Synergistic Effect of Polyphenol-Rich Complex of Plant and Green Propolis Extracts with Antibiotics against Respiratory Infections Causing Bacteria
Abstract
:1. Introduction
2. Results
2.1. Total Content of Polyphenols
2.2. Chemical Composition
2.3. Antibacterial Activity
2.4. Combined Effect of GoImmune Strong® Complex and Antibiotics
2.4.1. Determination of Fractional Inhibitory Concentrations (FIC) and FIC Index (FICI)
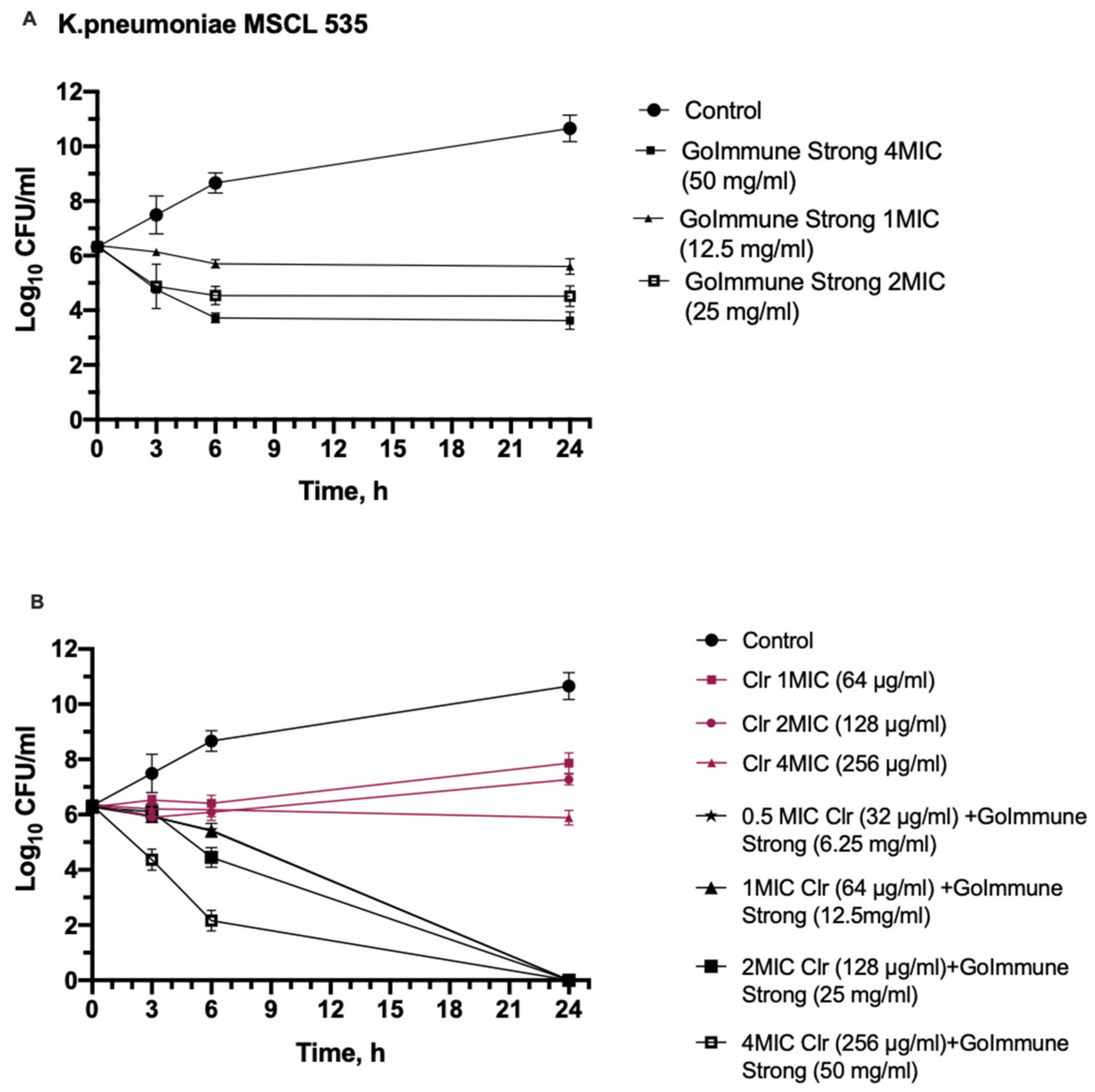
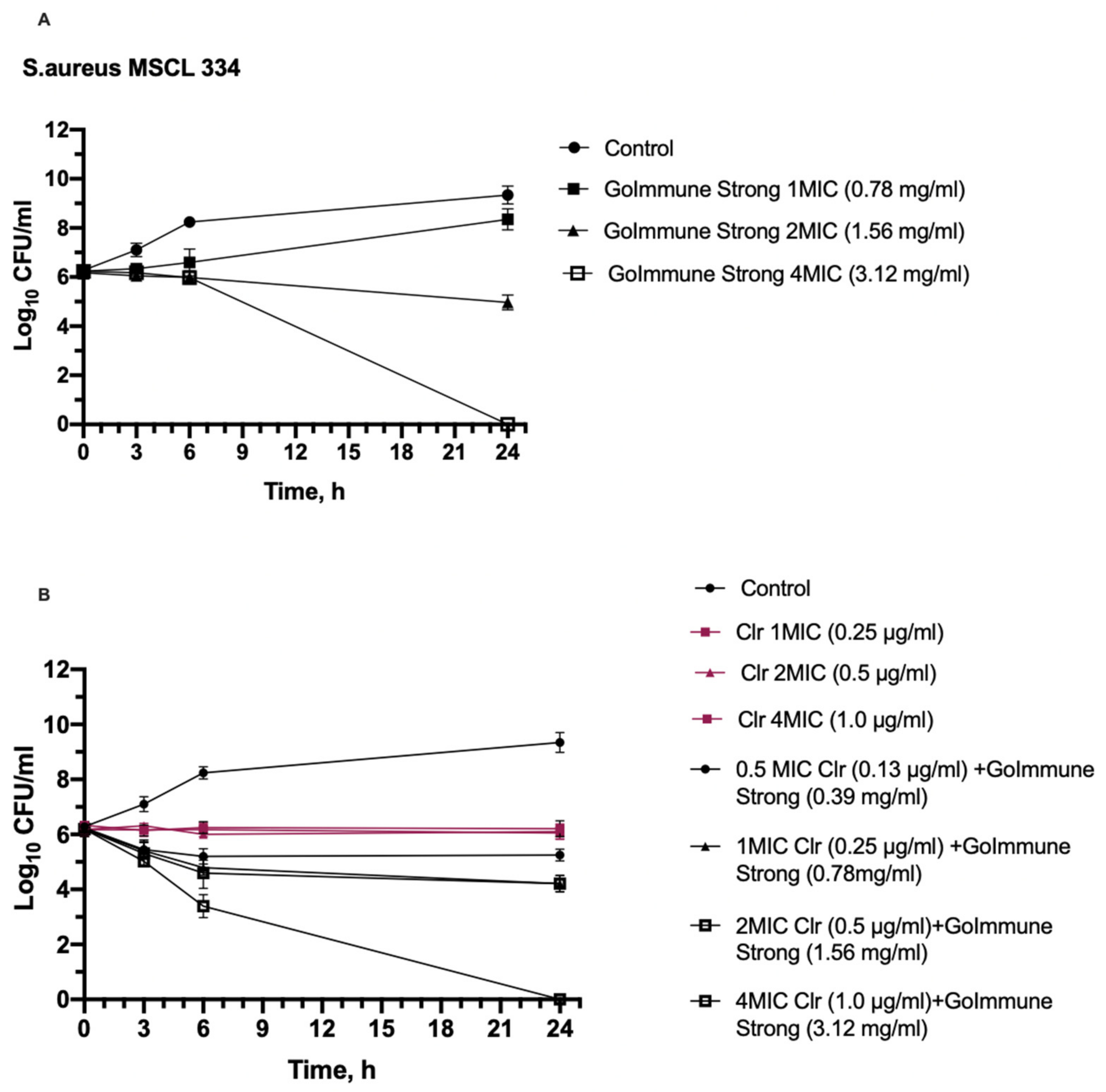
2.4.2. Time-Kill Assay
3. Discussion
4. Materials and Methods
4.1. Extracts and Their Preparation for the Tests
4.2. Total Phenolic Content
4.3. Chromatographic Analysis
4.3.1. Preparation of Samples and Standards
4.3.2. HPLC–TOF-HRMS Analysis
4.4. Determination of MIC and MBC
4.5. Determination of Fractional Inhibitory Concentrations (FIC) and FIC Index (FICI)
4.6. Time-Kill Assay
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement:
Informed Consent Statement
Data Availability Statement:
Conflicts of Interest
References
- Manohar, P.; Loh, B.; Nachimuthu, R.; Hua, X.; Welburn, S.C.; Leptihn, S. Secondary Bacterial Infections in Patients with Viral Pneumonia. Front. Med. 2020, 7, 420. [Google Scholar] [CrossRef] [PubMed]
- Santella, B.; Serretiello, E.; de Filippis, A.; Veronica, F.; Iervolino, D.; Dell’annunziata, F.; Manente, R.; Valitutti, F.; Santoro, E.; Pagliano, P.; et al. Lower Respiratory Tract Pathogens and Their Antimicrobial Susceptibility Pattern: A 5-Year Study. Antibiotics 2021, 10, 851. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Du, J.; Huang, C.; Li, H. Microbial Distribution and Antibiotic Susceptibility of Lower Respiratory Tract Infections Patients from Pediatric Ward, Adult Respiratory Ward, and Respiratory Intensive Care Unit. Front. Microbiol. 2020, 11, 1480. [Google Scholar] [CrossRef] [PubMed]
- Bakaletz, L.O. Viral–Bacterial Co-Infections in the Respiratory Tract. Curr. Opin. Microbiol. 2017, 35, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Coast, J. The True Cost of Antimicrobial Resistance. BMJ 2013, 346, f1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, B.L.; Brightling, C. Pharmacological Treatment of Bacterial Infections of the Respiratory Tract. Anaesth. Intensive Care Med. 2015, 16, 79–82. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Keman, D.; Soyer, F. Antibiotic-Resistant Staphylococcus Aureus Does Not Develop Resistance to Vanillic Acid and 2-Hydroxycinnamic Acid after Continuous Exposure In Vitro. ACS Omega 2019, 4, 15393–15400. [Google Scholar] [CrossRef] [Green Version]
- Ofosu, F.K.; Daliri, E.B.M.; Elahi, F.; Chelliah, R.; Lee, B.H.; Oh, D.H. New Insights on the Use of Polyphenols as Natural Preservatives and Their Emerging Safety Concerns. Front. Sustain. Food Syst. 2020, 4, 525810. [Google Scholar] [CrossRef]
- Clewell, A.E.; Béres, E.; Vértesi, A.; Glávits, R.; Hirka, G.; Endres, J.R.; Murbach, T.S.; Szakonyiné, I.P. A Comprehensive Toxicological Safety Assessment of an Extract of Olea europaea L. Leaves (BonoliveTM). Int. J. Toxicol. 2016, 35, 208–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savoia, D. Plant-Derived Antimicrobial Compounds: Alternatives to Antibiotics. Future Microbiol. 2012, 7, 979–990. [Google Scholar] [CrossRef] [Green Version]
- Patra, A.K. An Overview of Antimicrobial Properties of Different Classes of Phytochemicals. In Dietary Phytochemicals and Microbes; Springer: Dordrecht, The Netherlands, 2012; pp. 1–32. ISBN 978-94-007-3926-0. [Google Scholar]
- Sanhueza, L.; Melo, R.; Montero, R.; Maisey, K.; Mendoza, L.; Wilkens, M. Synergistic Interactions between Phenolic Compounds Identified in Grape Pomace Extract with Antibiotics of Different Classes against Staphylococcus Aureus and Escherichia Coli. PLoS ONE 2017, 12, e0172273. [Google Scholar] [CrossRef] [PubMed]
