Abstract
Novel plant-derived antimicrobials are of interest in dentistry, especially in the treatment of periodontitis, since the use of established substances is associated with side effects and concerns of antimicrobial resistance have been raised. Thus, the present study was performed to quantify the antimicrobial efficacy of crude plant extracts against Porphyromonas gingivalis, a pathogen associated with periodontitis. The minimal inhibitory concentrations (MICs) of Eucalyptus globulus leaf, Azadirachta indica leaf, Glycyrrhiza glabra root and Rheum palmatum root extracts were determined by broth microdilution for P. gingivalis ATCC 33277 according to CLSI (Clinical and Laboratory Standards Institute). The MICs for the E. globulus, A. indica and G. glabra extracts ranged from 64 mg/L to 1024 mg/L. The lowest MIC was determined for an ethanolic R. palmatum extract with 4 mg/L. The MIC for the anthraquinone rhein was also measured, as the antimicrobial activity of P. palmatum root extracts can be partially traced back to rhein. Rhein showed a remarkably low MIC of 0.125 mg/L. However, the major compounds of the R. palmatum root extract were not further separated and purified. In conclusion, R. palmatum root extracts should be further studied for the treatment of periodontitis.
1. Introduction
According to the Global Burden of Disease Study, severe periodontitis had a prevalence of nearly 10% of the global population in 2016 [1]. It is a chronic inflammatory disease of tooth-surrounding tissues causing soft tissue destruction and alveolar bone loss and, thus, may ultimately lead to tooth loss [2]. Furthermore, it has been associated with an increased risk of all-cause mortality and several diseases such as cardiovascular disease, cancer, diabetes mellitus, psoriasis and rheumatoid arthritis [3,4,5,6,7].
The initiation and progression of chronic periodontitis is a multifactorial process that is driven by Porphyromonas gingivalis, an obligate anaerobe Gram-negative rod that is part of the human oral flora [8]. Alongside being a pathogen in periodontitis, P. gingivalis may induce systemic inflammation and has been correlated with the development of systemic disorders such as cardiovascular and rheumatic disease [9].
The gold standard in the therapy of periodontitis is the mechanical subgingival instrumentation [10], although due to the complex anatomy of periodontal pockets and teeth, this procedure is unable to fully eradicate bacterial biofilms. The use of systemic antimicrobials is controversial and limited to aggressive and severe forms of periodontitis [11]. Thus, the adjuvant use of antiseptics such as chlorhexidine (CHX) mouthwash is a common part of the treatment concept for chronic periodontitis [12]. The main side effect of CHX is tooth staining [13]. Additionally, although not yet a clinical problem in dentistry, concerns about the development of bacterial resistance against CHX have been raised [14]. Therefore, there is increasing interest in novel antimicrobial agents for the local long-term treatment of chronic periodontitis. Plants are of high interest to discover new antibacterial agents, since they are naturally exposed to microbial infections and thus have developed various antimicrobial defense mechanisms. Various plant extracts have been used for centuries in traditional medicine and there is some clinical evidence for their potential to control periodontitis progression [15,16]. The most commonly discussed medicinal plants for use in dentistry are Eucalyptus globulus, Azadirachta indica, Glycyrrhiza glabra and Rheum palmatum.
E. globulus, known as fever tree, is a member of the Myrtaceae family. A chewing gum containing 0.6% E. globulus ethanolic leaf extract has shown statistically significant positive effects on various outcome parameters of gingivitis in a small double-blind randomized clinical trial [17].
A. indica (neem) belongs to the Meliaceae family and is a traditional medicinal herb used in India to maintain oral health [18,19]. Clinical trials with a low number of 20 subjects used either an A. indica extract-containing gel or non-absorbable neem oil chips as an adjunct to subgingival instrumentation. Both gel and chips led to further improvement on pocket probing depth and attachment level compared to subgingival instrumentation only [20,21]. Further, a systematic review found no statistical differences in treatment outcomes between mouth rinses containing A. indica extracts or CHX, although the quality of the randomized controlled trials included was low [22].
G. glabra (licorice) is a member of the Fabaceae family and one of the oldest plants of ayurvedic medicine [23]. The bioactive compounds of its roots had anti-inflammatory properties in vitro, although evidence for clinical efficacy in periodontitis is lacking so far [24].
R. palmatum (rhubarb) is a member of the Polygonaceae family. Its root extract has been used as “Dahuang” in traditional Chinese medicine for centuries [25]. However, convincing evidence for its clinical activity against periodontitis has not yet been demonstrated.
