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- Carocci, A.; Catalano, A.; Bruno, C.; Lentini, G.; Franchini, C.; De Bellis, M.; De Luca, A.; Conte Camerino, D. Synthesis and in vitro sodium channel blocking activity evaluation of novel homochiral mexiletine analogs. Chirality 2010, 22, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, T.F.F.; da Silva, C.M.; Dos Santos, L.B.F.; Santos, D.A.; Silva, L.M.; Fuchs, B.B.; Mylonakis, E.; Martins, C.V.B.; de Resende-Stoianoff, M.A.; de Fátima, Â. Cinnamyl Schiff bases: Synthesis, cytotoxic effects and antifungal activity of clinical interest. Lett. Appl. Microbiol. 2020, 71, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mi, Y.; Li, Q.; Dong, F.; Guo, Z. Synthesis of Schiff bases modified inulin derivatives for potential antifungal and antioxidant applications. Int. J. Biol. Macromol. 2020, 143, 714–723. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Q.; Wang, G.; Dong, F.; Zhou, H.; Zhang, J. Synthesis, characterization, and antifungal activity of novel inulin derivatives with chlorinated benzene. Carbohydr. Polym. 2014, 99, 469–473. [Google Scholar] [CrossRef]
- Hamad, A.; Chen, Y.; Khan, M.A.; Jamshidi, S.; Saeed, N.; Clifford, M.; Hind, C.; Sutton, J.M.; Rahman, K.M. Schiff bases of sulphonamides as a new class of antifungal agent against multidrug-resistant Candida auris. Microbiol. Open 2021, 10, e1218. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and mechanisms of antifungal resistance. Antibiotics 2020, 9, 312. [Google Scholar] [CrossRef]
- Chowdhary, A.; Tarai, B.; Singh, A.; Sharma, A. Multidrug-resistant Candida auris infections in critically Ill coronavirus disease patients, India, April–July 2020. Emerg. Infect. Dis. 2020, 26, 2694. [Google Scholar] [CrossRef]
- Bhagwatrao Biradar, S.; Vithal Narte, D.; Pradip Kale, R.; Momin, K.I.; Sudewad, M.S.; Tayade, K.C.; Palke, D.G. Synthesis, spectral and biological studies of DHA Schiff bases. J. Appl. Organometal. Chem. 2021, 1, 41–47. [Google Scholar]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan derivatives and their application in biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [Green Version]
- Hamed, A.A.; Abdelhamid, I.A.; Saad, G.R.; Elkady, N.A.; Elsabee, M.Z. Synthesis, characterization and antimicrobial activity of a novel chitosan Schiff bases based on heterocyclic moieties. Int. J. Biol. Macromol. 2020, 153, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Elhag, M.; Abdelwahab, H.E.; Mostafa, M.A.; Nasr, A.Z.; El Sadek, M.M. Synthesis and characterization of chitosan-pyrazoloquinoxaline Schiff bases for Cr(VI) removal from wastewater. Int. J. Biol. Macromol. 2020, 163, 2180–2188. [Google Scholar] [PubMed]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. The double face of metals: The intriguing case of chromium. Appl. Sci. 2021, 11, 638. [Google Scholar] [CrossRef]