- Pancu, D.F.; Scurtu, A.; Macasoi, I.G.; Marti, D.; Mioc, M.; Soica, C.; Coricovac, D.; Horhat, D.; Poenaru, M.; Dehelean, C. Antibiotics: Conventional Therapy and Natural Compounds with Antibacterial Activity-a Pharmaco-Toxicological Screening. Antibiotics 2021, 10, 401. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruddaraju, L.K.; Pammi, S.V.N.; sankar Guntuku, G.; Padavala, V.S.; Kolapalli, V.R.M. A Review on Anti-Bacterials to Combat Resistance: From Ancient Era of Plants and Metals to Present and Future Perspectives of Green Nano Technological Combinations. Asian J. Pharm. Sci. 2020, 15, 42–59. [Google Scholar] [CrossRef]
- Coppo, E.; Marchese, A. Antibacterial Activity of Polyphenols. Curr. Pharm. Biotechnol. 2014, 15, 380–390. [Google Scholar] [CrossRef]
- Martin, D.; Konrad, M.; Adarkwah, C.C.; Kostev, K. Reduced Antibiotic Use after Initial Treatment of Acute Respiratory Infections with Phytopharmaceuticals- a Retrospective Cohort Study. Postgrad. Med. 2020, 132, 412–418. [Google Scholar] [CrossRef] [Green Version]
- Thielmann, J.; Kohnen, S.; Hauser, C. Antimicrobial Activity of Olea europaea Linné Extracts and Their Applicability as Natural Food Preservative Agents. Int. J. Food Microbiol. 2017, 251, 48–66. [Google Scholar] [CrossRef]
- Pereira, A.P.; Ferreira, I.C.F.R.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic Compounds and Antimicrobial Activity of Olive (Olea europaea L. Cv. Cobrançosa) Leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef]
- Sudjana, A.N.; D’Orazio, C.; Ryan, V.; Rasool, N.; Ng, J.; Islam, N.; Riley, T.V.; Hammer, K.A. Antimicrobial Activity of Commercial Olea europaea (Olive) Leaf Extract. Int. J. Antimicrob. Agents 2009, 33, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Cecere, M.; Skaltsounis, A.L.; Argyropoulou, A.; Hellwig, E.; Aligiannis, N.; Wittmer, A.; Al-Ahmad, A. High-Level Antimicrobial Efficacy of Representative Mediterranean Natural Plant Extracts against Oral Microorganisms. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Keskın, D.; Ceyhan, N.; Uğur, A.; Dbeys, A.D. Antimicrobial Activity and Chemical Constitutions of West Anatolian Olive (Olea europaea L.) Leaves. Agric. Environ. 2012, 10, 99–102. [Google Scholar]
- Lee, O.H.; Lee, B.Y. Antioxidant and Antimicrobial Activities of Individual and Combined Phenolics in Olea europaea Leaf Extract. Bioresour. Technol. 2010, 101, 3751–3754. [Google Scholar] [CrossRef]
- Hashmi, M.A.; Khan, A.; Hanif, M.; Farooq, U.; Perveen, S. Traditional Uses, Phytochemistry, and Pharmacology of Olea europaea (Olive). Evid.-Based Complementary Altern. Med. 2015, 2015, 541591. [Google Scholar] [CrossRef] [Green Version]
- Borjan, D.; Leitgeb, M.; Knez, Ž.; Hrnčič, M.K. Microbiological and Antioxidant Activity of Phenolic Compounds in Olive Leaf Extract. Molecules 2020, 25, 5946. [Google Scholar] [CrossRef]
- Lim, A.; Subhan, N.; Jazayeri, J.A.; John, G.; Vanniasinkam, T.; Obied, H.K. Plant Phenols as Antibiotic Boosters: In Vitro Interaction of Olive Leaf Phenols with Ampicillin. Phytother. Res. 2016, 30, 503–509. [Google Scholar] [CrossRef]
- Silici, S.; Kutluca, S. Chemical Composition and Antibacterial Activity of Propolis Collected by Three Different Races of Honeybees in the Same Region. J. Ethnopharmacol. 2005, 99, 69–73. [Google Scholar] [CrossRef]
- Ristivojević, P.; Dimkić, I.; Trifković, J.; Berić, T.; Vovk, I.; Milojkovič-Opsenica, D.; Stanković, S. Antimicrobial Activity of Serbian Propolis Evaluated by Means of MIC, HPTLC, Bioautography and Chemometrics. PLoS ONE 2016, 11, e0157097. [Google Scholar] [CrossRef]