Apart from their use in traditional medicine, at least some evidence for clinical efficacy in periodontitis or in vitro activity exists for most of the medicinal plants mentioned above. Given the fact that P. gingivalis is a major periodontal pathogen [8], we chose to investigate the antimicrobial activity of E. globulus, A. indica, G. glabra and R. palmatum extracts against P. gingivalis. The interpretation of studies reporting the antimicrobial activity of plant extracts is hindered by the fact that often insufficient techniques, such as disk diffusion, were used [26]. Therefore, we chose to compare the antimicrobial activity of extracts of the four plants against P. gingivalis using broth microdilution (BMD), as it represents the reference method of antimicrobial susceptibility testing [27].
To the best of our knowledge, no direct comparison of the antimicrobial activity of these extracts against P. gingivalis has been undertaken so far. The highest antimicrobial activity was found here for R. palmatum root extracts. Therefore, the anthraquinone rhein, a constituent of R. palmatum with known antimicrobial activity [28], was included in the analysis.
2. Materials and Methods
2.1. Plant Extracts
200 g of dried powder of pharmaceutical-grade E. globulus leaves (Bristol Botanicals, Bristol, UK), 200 g of dried powder of food-grade A. indica leaves (Vitafoodz, Waren, Germany) and 200 g of dried powder of pharmaceutical grade G. glabra roots (Bristol Botanicals) were extracted using either 600 mL 70% aqueous ethanol (Carl Roth, Karlsruhe, Germany) or 600 mL acetone (Carl Roth) for 24 h under continuous stirring. Insolvable parts were taken off using a 0.22 µm Stericup vacuum filtration system (Merck Millipore, Billerica, MA, USA). The extractant was then removed under reduced pressure at 40 °C using a rotary evaporator (Rotavapor R-210, Büchi, Essen, Germany). The soluble fraction was weighed and dissolved in DMSO (Carl Roth), achieving a stock concentration of 204.8 g/L. Aliquots were stored at −80 °C and diluted in DMSO to a final concentration of 20.48 g/L prior to use. Dried R. palmatum root extract (2.048 g, Paninkret, Pinneberg, Germany) was solved according to the instructions of the manufacturer in 100 mL 50% aqueous ethanol (Carl Roth), achieving a stock concentration of 20.48 g/L. The stock solution was stored at 4 °C. 2.048 g rhein (Sigma Aldrich, Darmstadt, Germany) was dissolved in 100 mL 0.1 M NaOH(aq.) (Carl Roth), achieving a stock concentration of 20.48 g/L. The stock solution was stored at room temperature protected from daylight.
2.2. Broth Microdilution (BMD)
P. gingivalis (DSM 20709, ATCC 33277) was obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ). The stock was stored in liquid nitrogen using the cryobank system from Mast Diagnostika (Reinfeld, Germany). A working aliquot was held at −70 °C and used for sub-culturing the strain each week at 37 °C on Schaedler agar (Becton Dickinson, Heidelberg, Germany) under anaerobic conditions (90% N2, 10% CO2, 10% H2). A fresh 48 h-old isolation of P. gingivalis was used for the inoculation of BMD. Anaerobiosis was controlled by indicators. All susceptibility tests were performed according to CLSI (Clinical and Laboratory Standards Institute) [27] with the exception that Wilkins–Chalgren broth (Merlin, Bornheim-Hersel, Germany) was used instead of supplemented Brucella broth. The minimal inhibitory concentration (MIC) of each test substance was determined as the lowest concentration that prevented the visible growth of P. gingivalis ATCC 33277 after 48 h of incubation and was expressed in mg/L. An inoculum with turbidity equal to 0.5 McFarland was used. Serial dilutions revealed that such a suspension contained approximately 1 × 107 P. gingivalis ATCC 33277 colony-forming units (CFU)/mL. The bacterial colonies were suspended in 1.5 mL sterile 0.9% NaCl(aq.) solution until a turbidity of 0.5 McFarland was reached. The turbidity was controlled using a photometric device (DensiCheck Plus, bioMerieux, Nürtingen, Germany). The bacterial suspension was then diluted 1:10 in Wilkins–Chalgren broth. 50 µL were used to inoculate the wells of a polypropylene microdilution tray (Greiner, Frickenhausen, Germany). 1 mL of the test substance stock (20.48 g/L) was diluted in 9 mL Wilkins–Chalgren broth. 50 µL was mixed with 50 µL of the bacterial suspension, reaching a final test substance concentration of 1024 mg/L. The bacterial inoculum was equivalent to 5 × 105 CFU contained in a total test volume of 100 µL. Lower test substance concentrations were reached by serial dilution. Each test substance concentration was tested in quadruples or octuples depending on the respective experiment. Each experiment was repeated five times. Control rows contained 100 µL of Wilkens–Chalgren broth alone, 100 µL of the bacterial inoculum alone as growth control, or 100 µL of the test substance alone. Microdilution trays were covered with a perforated plastic foil (reference number M/B3-002-040, Sifin Diagnostics, Berlin, Germany) and incubated for 48 h in an anaerobic jar under anaerobic conditions (see above). Selected wells were sub-cultured on Schaedler agar as purity controls. The growth of bacteria other than P. gingivalis was not observed. The control experiments were performed with the respective solvent (DMSO, 50% aqueous ethanol, 0.1 M NaOH(aq.)) alone and repeated five times. Growth inhibition due to the solvent alone was not detected.