- Barbosa, H.F.G.; Attjioui, M.; Ferreira, A.P.G.; Dockal, E.R.; El Gueddari, N.E.; Moerschbacher, B.M.; Cavalheiro, É.T.G. Synthesis, characterization and biological activities of biopolymeric Schiff bases prepared with chitosan and salicylaldehydes and their Pd(II) and Pt(II) complexes. Molecules 2017, 22, 1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, L.; Tan, W.; Wang, G.; Li, Q.; Dong, F.; Guo, Z. The antioxidant and antifungal activity of chitosan derivatives bearing Schiff bases and quaternary ammonium salts. Carbohydr. Pol. 2019, 226, 115256. [Google Scholar] [CrossRef]
- Hassan, M.A.; Omer, A.M.; Abbas, E.; Baset, W.M.; Tamer, T.M. Preparation, physicochemical characterization and antimicrobial activities of novel two phenolic chitosan Schiff base derivatives. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.S.; Kenawy, E.R.; Sonbol, F.I.; Sun, J.; Al-Etewy, M.; Ali, A.; Huizi, L.; El-Zawawy, A.N. Pharmaceutical potential of a novel chitosan derivative Schiff base with special reference to antibacterial, anti-biofilm, antioxidant, anti-inflammatory, hemocompatibility and cytotoxic activities. Pharm. Res. 2018, 36, 5. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, J.; Tan, W.; Wang, G.; Li, Q.; Dong, F.; Guo, Z. Antifungal activity of double Schiff bases of chitosan derivatives bearing active halogeno-benzenes. Int. J. Biol. Macromol. 2021, 179, 292–298. [Google Scholar] [CrossRef]
- Kesharwani, P.; Gorain, B.; Choudhury, H.; Pandey, M.; Low, S.Y.; Tan, S.A.; Ling, E.C.S.; Lim, Y.K.; Chin, C.M.; Lee, P.Y.; et al. Nanotechnology based approaches for anti-diabetic drugs delivery. Diabetes Res. Clin. Pract. 2018, 136, 52–77. [Google Scholar] [CrossRef]
- Ceramella, J.; Mariconda, A.; Iacopetta, D.; Saturnino, C.; Barbarossa, A.; Caruso, A.; Rosano, C.; Sinicropi, M.S.; Longo, P. From coins to cancer therapy: Gold, silver and copper complexes targeting human topoisomerases. Bioorg. Med. Chem. Lett. 2020, 30, 126905–126916. [Google Scholar] [CrossRef]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The potential of silver nanoparticles for antiviral and antibacterial applications: A mechanism of action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, S.; Yin, J.; Yang, J.; Li, Q.; Zheng, W.; Liu, S.; Jiang, X. The density of surface coating can contribute to different antibacterial activities of gold nanoparticles. Nano Lett. 2020, 20, 5036–5042. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xu, B.; Yao, H.; Lu, X.; Tan, Y.; Wang, B.; Wang, X.; Yang, Z. Schiff-linked PEGylated doxorubicin prodrug forming pH-responsive nanoparticles with high drug loading and effective anticancer therapy. Front. Oncol. 2021, 11, 890. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, M.; Morsali, A.; Bozorgmehr, M.R.; Beyramabadi, S.A. Quantum chemical studies of chitosan nanoparticles as effective drug delivery systems for 5-fluorouracil anticancer drug. J. Mol. Liq. 2020, 302, 112495. [Google Scholar] [CrossRef]