- Torres, A.R.; Sandjo, L.P.; Friedemann, M.T.; Tomazzoli, M.M.; Maraschin, M.; Mello, C.F.; Santos, A.R.S. Chemical Characterization, Antioxidant and Antimicrobial Activity of Propolis Obtained from Melipona Quadrifasciata Quadrifasciata and Tetragonisca Angustula Stingless Bees. Braz. J. Med. Biol. Res. 2018, 51, e7118. [Google Scholar] [CrossRef]
- Popova, M.; Trusheva, B.; Bankova, V. Propolis of Stingless Bees: A Phytochemist’s Guide through the Jungle of Tropical Biodiversity. Phytomedicine 2021, 86, 153098. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.; Giannopoulou, E.; Skalicka-Wózniak, K.; Graikou, K.; Widelski, J.; Bankova, V.; Kalofonos, H.; Sivolapenko, G.; Gaweł-Bȩben, K.; Antosiewicz, B.; et al. Characterization and Biological Evaluation of Propolis from Poland. Molecules 2017, 22, 1159. [Google Scholar] [CrossRef] [PubMed]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavri, A.; Abramovič, H.; Polak, T.; Bertoncelj, J.; Jamnik, P.; Možina, S.S.; Jeršek, B. Chemical Properties and Antioxidant and Antimicrobial Activities of Slovenian Propolis. Chem. Biodivers. 2012, 9, 1545–1558. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sankarapandian, K.; Cheng, Y.; Woo, S.O.; Kwon, H.W.; Perumalsamy, H.; Ahn, Y.J. Relationship between Total Phenolic Contents and Biological Properties of Propolis from 20 Different Regions in South Korea. BMC Complementary Altern. Med. 2016, 16, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nainu, F.; Masyita, A.; Bahar, M.A.; Raihan, M.; Prova, S.R.; Mitra, S.; Emran, T.B.; Simal-Gandara, J. Pharmaceutical Prospects of Bee Products: Special Focus on Anticancer, Antibacterial, Antiviral, and Antiparasitic Properties. Antibiotics 2021, 10, 822. [Google Scholar] [CrossRef]
- Campos, J.F.; das Santos, U.P.; da Rocha, P.D.S.; Damião, M.J.; Balestieri, J.B.P.; Cardoso, C.A.L.; Paredes-Gamero, E.J.; Estevinho, L.M.; de Picoli Souza, K.; dos Santos, E.L. Antimicrobial, Antioxidant, Anti-Inflammatory, and Cytotoxic Activities of Propolis from the Stingless Bee Tetragonisca Fiebrigi (Jataí). Evid.-Based Complementary Altern. Med. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Seidel, V.; Peyfoon, E.; Watson, D.G.; Fearnley, J. Comparative Study of the Antibacterial Activity of Propolis from Different Geographical and Climatic Zones. Phytother. Res 2008, 22, 1256–1263. [Google Scholar] [CrossRef]
- Berretta, A.A.; Nascimento, A.P.; Bueno, P.C.P.; Leite Vaz, M.M.d.O.L.; Marchetti, J.M. Propolis Standardized Extract (EPP-AF®), an Innovative Chemically and Biologically Reproducible Pharmaceutical Compound for Treating Wounds. Int. J. Biol. Sci. 2012, 8, 512–521. [Google Scholar] [CrossRef]
- Wojtyczka, R.D.; Dziedzic, A.; Idzik, D.; Kepa, M.; Kubina, R.; Kabała-Dzik, A.; Smoleń-Dzirba, J.; Stojko, J.; Sajewicz, M.; Wasik, T.J. Susceptibility of Staphylococcus Aureus Clinical Isolates to Propolis Extract Alone or in Combination with Antimicrobial Drugs. Molecules 2013, 18, 9623–9640. [Google Scholar] [CrossRef] [Green Version]
- Orsi, R.O.; Fernandes, A.; Bankova, V.; Sforcin, J.M. Antibacterial Effects of Brazilian and Bulgarian Propolis and Synergistic Effects with Antibiotics Acting on the Bacterial DNA and Folic Acid. Nat. Prod. Res. 2012, 26, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Orsi, R.O.; Fernandes, A.; Bankova, V.; Sforcin, J.M. The Effects of Brazilian and Bulgarian Propolis in Vitro against Salmonella Typhi and Their Synergism with Antibiotics Acting on the Ribosome. Nat. Prod. Res. 2012, 26, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Grecka, K.; Kuś, P.M.; Okińczyc, P.; Worobo, R.W.; Walkusz, J.; Szweda, P. The Anti-Staphylococcal Potential of Ethanolic Polish Propolis Extracts. Molecules 2019, 24, 1732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takaisi-Kikuni, N.B.; Schilcher, H. Electron Microscopic and Microcalorimetric Investigations of the Possible Mechanism of the Antibacterial Action of a Defined Propolis Provenance. Planta Med. 1994, 60, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.; Kaminska, D.; Matuszewska, E.; Hołderna-Kedzia, E.; Rogacki, J.; Matysiak, J. Promising Antimicrobial Properties of Bioactive Compounds from Different Honeybee Products. Molecules 2021, 26, 4007. [Google Scholar] [CrossRef]