2.3. Quality Control
The performance of the anaerobic test system was monitored by the concomitant use of the control strains Bacteroides fragilis ATCC 25285 and B. thetaiotaomicron ATCC2 9741 and the MICRONAUT-S anaerobes MIC plates (reference number E1-085-040, Merlin). B. fragilis and B. thetaiotaomicron were cultured on Columbia blood agar (Becton Dickinson) at 37 °C under an anaerobic atmosphere for 24 h (B. fragilis) or 48 h (B. thetaiotaomicron) and used to inoculate MICRONAUT-S anaerobes microtiter plates according to the instructions of the manufacturer. The MICs were determined after an incubation of 24 h (B. fragilis) or 48 h (B. thetaiotaomicron) under anaerobic conditions using a Tecan Sunrise microplate reader (Tecan, Crailsheim, Germany) and the MCN6 software (Merlin). Quality control ranges for the respective antimicrobials were applied according to the instructions of the manufacturer (Supplementary Materials Table S1). The quality controls were performed weekly and quality control ranges were always reached.
2.4. HPLC (High-Performance Liquid Chromatography) Analysis
The analysis of the crude extracts was performed on an Agilent Infinity II 1260 system with a diode array detector. An ACE C18-PFP column (150 mm × 4.6 mm, 3 μm, 40 °C) was applied as stationary phase. A gradient mixture of acetonitrile and water (containing 0.1% formic acid) with a constant flow rate of 1.0 mL/min was used as an eluent with the following linear gradient elution program: 0 min: 99% H2O and 1% MeCN, 30 min: 5% H2O and 95% MeCN, 40 min: 5% H2O and 95% MeCN. For HPLC analysis, the material was dissolved in a 1:1 (v/v) MeCN/H2O solution arriving at a concentration of 1.02 mg/mL. The resulting samples were filtered over a Macherey–Nagel syringe filter with a PTFE membrane (0.2 µm pore size) prior to injection with an injection volume of 3 μL.
3. Results
3.1. BMD
The median MICs of E. globulus, A. indica, G. glabra and R. palmatum extracts for P. gingivalis ATCC 33277 from five independent experiments are given in Table 1. The MICs of E. globulus leaf, A. indica leaf and G. glabra root extracts were lower when acetone was used as extractant compared to 70% aqueous ethanol (Table 1). This was most striking for the G. glabra root extracts where the median MIC of the 70%-ethanolic extract had a 16-fold higher MIC than the respective acetone extract (1024 mg/L versus 64 mg/L). The lowest MIC of all plant extracts (4 mg/L) was found for the R. palmatum extract dissolved in 50% aqueous ethanol.

Table 1.
MICs of the E. globulus, A. indica, G. glabra, R. palmatum extracts and rhein for P. gingivalis ATCC 33277. The median MIC from five independent experiments is shown.
For R. palmatum root extract, it is known that its antimicrobial activity against P. gingivalis can be traced back partially to the anthraquinone rhein [29]. Therefore, the MIC of rhein dissolved in 0.1 M NaOH(aq.) for P. gingivalis ATCC 33277 was determined. The median MIC of rhein from five independent experiments was 0.125 mg/L (Table 1, Figure 1).
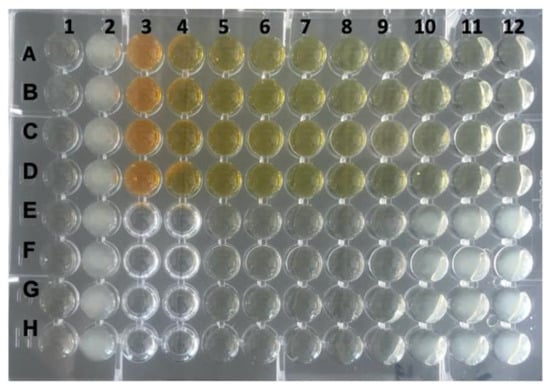
Figure 1.