- Sen, P.; Nyokong, T. Enhanced photodynamic inactivation of Staphylococcus aureus with Schiff base substituted zinc phthalocyanines through conjugation to silver nanoparticles. J. Mol. Struct. 2021, 1232, 130012. [Google Scholar] [CrossRef]
- Minhaz, A.; Khan, N.; Jamila, N.; Javed, F.; Imran, M.; Shujah, S.; Khan, S.N.; Atlas, A.; Shah, M.R. Schiff base stabilized silver nanoparticles as potential sensor for Hg(II)detection, and anticancer and antibacterial agent. Arab. J. Chem. 2020, 13, 8898–8908. [Google Scholar] [CrossRef]
- Elmehbad, N.Y.; Mohamed, N.A. Designing, preparation and evaluation of the antimicrobial activity of biomaterials based on chitosan modified with silver nanoparticles. Int. J. Biol. Macromol. 2020, 151, 92–103. [Google Scholar] [CrossRef]
- Ahmed, M.E.; Mohamed, H.M.; Mohamed, M.I.; Kandile, N.G. Sustainable antimicrobial modified chitosan and its nanoparticles hydrogels: Synthesis and characterization. Int. J. Biol. Macromol. 2020, 162, 1388–1397. [Google Scholar] [CrossRef]
- Montaser, A.S.; Wassel, A.R.; Al-Shaye’a, O.N. Synthesis, characterization and antimicrobial activity of Schiff bases from chitosan and salicylaldehyde/TiO2 nanocomposite membrane. Int. J. Biol. Macromol. 2019, 124, 802–809. [Google Scholar] [CrossRef]
- Boroumand, H.; Badie, F.; Mazaheri, S.; Seyedi, Z.S.; Nahand, J.S.; Nejati, M.; Baghi, H.B.; Abbasi-Kolli, M.; Badehnoosh, B.; Ghandali, M.; et al. Chitosan-based nanoparticles against viral infections. Front. Cell Infect. Microbiol. 2021, 11, 643953. [Google Scholar] [CrossRef]
- Abdel-Monem, R.A.; Khalil, A.M.; Darwesh, O.M.; Hashim, A.I.; Rabie, S.T. Antibacterial properties of carboxymethyl chitosan Schiff-base nanocomposites loaded with silver nanoparticles. J. Macromol. Sci. Part A 2020, 57, 145–155. [Google Scholar] [CrossRef]
- Cao, Y.; Alamri, S.; Rajhi, A.A.; Anqi, A.E.; Khalaji, A.D. New chitosan Schiff base and its nanocomposite: Removal of methyl green from aqueous solution and its antibacterial activities. Int. J. Biol. Macromol. 2021, 192, 1–6. [Google Scholar] [CrossRef] [PubMed]
| Structure | Compd | Antimicrobial Activity | Ref |
|---|---|---|---|
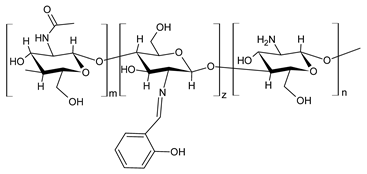 | 44 | MIC = 25 µg/mL (P. syringae) MIC = 50 µg/mL (F. graminearum) | [75] |
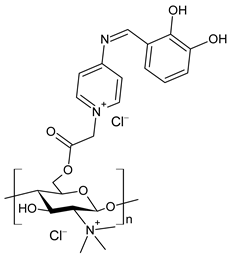 | 45 | II > 90% | [76] |
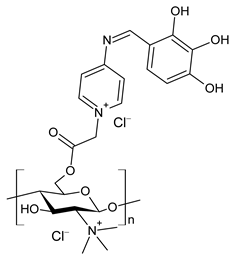 | 46 | II > 90% | [76] |
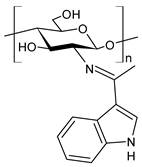 | 47 | IZD = 17.7 mm (E. coli) IZD = 17.2 mm (P. aeruginosa) IZD = 17.1 mm (Salmonella spp.) IZD = 18.9 mm (S. aureus) IZD = 18.1 mm (C. albicans) | [77] |
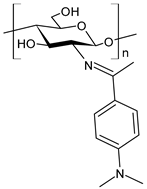 | 48 | IZD = 13.7 mm (E. coli) IZD = 14.4 mm (P. aeruginosa) IZD = 14.7 mm (Salmonella spp.) IZD = 15.1 mm (S. aureus) IZD = 15.5 mm (C. albicans) | [77] |