- Mirzoeva, O.K.; Grishanin, R.N.; Calder, P.C. Microbiological Research Antimicrobial Action of Propolis and Some of Its Components: The Effects on Growth, Membrane Potential and Motility of Bacteria. Microbiol. Res. 1997, 152, 239–246. [Google Scholar] [CrossRef]
- AL-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Antimicrobial Activities of European Propolis Collected from Various Geographic Origins Alone and in Combination with Antibiotics. Medicines 2018, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Stepanović, S.; Antić, N.; Dakić, I.; Svabić-Vlahović, M. In Vitro Antimicrobial Activity of Propolis and Synergism between Propolis and Antimicrobial Drugs. Microbiol. Res. 2003, 158, 353–357. [Google Scholar] [CrossRef]
- Salatino, A.; Salatino, M.L.F.; Negri, G. How Diverse Is the Chemistry and Plant Origin of Brazilian Propolis? Apidologie 2021, 52, 1075–1097. [Google Scholar] [CrossRef]
- Bittencourt, M.L.F.; Ribeiro, P.R.; Franco, R.L.P.; Hilhorst, H.W.M.; de Castro, R.D.; Fernandez, L.G. Metabolite Profiling, Antioxidant and Antibacterial Activities of Brazilian Propolis: Use of Correlation and Multivariate Analyses to Identify Potential Bioactive Compounds. Food Res. Int. 2015, 76, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Dantas Silva, R.P.; Machado, B.A.S.; Barreto, G.d.A.; Costa, S.S.; Andrade, L.N.; Amaral, R.G.; Carvalho, A.A.; Padilha, F.F.; Barbosa, J.D.V.; Umsza-Guez, M.A. Antioxidant, Antimicrobial, Antiparasitic, and Cytotoxic Properties of Various Brazilian Propolis Extracts. PLoS ONE 2017, 12, e0172585. [Google Scholar] [CrossRef] [PubMed]
- Nader, R.A.; Mackieh, R.; Wehbe, R.; El Obeid, D.; Sabatier, J.M.; Fajloun, Z. Beehive Products as Antibacterial Agents: A Review. Antibiotics 2021, 10, 717. [Google Scholar] [CrossRef] [PubMed]
- El-Hawary, S.S.; Taher, M.A.; Amin, E.; AbouZid, S.F.; Mohammed, R. Genus Tabebuia: A Comprehensive Review Journey from Past Achievements to Future Perspectives. Arab. J. Chem. 2021, 14, 103046. [Google Scholar] [CrossRef]
- Jiménez-González, F.J.; Veloza, L.A.; Sepúlveda-Arias, J.C. Anti-Infectious Activity in Plants of the Genus Tabebuia. Univ. Sci. 2013, 18, 257–267. [Google Scholar] [CrossRef] [Green Version]
- Pereira, E.M.; de Barros Machado, T.; Ramos Leal, I.C.; Jesus, D.M.; de Almeida Damaso, C.R.; Pinto, A.V.; Giambiagi-deMarval, M.; Kuster, R.M.; Netto dos Santos, K.R. Tabebuia Avellanedae Naphtoquinones: Activity against Methicillin-Resistant Staphylococcal Strains, Cytotoxic Activity and in Vivo Dermal Irritability Analysis. Ann. Clin. Microbiol. Antimicrob. 2006, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Hamed, A.N.E.; Mahmoud, B.K.; Samy, M.N.; Kamel, M.S. An Extensive Review on Genus “Tabebuia”, Family Bignoniaceae: Phytochemistry and Biological Activities (1967 to 2018). J. Herb. Med. 2020, 24, 100410. [Google Scholar] [CrossRef]
- Ghomari, O.; Sounni, F.; Massaoudi, Y.; Ghanam, J.; Drissi Kaitouni, L.B.; Merzouki, M.; Benlemlih, M. Phenolic Profile (HPLC-UV) of Olive Leaves According to Extraction Procedure and Assessment of Antibacterial Activity. Biotechnol. Rep. 2019, 23, e00347. [Google Scholar] [CrossRef]
- Jimenez, P.; Masson, L.; Barriga, A.; Chávez, J.; Robert, P. Oxidative Stability of Oils Containing Olive Leaf Extracts Obtained by Pressure, Supercritical and Solvent-Extraction. Eur. J. Lipid Sci. Technol. 2011, 113, 497–505. [Google Scholar] [CrossRef]