Determination of the MIC of rhein for P. gingivalis strain ATCC 33277. Column 1 served as negative control (Wilkins–Chalgren broth only), column 2 contained the growth control (P. gingivalis only) and column 3 wells A–D contained the test substance alone (rhein at a concentration of 2048 mg/L). A serial dilution of rhein was performed in quadruples, starting in column 4 wells A–D (concentration of 1024 mg/L) to column 12 wells A–D. The last row with prevented visible growth was column 9 wells E–H (MIC = 0.125 mg/L). Columns 3 and 4 wells E–H were left empty.
The solvents used (DMSO, 50% aqueous ethanol and 0.1 M NaOH(aq.) showed no significant antimicrobial activity with MICs above 1024 mg/L (Table 2).

Table 2.
MICs of the solvent controls DMSO, 50% aqueous ethanol and 0.1 M NaOH(aq.) Five independent experiments yielded the same MIC of >1024 mg/L for all three solvents used.
All extracts were further characterized by HPLC–MS. This was of special interest for the R. palmatum extract with regard to its rhein content and, considering that the oral enzymes are able to cleave glycosidic bonds, also its rhein glucoside content [30,31].
3.2. HPLC
HPLC with diode array detection was performed to reveal the chemical fingerprints of the plant extracts tested herein. This will allow the comparison with upcoming samples of the same plant species in the future. As mentioned above, the acetone extracts of E. globulus leaves, A. indica leaves and G. glabra roots had lower MICs than the respective ethanol extracts. When comparing the chromatograms of the ethanol and acetone extracts, it is evident that the chemical complexity of the acetone samples is generally higher than that of the corresponding ethanol samples, which can be deduced from the number of peaks (Supplementary Materials Figures S1–S3, Tables S2–S7). In addition, it can be observed that more peaks with higher retention times appear in the acetone samples than in the peaks with lower retention times, with the former generally being attributed to more lipophilic substances (e.g., essential oils). However, a detailed component analysis was not performed. The chromatogram and the peak list of the rhubarb extract are shown in Figure 2 and Table S8.
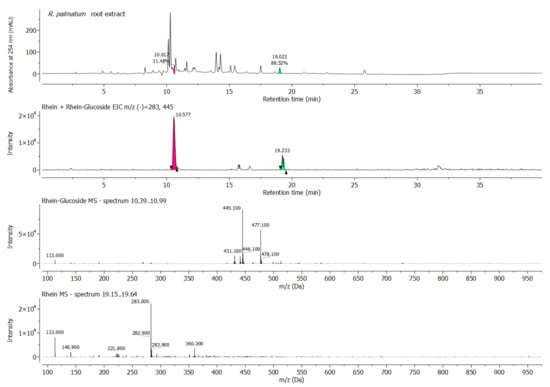
Figure 2.
HPLC chromatogram (monitored at 254 nm), respective total ion current (TIC) ESI–MS (–) and extracted ESI–MS (–) spectrum of R. palmatum root sample at 10.39–10.99 min (pink, rhein-glucoside) and 19.21–19.34 min (green, rhein).
In the instance of the R. palmatum root extract, it was suspected that its antimicrobial activity against P. gingivalis was partially derived from the presence of the anthraquinone rhein [29] and possibly its glycosylated form as well. This prompted us to further analyze the extract for its rhein content, for which purpose a sample of pure rhein was prepared and analyzed via HPLC. By comparing the retention time of rhein with the signals observed for the R. palmatum extract, it was noted that rhein was indeed present in the rhubarb sample, but in a relatively low concentration (Figure 2 and Figure S4, Tables S8 and S9). The comparison of the UV signal levels between the rhubarb root and the pure rhein sample indicated a rhein concentration of 5% in the R. palmatum extract. This nicely correlated with the BMD results, where the MIC ratio of the R. palmatum extract (4 mg/L) and rhein (0.125 mg/L) was 32. However, the major compounds of the R. palmatum root extract were not further separated and purified.
An extracted ion chromatogram (EIC) of rhein glucoside (m/z [M − H]− = 445.1) and rhein (m/z [M − H]− = 283.0) confirmed the presence of both forms (Figure 2). The m/z values of other known R. palmatum anthraquinones were also detected, but could not be individually assigned.
4. Discussion
This study was designed to evaluate plant extracts regarding their antimicrobial activity against P. gingivalis in vitro. BMD according to CLSI was chosen as the method, since it is standardized and therefore allows the comparison of the antimicrobial activity against P.gingivalis between the extracts tested. Many previous in vitro studies performed with E. globulus, A. indica, G. glabra or R. palmatum extracts do not allow a direct comparison between their antimicrobial activity due to the variety of methods used and the parameters applied, such as solvents, inocula, culture media and incubation periods. A common method used in other studies is disk diffusion. Due to their non-polarity, the compounds of plant extracts with the highest antimicrobial activity do not diffuse well in agar-based media. Therefore, disk diffusion is not appropriate to study the antimicrobial activity of plant extracts [26].