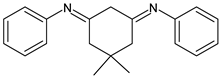 | 49 | IZD = 28.5 mm (MDR-SA-04) IZD = 22.1 mm (MDR-PA-09) | [78] |
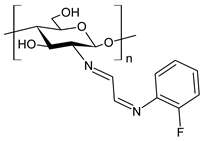 | 50 | II = 96.7% (B. cinerea at 1.0 mg/mL) | [79] |
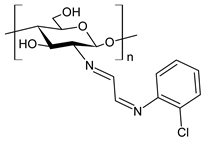 | 51 | II = 96.0% (B. cinerea at 1.0 mg/mL) | [79] |
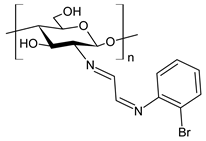 | 52 | II = 95.8% (B. cinerea at 1.0 mg/mL) | [79] |
| Structure | SB or SB/Nanoparticle | Antimicrobial Activity | Ref |
|---|---|---|---|
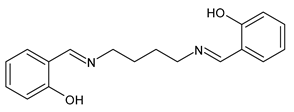 | 53 | IZD = 8.0 mm (S. aureus) IZD = 9.0 mm (B. subtilis) IZD = 7.0 mm (P. aeruginosa) IZD = 7.0 mm (E. coli) | [87] |
| 53-AgNPs | IZD = 18.0 mm (S. aureus) IZD = 18.0 mm (B. subtilis) IZD = 10.0 mm (P. aeruginosa) IZD = 10.0 mm (E. coli) | [87] | |
| Nanoparticles of Schiff bases with chitosan | |||
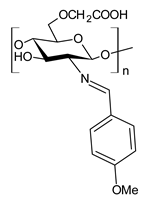 | 54 | IZD = 10 mm (B. subtilis) IZD = 11 mm (S. aureus) IZD = 10 mm (S. faecalis) IZD = 12 mm (E. coli) IZD = 13 mm (N. gonorrhoeae) IZD = 13 mm (P. aeruginosa) | [92] |
| 54-AgNO3 | IZD = 15 mm (B. subtilis) IZD = 15 mm (S. aureus) IZD = 14 mm (S. faecalis) IZD = 21 mm (E. coli) IZD = 19 mm (N. gonorrhoeae) IZD = 20 mm (P. aeruginosa) | [92] | |
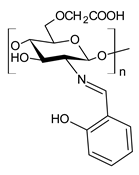 | 55 | IZD = 9 mm (B. subtilis) IZD = 10 mm (S. aureus) IZD = 10 mm (S. faecalis) IZD = 11 mm (E. coli) IZD = 12 mm (N. gonorrhoeae) IZD = 12 mm (P. aeruginosa) | [92] |
| 55-AgNO3 | IZD = 13 mm (B. subtilis) IZD = 13 mm (S. aureus) IZD = 14 mm (S. faecalis) IZD = 15 mm (E. coli) IZD = 14 mm (N. gonorrhoeae) IZD = 16 mm (P. aeruginosa) | [92] | |
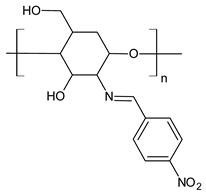 | 56 | MIC and/or IZD not given. Only photos are shown | [93] |
 | 56-NiFe | MIC and/or IZD not given. Only photos are shown | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceramella, J.; Iacopetta, D.; Catalano, A.; Cirillo, F.; Lappano, R.; Sinicropi, M.S. A Review on the Antimicrobial Activity of Schiff Bases: Data Collection and Recent Studies. Antibiotics 2022, 11, 191. https://doi.org/10.3390/antibiotics11020191
Ceramella J, Iacopetta D, Catalano A, Cirillo F, Lappano R, Sinicropi MS. A Review on the Antimicrobial Activity of Schiff Bases: Data Collection and Recent Studies. Antibiotics. 2022; 11(2):191. https://doi.org/10.3390/antibiotics11020191
Chicago/Turabian StyleCeramella, Jessica, Domenico Iacopetta, Alessia Catalano, Francesca Cirillo, Rosamaria Lappano, and Maria Stefania Sinicropi. 2022. "A Review on the Antimicrobial Activity of Schiff Bases: Data Collection and Recent Studies" Antibiotics 11, no. 2: 191. https://doi.org/10.3390/antibiotics11020191
APA StyleCeramella, J., Iacopetta, D., Catalano, A., Cirillo, F., Lappano, R., & Sinicropi, M. S. (2022). A Review on the Antimicrobial Activity of Schiff Bases: Data Collection and Recent Studies. Antibiotics, 11(2), 191. https://doi.org/10.3390/antibiotics11020191