- Poudyal, H.; Campbell, F.; Brown, L. Olive Leaf Extract Attenuates Cardiac, Hepatic, and Metabolic Changes in High Carbohydrate-, High Fat-Fed Rats. J. Nutr. 2010, 140, 946–953. [Google Scholar] [CrossRef] [Green Version]
- Korukluoglu, M.; Sahan, Y.; Yigit, A.; Ozer, E.T.; GÜCer, S. Antibacterial Activity and Chemical Constitutions of Olea europaea l. Leaf Extracts. J. Food Processing Preserv. 2010, 34, 383–396. [Google Scholar] [CrossRef]
- Mylonaki, S.; Kiassos, E.; Makris, D.P.; Kefalas, P. Optimisation of the Extraction of Olive (Olea europaea) Leaf Phenolics Using Water/Ethanol-Based Solvent Systems and Response Surface Methodology. Anal. Bioanal. Chem. 2008, 392, 977–985. [Google Scholar] [CrossRef]
- Lipid Maps. Available online: https://www.lipidmaps.org (accessed on 7 January 2022).
- Andrikopoulos, N.K.; Salta, F.N.; Mylona, A.; Chiou, A.; Boskou, G. Oxidative Stability of Edible Vegetable Oils Enriched in Polyphenols with Olive Leaf Extract. Food Sci. Technol. Int. 2007, 13, 413–421. [Google Scholar] [CrossRef]
- Dekanski, D.; Janićijević-Hudomal, S.; Tadić, V.; Marković, G.; Arsić, I.; Mitrović, D.M. Phytochemical Analysis and Gastroprotective Activity of an Olive Leaf Extract. J. Serb. Chem. Soc. 2009, 74, 367–377. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Mohammed, R.; Tawfike, A.F.; AbouZid, S.F.; Taher, M.A.; Abdelmohsen, U.R.; Amin, E. Metabolic Profiling of Cytotoxic Metabolites from Five Tabebuia Species Supported by Molecular Correlation Analysis. Sci. Rep. 2021, 11, 8405. [Google Scholar] [CrossRef] [PubMed]
- Nocchi, S.R.; Kato, N.N.; de Almeida, J.M.; Ferreira, A.M.T.; Toffoli-Kadri, M.C.; de Freitas Meirelles, L.E.; Damke, G.M.Z.F.; Consolaro, M.E.L.; Rigo, G.V.; Macedo, A.J.; et al. Pharmacological Properties of Specioside from the Stem Bark of Tabebuia Aurea. Rev. Bras. De Farmacogn. 2020, 30, 118–122. [Google Scholar] [CrossRef]
- Inouye, S.; Yamaguchi, H.; Takizawa, T. Screening of the Antibacterial Effects of a Variety of Essential Oils on Respiratory Tract Pathogens, Using a Modified Dilution Assay Method. J. Infect. Chemother. 2001, 7, 251–254. [Google Scholar] [CrossRef]
- de Abreu, M.B.; Temraz, A.; Vassallo, A.; Braca, A.; de Tommasi, N. Phenolic Glycosides from Tabebuia Argentea and Catalpa Bignonioides. Phytochem. Lett. 2014, 7, 85–88. [Google Scholar] [CrossRef]
- Takahashi, S.; Kawakami, S.; Sugimoto, S.; Matsunami, K.; Otsuka, H. Lignan Glycosides and Phenolic Compound Glycosides from the Branches of Tabebuia Chrysotricha. Am. J. Plant Sci. 2015, 6, 676–684. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, Y.; Eguchi, T.; Murasaki, C.; Ohashi, Y.; Kakinuma, K.; Takagaki, H.; Abe, M.; Inazawa, K.; Yamazaki, K.; Ikekawa, N.; et al. Studies on the Structure and Stereochemistry of Cytotoxic Furanonaphthoquinones from Tabebuia Impetiginosa: 5-and 8-Hydroxy-2-(1-Hyd Roxyethyl)Naphtho[2,3-b]f Uran-4,g-Diones. J. Chem. Soc., Perkin Trans. 1 1991, 10, 2323–2327. [Google Scholar] [CrossRef]
- de Morais, S.K.R.; Silva, S.G.; Portela, C.N.; Nunomura, S.M.; Quignard, E.L.J.; Pohlit, A.M. Bioactive Dihydroxyfuranonaphthoquinones from the Bark of Tabebuia Incana A.H. Gentry (Bignoniaceae) and HPLC Analysis of Commercial Pau d’arco and Certified T.Incana Bark Infusions. Acta Amaz. 2007, 37, 99–102. [Google Scholar] [CrossRef]
- Szliszka, E.; Kucharska, A.Z.; Sokół-Łętowska, A.; Mertas, A.; Czuba, Z.P.; Król, W. Chemical Composition and Anti-Inflammatory Effect of Ethanolic Extract of Brazilian Green Propolis on Activated J774A.1 Macrophages. Evid.-Based Complementary Altern. Med. 2013, 2013, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Shimazawa, M.; Mishima, S.; Hara, H. Water Extract of Propolis and Its Main Constituents, Caffeoylquinic Acid Derivatives, Exert Neuroprotective Effects via Antioxidant Actions. Life Sci. 2007, 80, 370–377. [Google Scholar] [CrossRef] [PubMed]