The antimicrobial activity of Eucalyptus essential oil or an E. globulus ethanolic leaf extract against P. gingivalis has been shown using disk diffusion [32,33]. However, the inhibition zones reported cannot be compared due to the reasons discussed above. In a study performed by Nagata et al., the growth of P. gingivalis ATCC 33277 was inhibited by macrocarpals A or B purified from a 60% ethanolic E. globulus leaf extract at a concentration of 1 mg/L [34]. This is much lower than in our study, as we measured a MIC of 128 mg/L for the acetone extract and of 256 mg/L for the ethanol extract, but it is reasonable that purified compounds exhibit stronger antimicrobial activities than crude extracts.
An ethanolic A. indica leaf extract showed a MIC of 500 mg/L against P. gingivalis ATCC 33277 in a previous study [35], which is comparable to our results of 1024 mg/L for the ethanol and 256 mg/L for the acetone extract.
A MIC of 62.5 mg/L of a 95% ethanol G. glabra root extract was reported. for P. gingivalis ATCC 33277 [36]. This matches the MIC of 64 mg/L we measured for the acetone extract, although our 70% ethanol extract showed a higher MIC of 1024 mg/L. This might be explained by the higher ethanol concentration used in the study mentioned above.
An 100% ethanolic extract of R. palmatum root showed a MIC of 500 mg/L for P. gingivalis ATCC 33277 in a previous study [29]. In our study, a 80%/50% R. palmatum root extract had the highest antimicrobial activity of all the extracts tested, with a MIC of 4 mg/L. The difference might be partially explained by the higher inoculum of 0.2 OD660 used by Liao et al. [29].
It was suspected that the antimicrobial activity of R. palmatum root extracts against P. gingivalis was partially derived from the presence of the anthraquinone rhein [29]. In a study by Azelmat et al., rhein showed a MIC of 2.5 mg/L for P. gingivalis ATCC 33277 [28]. In our study, we measured a MIC of 0.125 mg/L for rhein, which again can be partially explained by the higher inoculum of 0.2 OD660 used by Azelmat et al. [28].
A study investigating the antimicrobial activity of R. palmatum root extract demonstrated that anthraquinones others than rhein, such as emodin, aloe-emodin, physcion and chrysophanol, inhibited the growth of Salmonella Typhimurium [37]. Rhein had the lowest MIC against S. Typhimurium. In our study, the comparison of the UV signal levels between the rhubarb root and the pure rhein sample indicated a rhein concentration of 5% in the R. palmatum extract. This nicely correlated with the BMD results, where the MIC ratio of the R. palmatum extract (4 mg/L) and rhein (0.125 mg/L) was 32. Therefore, we hypothesize that in our setting, rhein might have been responsible for a relevant part of the antimicrobial activity. However, the major compounds of the R. palmatum root extract were not further separated and purified.
In a study investigating 56 clinical isolates of P. gingivalis, the MIC90 for amoxicillin/clavulanic acid was 0.5/0.25 mg/L, for clindamycin 0.25 m/L and for metronidazole 2 mg/L [38], which is interpreted as susceptible for systemic use according to CLSI 2021 [39]. Therefore, the local application of R. palmatum root extract, for which we determined a MIC of 4 mg/L, might be promising in inhibiting the growth of P. gingivalis in vivo. However, when treating periodontitis, it must be considered that the antimicrobial effect on the bacteria in biofilms cannot be necessarily inferred from the growth inhibitory effect on planktonic cells. Further, periodontitis is a multifactorial process that is not caused by P. gingivalis alone [8]. Given the potential toxicity and carcinogenicity of R. palmatum [40], local tolerance of R. palmatum root extracts has to be demonstrated. Further, their clinical efficacy remains to be shown before they could become an alternative to CHX. At least, the in vitro MIC of CHX for P. gingivalis ATCC 33277 was reported to be 4 mg/L [41], which is the same as for the R. palmatum root extract tested in our study.