- de Moura, S.A.; Negri, G.; Salatino, A.; Lima, L.D.; Dourado, L.P.; Mendes, J.B.; Andrade, S.P.; Ferreira, M.A.; Cara, D.C. Aqueous Extract of Brazilian Green Propolis: Primary Components, Evaluation of Inflammation and Wound Healing by Using Subcutaneous Implanted Sponges. Evid. -Based Complementary Altern. Med. 2011, 2011, 748283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellati, F.; Orlandini, G.; Pinetti, D.; Benvenuti, S. HPLC-DAD and HPLC-ESI-MS/MS Methods for Metabolite Profiling of Propolis Extracts. J. Pharm. Biomed. Anal. 2011, 55, 934–948. [Google Scholar] [CrossRef] [PubMed]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Dieases (ESCMID). EUCAS Definitive document E.Def 1.2, May 2000: Terminology Relating to Methods for the Determination of Susceptibility of Bacteria to Antimicrobial Agents European Committee ForAntimicrobial SusceptibilityTesting (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Clin. Microbiol. Infect. 2000, 6, 503–508. [Google Scholar] [CrossRef] [Green Version]
- Odds, F.C. Synergy, Antagonism, and What the Chequerboard Puts between Them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Fratini, F.; Mancini, S.; Turchi, B.; Friscia, E.; Pistelli, L.; Giusti, G.; Cerri, D. A Novel Interpretation of the Fractional Inhibitory Concentration Index: The Case Origanum Vulgare L. and Leptospermum Scoparium J. R. et G. Forst Essential Oils against Staphylococcus Aureus Strains. Microbiol. Res. 2017, 195, 11–17. [Google Scholar] [CrossRef]
- Borges, A.; Abreu, A.C.; Dias, C.; Saavedra, M.J.; Borges, F.; Simões, M. New Perspectives on the Use of Phytochemicals as an Emergent Strategy to Control Bacterial Infections Including Biofilms. Molecules 2016, 21, 877. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Kruthiventi, A.K.; Doble, M. Synergism between Natural Products and Antibiotics against Infectious Diseases. Phytomedicine 2008, 15, 639–652. [Google Scholar] [CrossRef]
- Elnahas, R.A.; Elwakil, B.H.; Elshewemi, S.S.; Olama, Z.A. Egyptian Olea europaea Leaves Bioactive Extract: Antibacterial and Wound Healing Activity in Normal and Diabetic Rats. J. Tradit. Complementary Med. 2021, 11, 427–434. [Google Scholar] [CrossRef]
- Massaro, C.F.; Simpson, J.B.; Powell, D.; Brooks, P. Chemical Composition and Antimicrobial Activity of Honeybee (Apis Mellifera Ligustica) Propolis from Subtropical Eastern Australia. Sci. Nat. 2015, 102, 68. [Google Scholar] [CrossRef] [PubMed]
- Nina, N.; Quispe, C.; Jiménez-Aspee, F.; Theoduloz, C.; Feresín, G.E.; Lima, B.; Leiva, E.; Schmeda-Hirschmann, G. Antibacterial Activity, Antioxidant Effect and Chemical Composition of Propolis from the Región Del Maule, Central Chile. Molecules 2015, 20, 18144–18167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azaizeh, H.; Tafesh, A.; Najami, N.; Jadoun, J.; Halahlih, F.; Riepl, H. Synergistic Antibacterial Effects of Polyphenolic Compounds from Olive Mill Wastewater. Evid.-Based Complementary Altern. Med. 2011, 2011, 431021. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.J.; Yan, H.J.; He, J.; Liu, Y.Y. Antibacterial Activities of Polyphenols from Olive Leaves against Klebsiella Pneumoniae. In Proceedings of the IOP Conference Series: Earth and Environmental Science, 6th International Symposium on Energy Science and Chemical Engineering, Harbin, China, 22–24 January 2021; IOP Publishing Ltd.: Bristol, UK, 2021; Volume 680. [Google Scholar] [CrossRef]
- Bisignano, G.; Tomaino, A.T.; lo Cascio, R.; Crisafi, G.; Uccella, N.; Saija, A. On the In-Vitro Antimicrobial Activity of Oleuropein and Hydroxytyrosol. J. Pharm. Pharm. 1999, 51, 971–974. [Google Scholar] [CrossRef]
- Guzman, J.D. Natural Cinnamic Acids, Synthetic Derivatives and Hybrids with Antimicrobial Activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef]
- Kubica, P.; Szopa, A.; Kokotkiewicz, A.; Miceli, N.; Taviano, M.F.; Maugeri, A.; Cirmi, S.; Synowiec, A.; Gniewosz, M.; Elansary, H.O.; et al. Production of Verbascoside, Isoverbascoside and Phenolic Acids in Callus, Suspension, and Bioreactor Cultures of Verbena Officinalis and Biological Properties of Biomass Extracts. Molecules 2020, 25, 5609. [Google Scholar] [CrossRef]
- Cho, J.-Y.; Moon, J.-H.; Seong, K.-Y.; Park, K.-H. Antimicrobial Activity of 4-Hydroxybenzoic Acid and Trans 4-Hydroxycinnamic Acid Isolated and Identified from Rice Hull. Biosci. Biotechnol. Biochem. 1998, 62, 2273–2276. [Google Scholar] [CrossRef] [Green Version]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure-Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef]
| TAE | OEE | GPE | GoImmune Strong® |
|---|---|---|---|
| 23.56 ± 3.37 | 171.69 ± 27.50 | 13.03 ± 2.12 | 63.37 ± 10.89 |
| Compound | Formula | (M–H)− | RT, min | Refs. | Quantity Mean ± SD, μg/g DW |
|---|---|---|---|---|---|
| Hydroxytyrosol b,c | C8H10O3 | 153.0557 | 7.12 | [27,58] | 1695 ± 11 d |
| Hydroxytyrosol glucoside b,c | C14H20O8 | 315.1085 | 7.78 | [59] | 203 ± 8 d |
| Oleoside b,c | C16H22O11 | 389.1089 | 7.94 | [60] | 44 ± 6 k |
| 4-Hydroxybenzoic acid a | C7H6O3 | 137.0244 | 8.35 | [61] | 41 ± 3 |
| Aesculin b,c | C15H16O9 | 339.0722 | 8.59 | [60] | 21.8 ± 0.9 d |
| Chlorogenic acid a | C16H18O9 | 353.0878 | 9.01 | [59] | 17 ± 2 |
| NI c | – | 377.1487 | 9.39 | – | NQ |
| Luteolin rutinoside b,c | C27H30O16 | 609.1461 | 9.61 | [62] | 5.7 ± 0.5 j |
| C10 isoprenoidc | C17H24O11 | 403.1246 | 9.67 | [63] | NQ |
| NI c | – | 305.0739 | 9.95 | NQ | |
| Demethyloleuropein b,c | C24H30O13 | 525.1614 | 10.05 | [63] | 4.0 ± 0.2 k |
| Hydroxyphenylacetic acid b,c | C8H8O3 | 151.0401 | 10.09 | [64] | 0.858 ± 0.004 d |
| Rutin a | C27H30O16 | 609.1461 | 10.18 | [21,27,58] | 7.5 ± 0.2 |
| Verbascoside b,c | C29H36O15 | 623.1981 | 10.22 | [21,27] | 120.4 ± 0.6 e |
| p-Coumaric acid a | C9H8O3 | 163.0401 | 10.26 | [58,61] | 1.80 ± 0.04 |
| Luteolin O-glucoside b,c | C21H20O11 | 447.0933 | 10.41 | [27,58,60] | 31.0 ± 0.6 j |
| Isoverbascoside b,c | C29H36O15 | 623.1981 | 10.49 | [27] | 18.8 ± 1.3 e |
| Ferulic acid a | C10H10O4 | 193.0506 | 10.60 | [58,61] | 1.6 ± 0.2 |
| Apigenin 7-O-glucoside b,c | C21H20O10 | 431.0984 | 10.88 | [21,27] | 4.6 ± 0.4 j |
| Luteolin O-glucoside b,c | C21H20O11 | 447.0933 | 10.93 | [21,27,60] | 17.3 ± 1.1 j |
| Elenolic acid b,c | C11H14O6 | 241.0718 | 11.03 | [59] | 7.2 ± 0.4 k |
| Oleuropein a | C25H32O13 | 539.1770 | 11.10 | [27,58,59,60] | 8681 ± 85 |
| NI c | – | 601.2146 | 11.61 | – | NQ |
| Elenolic acid derivative b,c | – | 241.0718 | 12.16 | – | 2.5 ± 0.3 k |
| Luteolin b,c | C15H10O6 | 285.0405 | 12.20 | [58,60] | 18 ± 2 j |
| Quercetin b,c | C15H10O7 | 301.0354 | 12.22 | [58] | 21 ± 2 j |
| NI c | – | 377.1253 | 12.47 | – | NQ |
| Apigenin derivative b,c | – | 269.0455 | 12.96 | – | 1.1 ± 0.2j |
| NI c | – | 377.1255 | 13.23 | – | 208 ± 12 d |
| NI c | – | 377.1255 | 13.45 | – | 53 ± 7 d |
| Chrysoeriol b,c | C16H12O6 | 299.0561 | 14.89 | [65] | 4.5 ± 0.2 j |
| NI c | – | 545.1911 | 14.94 | – | NQ |
| NI c | – | 721.3611 | 15.80 | – | NQ |
| Apigenin derivative b,c | – | 269.0455 | 16.02 | – | 1.8 ± 0.3 j |
| C30 isoprenoid c | C30H48O4 | 471.3480 | 17.64 | [63] | NQ |
| NI c | – | 401.3017 | 20.46 | – | NQ |
| NI c | – | 401.3014 | 20.99 | – | NQ |
| Compound | Formula | (M–H)− | RT, min | Refs. | Quantity Mean ± SD, μg/g DW |
|---|---|---|---|---|---|
| Protocatechuic acid derivative c | – | 153.0193 * | 6.46 | – | 52 ± 7 l |
| Protocatechuic acid a | C7H6O4 | 153.0193 | 7.37 | – | 87 ± 9 |