5. Conclusions
Extracts of E. globulus, A. indica (neem), G. glabra (licorice), R. palmatum (rhubarb) and rhein showed antimicrobial activity against P. gingivalis, making them promising candidates for further study in the treatment of periodontitis. The lowest MIC of all plant extracts was found for the R. palmatum extract (4 mg/L) and for the anthraquinone rhein (0.125 mg/L). The R. palmatum extract had a rhein concentration of 5%. The MIC ratio of both of them was 32. Therefore, we hypothesize rhein might have been responsible for a relevant part of the antimicrobial activity. However, the major compounds of the R. palmatum root extract were not further separated and purified.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics11020186/s1. Figure S1: HPLC chromatograms (monitored at 254 nm) of E. globulus leaf acetone and 70% ethanol extracts. Figure S2: HPLC chromatograms (monitored at 254 nm) of A. indica leaf acetone and 70% ethanol extracts. Figure S3: HPLC chromatograms (monitored at 254 nm) of G. glabra root acetone and 70% ethanol extracts. Figure S4: HPLC chromatogram (monitored at 254 nm), respective total ion current (TIC) ESI–MS (–) and extracted ESI–MS (–) spectrum at 19.21–19.37 min (green, rhein) of a commercial rhein sample (Sigma Aldrich). Table S1: Quality control ranges for B. fragilis and B. thetaiotaomicron. Table S2: Peak list of the E. globulus leaf acetone extract chromatogram at 254 nm with a minimum area threshold of 0.5%. Table S3: Peak list of the E. globulus leaf 70% ethanol extract chromatogram at 254 nm with a minimum area threshold of 0.5%. Table S4: Peak list the of the A. indica leaf acetone extract chromatogram at 254 nm with a minimum area threshold of 0.5%. Table S5: Peak list of the A. indica leaf 70% ethanol extract chromatogram at 254 nm with a minimum area threshold of 0.5%. Table S6: Peak list of the of the G. glabra root acetone extract chromatogram at 254 nm with a minimum area threshold of 0.5%. Table S7: Peak list of the of the G. glabra root 70% ethanol extract chromatogram at 254 nm with a minimum area threshold of 0.5%. Table S8: Peak list of the of the R. palmatum root extract chromatogram at 254 nm with a minimum area threshold of 0.5%. Table S9: Peak list of the of rhein chromatogram at 254 nm with a minimum area threshold of 0.5%.
Author Contributions
L.K.M.-H., F.D.v.L., N.V., J.G., T.O. and J.D. conceived and designed the experiments; L.K.M.-H., N.V., J.G. and F.D.v.L. performed the experiments; L.K.M.-H., N.V., J.G., T.O. and F.D.v.L. analyzed the data; L.K.M.-H., F.D.v.L. and T.O. contributed reagents/materials/analysis tools; L.K.M.-H., N.V., J.G. and F.D.v.L. wrote the original draft of the paper; L.K.M.-H. and F.D.v.L. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the grant “Startup Innovativ” of the Ministry of EconoMIC of Rhineland-Palatinate.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained in this manuscript or supplementary materials.
Conflicts of Interest
L.K.M.-H. is holder of the “Startup Innovativ” grant of the Ministry of Economics, Rhineland-Palatinate, and a mouth rinse containing R. palmatum that was launched in 06/2021. The other authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Abbreviations
BMD: broth microdilution; MIC: minimal inhibitory concentration.
References
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 328 Diseases and Injuries for 195 Countries, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal Diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Romandini, M.; Baima, G.; Antonoglou, G.; Bueno, J.; Figuero, E.; Sanz, M. Periodontitis, Edentulism, and Risk of Mortality: A Systematic Review with Meta-analyses. J. Dent. Res. 2021, 100, 37–49. [Google Scholar] [CrossRef]
- LaMonte, M.J.; Genco, R.J.; Hovey, K.M.; Wallace, R.B.; Freudenheim, J.L.; Michaud, D.S.; Mai, X.; Tinker, L.F.; Salazar, C.R.; Andrews, C.A.; et al. History of Periodontitis Diagnosis and Edentulism as Predictors of Cardiovascular Disease, Stroke, and Mortality in Postmenopausal Women. J. Am. Heart Assoc. 2017, 6, e004518. [Google Scholar] [CrossRef]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 1414. [Google Scholar] [CrossRef]
- de Oliveira Ferreira, R.; de Brito Silva, R.; Magno, M.B.; Carvalho Almeida, A.; Fagundes, N.C.F.; Maia, L.C.; Lima, R.R. Does Periodontitis Represent a Risk Factor for Rheumatoid Arthritis? A Systematic Review and Meta-Analysis. Ther. Adv. Musculoskelet. Dis. 2019, 11, 1759720x19858514. [Google Scholar] [CrossRef]
- Ungprasert, P.; Wijarnpreecha, K.; Wetter, D.A. Periodontitis and Risk of Psoriasis: A Systematic Review and Meta-Analysis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 857–862. [Google Scholar] [CrossRef]
- How, K.Y.; Song, K.P.; Chan, K.G. Porphyromonas gingivalis: An Overview of Periodontopathic Pathogen below the Gum Line. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef]
- Fiorillo, L.; Cervino, G.; Laino, L.; D’Amico, C.; Mauceri, R.; Tozum, T.F.; Gaeta, M.; Cicciù, M. Porphyromonas gingivalis, Periodontal and Systemic Implications: A Systematic Review. Dent. J. 2019, 7, 114. [Google Scholar] [CrossRef]
- Cobb, C.M.; Sottosanti, J.S. A Re-Evaluation of Scaling and Root Planing. J. Periodontol. 2021, 92, 1370–1378. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 4–60. [Google Scholar] [CrossRef]
- da Costa, L.F.N.P.; Amaral, C.d.S.F.; Barbirato, D.d.S.; Leão, A.T.T.; Fogacci, M.F. Chlorhexidine Mouthwash as an Adjunct to Mechanical Therapy in Chronic Periodontitis: A Meta-Analysis. J. Am. Dent. Assoc. 2017, 148, 308–318. [Google Scholar] [CrossRef]
- al-Tannir, M.A.; Goodman, H.S. A Review of Chlorhexidine and Its Use in Special Populations. Spec. Care Dentist. 1994, 14, 116–122. [Google Scholar] [CrossRef]
- Cieplik, F.; Jakubovics, N.S.; Buchalla, W.; Maisch, T.; Hellwig, E.; Al-Ahmad, A. Resistance Toward Chlorhexidine in Oral Bacteria—Is There Cause for Concern? Front. Microbiol. 2019, 10, 587. [Google Scholar] [CrossRef]
- Eid Abdelmagyd, H.A.; Ram Shetty, D.S.; Musa Musleh Al-Ahmari, D.M. Herbal Medicine as Adjunct in Periodontal Therapies—A Review of Clinical Trials in Past Decade. J. Oral Biol. Craniofac. Res. 2019, 9, 212–217. [Google Scholar] [CrossRef]
- Surathu, N.; Kurumathur, A.V. Traditional Therapies in the Management of Periodontal Disease in India and China. Periodontol 2000 2011, 56, 14–24. [Google Scholar] [CrossRef]
- Nagata, H.; Inagaki, Y.; Tanaka, M.; Ojima, M.; Kataoka, K.; Kuboniwa, M.; Nishida, N.; Shimizu, K.; Osawa, K.; Shizukuishi, S. Effect of Eucalyptus Extract Chewing Gum on Periodontal Health: A Double-Masked, Randomized Trial. J. Periodontol. 2008, 79, 1378–1385. [Google Scholar] [CrossRef]
- Kaushik, A.; Tanwar, R.; Kaushik, M. Ethnomedicine: Applications of Neem (Azadirachta indica) in Dentistry. Dent. Hypotheses 2012, 3, 112–114. [Google Scholar] [CrossRef]
- Imam, H.; Azad, H.; Makbul, S. Neem (Azadirachta indica A. Juss)-A Nature’s Drugstore: An Overview. Int. Res. J. Biol. Sci. 2012, 1, 76. [Google Scholar]
- Antony, V.; Prasad, D.; Khan, R. Evaluation of the Efficacy of Azadirachta Indica (Neem) Extract Gel as a Local Drug Delivery in the Treatment of Patients with Chronic Periodontitis. A Double Blind Randomised Clinical Trial. J. Med. Dent. Sci. 2013, 3, 2250–3013. [Google Scholar] [CrossRef]
- Vennila, K.; Elanchezhiyan, S.; Ilavarasu, S. Efficacy of 10% whole Azadirachta indica (Neem) Chip as an Adjunct to Scaling and Root Planing in Chronic Periodontitis: A Clinical and Microbiological Study. Indian J. Dent. Res. 2016, 27, 15–21. [Google Scholar] [CrossRef]
- Dhingra, K.; Vandana, K.L. Effectiveness of Azadirachta indica (Neem) Mouthrinse in Plaque and Gingivitis Control: A Systematic Review. Int. J. Dent. Hyg. 2017, 15, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Fatima, A.; Faridi, U.; Negi, A.S.; Shanker, K.; Kumar, J.; Rahuja, N.; Luqman, S.; Sisodia, B.; Saikia, D.; et al. Antimicrobial Potential of Glycyrrhiza glabra Roots. J. Ethnopharmacol. 2008, 116, 377–380. [Google Scholar] [CrossRef]
- Messier, C.; Epifano, F.; Genovese, S.; Grenier, D. Licorice and its Potential Beneficial Effects in Common Oro-Dental Diseases. Oral Dis. 2011, 18, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Zuo, J.; Guo, F.; Dong, D. What We Already Know About Rhubarb: A Comprehensive Review. Chin. Med. 2020, 15, 88. [Google Scholar] [CrossRef]
- Eloff, J.N. Avoiding Pitfalls in Determining Antimicrobial Activity of Plant Extracts and Publishing the Results. BMC Complement. Altern Med. 2019, 19, 106. [Google Scholar] [CrossRef] [PubMed]
- CLSI. M11: Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria, 9th ed.; Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Azelmat, J.; Larente, J.F.; Grenier, D. The Anthraquinone Rhein Exhibits Synergistic Antibacterial Activity in Association with Metronidazole or Natural Compounds and Attenuates Virulence Gene Expression in Porphyromonas gingivalis. Arch. Oral Biol. 2015, 60, 342–346. [Google Scholar] [CrossRef]
- Liao, J.; Zhao, L.; Yoshioka, M.; Hinode, D.; Grenier, D. Effects of Japanese Traditional Herbal Medicines (Kampo) on Growth and Virulence Properties of Porphyromonas gingivalis and Viability of Oral Epithelial Cells. Pharm. Biol. 2013, 51, 1538–1544. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, P.; Xu, L.; He, C.; Peng, Y.; Xiao, P. Evaluation of the content variation of anthraquinone glycosides in rhubarb by UPLC-PDA. Chem. Cent. J. 2013, 7, 170. [Google Scholar] [CrossRef]
- Moreau, J.P.; Moreau, S.; Skinner, S. Comparative physiological disposition of some anthraquinone glycosides and aglycones. Biopharm. Drug Dispos. 1985, 6, 325–334. [Google Scholar] [CrossRef]
- Hans, V.M.; Grover, H.S.; Deswal, H.; Agarwal, P. Antimicrobial Efficacy of Various Essential Oils at Varying Concentrations against Periopathogen Porphyromonas gingivalis. J. Clin. Diagn. Res. 2016, 10, Zc16–zc19. [Google Scholar] [CrossRef] [PubMed]
- Bankur, P.K.; Mathew, M.; Almalki, S.A.; Jalaluddin, M.; Jayanti, I.; Durgaraju, M. An In Vitro Evaluation of Antibacterial Efficacy of Various Concentration of Eucalyptus globulus Leaf Extract on Periodontal Pathogens. J. Contemp. Dent. Pract. 2019, 20, 1041–1044. [Google Scholar] [PubMed]
- Nagata, H.; Inagaki, Y.; Yamamoto, Y.; Maeda, K.; Kataoka, K.; Osawa, K.; Shizukuishi, S. Inhibitory Effects of Macrocarpals on the Biological Activity of Porphyromonas gingivalis and Other Periodontopathic Bacteria. Oral Microbiol. Immunol. 2006, 21, 159–163. [Google Scholar] [CrossRef]
- Matson Robles, A.; Alejandra, H.; Diaz, A. In vitro Antibacterial Activity of Maclura tinctoria and Azadirachta indica against Streptococcus mutans and Porphyromonas gingivalis. Br. J. Pharm. Res. 2015, 7, 291–298. [Google Scholar] [CrossRef]
- Suwannakul, S. Antibacterial Activities of Glycyrrhiza glabra Linn. (Licorice) Root Extract against Porphyromonas gingivalis and Its Inhibitory Effects on Cysteine Proteases and Biofilms. J. Dent. Indones. 2017, 24, 85–92. [Google Scholar] [CrossRef]
- Wu, X. Antibacterial Activities of Rhubarb Extract and the Bioactive Compounds against Salmonella. Int. J. Nutr. Sci. Food Technol. 2015, 1, 1–9. [Google Scholar] [CrossRef][Green Version]
- Kulik, E.M.; Thurnheer, T.; Karygianni, L.; Walter, C.; Sculean, A.; Eick, S. Antibiotic Susceptibility Patterns of Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis Strains from Different Decades. Antibiotics 2019, 8, 253. [Google Scholar] [CrossRef]
- CLSI. M100: Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- Shukla, V.; Asthana, S.; Gupta, P.; Dwivedi, P.D.; Tripathi, A.; Das, M. Chapter One—Toxicity of Naturally Occurring Anthraquinones. Adv. Mol. Toxicol. 2017, 11, 1–50. [Google Scholar]
- Kulik, E.M.; Waltimo, T.; Weiger, R.; Schweizer, I.; Lenkeit, K.; Filipuzzi-Jenny, E.; Walter, C. Development of Resistance of Mutans Streptococci and Porphyromonas gingivalis to Chlorhexidine Digluconate and Amine Fluoride/Stannous Fluoride-containing Mouthrinses, in vitro. Clin. Oral Investig. 2015, 19, 1547–1553. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).