| 2,4-dimethoxyphenyl 1-O-β-D-apiofuranosyl-(1–6)-β-D-glucopyranoside b,c | C19H28O12 | 447.1509 | 7.95 | [66] | 5.61 ± 0.06 d |
| 4-Hydroxybenzoic acid a | C7H6O3 | 137.0244 | 8.35 | [54,57] | 20.5 ± 0.5 |
| Epiaucubin b,c | C15H22O9 | 345.1191 | 8.41 | [66] | 3.0 ± 0.4 k |
| NI c | – | 461.1284 | 8.49 | – | 121 ± 9 d |
| NI c | – | 487.1431 | 8.55 | NQ | |
| C10 isoprenoids c | C19H28O11 | 431.1559 | 8.67 | [63] | NQ |
| Hydroxy benzoic acid isomer b,c | C7H6O3 | 137.0244 | 8.70 | [54] | 521 ± 46 m |
| Protocatechuic acid derivative c | – | 153.0193 * | 8.73 | – | 9.4 ± 0.3 l |
| NI c | – | 523.1660 | 9.00 | – | NQ |
| Chlorogenic acid a | C16H18O9 | 353.0878 | 9.05 | [54] | 0.49 ± 0.02 |
| Protocatechuic acid derivative c | – | 153.0193 * | 9.13 | – | 9.4 ± 0.3 l |
| Specioside b,c | C24H28O12 | 507.1508 | 9.16 | [67] | 2.6 ± 0.2 k |
| 10-O-(4-methoxybenzoyl)-impetiginoside A b,c | C23H28O12 | 495.1508 | 9.37 | [66] | 3.1 ± 0.3 k |
| Caffeic acid a | C9H8O4 | 179.0350 | 9.39 | [54] | 6.87 ± 0.04 |
| Flavone or flavonol c | C29H28O11 | 551.1559 | 9.41 | [63] | 30 ± 4 j |
| NI c | – | 639.1926 | 9.61 | – | NQ |
| NI c | – | 521.1521 | 9.74 | – | 62 ± 6 d |
| Calyxin c | C35H34O8 | 581.2181 | 9.83 | [68] | 14.6 ± 0.4 i |
| C15 isoprenoids c | C15H16O6 | 291.0874 | 10.11 | [68] | NQ |
| Verbascoside b,c | C29H36O15 | 623.1976 | 10.22 | [54] | 355 ± 14 e |
| p-coumaric acid a | C9H8O3 | 163.0401 | 10.26 | [54] | 2.20 ± 0.04 |
| 1-benzyl-[6-p-hydroxybenzoyl]-b-D-glucopyranosyl-(1–3)-b-D-glucopyranoside b,c or 2-(4-hydroxyphenyl)ethy1, 1-O-β-D-[5-O-(4-hydroxybenzoyl)]-apiofuranosyl-(1–6)-β-D-glucopyranoside b,c | C26H32O13 | 551.1770 | 10.40 | [66,69] | 26.0 ± 1.1 d |
| 3,4-dimethoxyphenyl 1-O-β-D-[5-O-(4- methoxybenzoyl)]-apiofuranosyl-(1–6)-β-D-glucopyranoside b,c | C27H34O14 | 581.1875 | 10.43 | [66] | 2.11 ± 0.09 d |
| Astragalin b,c | C21H20O11 | 447.0933 | 10.46 | [69] | 15.1 ± 0.8 j |
| 5,7-Dihydroxy-3’,4’-dimethoxyflavanone 7-rutinoside c | C29H36O15 | 623.1981 | 10.50 | [63] | 265 ± 20 i |
| Isoverbascoside b,c | C29H36O15 | 623.1976 | 10.53 | [54] | 279 ± 3 e |
| 6-O-(4-methoxybenzoyl)-5,7-bisdeoxycynanchoside b,c | C23H30O12 | 497.1664 | 10.56 | [66] | 5.8 ± 0.3 k |
| Veratic acid b,c | C9H10O4 | 181.0506 | 10.64 | [54,57] | 68 ± 7 n |
| C15 isoprenoid c | C15H16O6 | 291.0857 | 10.74 | – | NQ |
| Phenolic compound glycoside b,c | C27H34O15 | 597.1825 | 10.81 | [70] | 24 ± 3 |
| Veratic acid derivative c | – | 181.0506 * | 11.02 | – | 15 ± 3 n |
| 2-(4-hydroxyphenyl)ethyl, 1-O-β-D-[5-O-(3,4-dimethoxybenzoyl)]-apiofuranosyl-(1–6)-β-D-glucopyranoside b,c | C28H36O14 | 595.2031 | 11.04 | [66] | 70.3 ± 1.2 d |
| NI c | – | 539.1779 | 11.15 | – | 62 ± 2 d |
| Quercetin 5,7,3’,4’-tetramethyl ether Quercetin 5,7,3’,4’-tetramethyl ether 3-rutinoside c | C31H38O16 | 665.2087 | 11.27 | [63] | 230 ± 19 j |
| 2-(4-hydroxyphenyl)ethyl 1-O-β-D-[5-O-(4 methoxybenzoyl)]-apiofuranosyl-(1–6)-β-D-glucopyranoside b,c | C27H34O13 | 565.1926 | 11.49 | [66] | 11.8 ± 1.5 d |
| NI c | – | 527.1768 | 11.55 | – | 74.1 ± 1.4 d |
| Ferulic acid derivative c | – | 193.0506 * | 11.92 | – | 112 ± 15 h |
| Kaempferol deivative c | – | 285.0405 * | 12.24 | – | 3.0 ± 0.3 j |
| NI c | – | 327.2181 | 12.70 | – | NQ |
| NI c | – | 329.2345 | 13.14 | – | NQ |
| 5-hydroxy-2-(1-hydroxyethyl)naphtho [2,3-b]furan-4,9-dione or 8-hydroxy-2-(1-hydroxyethyl)naphtho [2,3-b]furan-4,9-dione b,c | C14H10O5 | 257.0455 | 13.37 | [71,72] | NQ |
| Alkyl hydroquinone or derivative c | C17H26O4 | 293.1758 | 14.72 | [63] | NQ |
| NI c | – | 385.2556 | 16.19 | – | NQ |
| NI c | – | 285.2042 | 16.42 | – | NQ |
| NI c | – | 311.2196 | 17.02 | – | NQ |
| NI c | – | 327.1776 | 17.14 | – | NQ |
| Compound | Formula | (M–H)− | RT, min | Refs. | Quantity Mean ± SD, μg/g DW |
|---|---|---|---|---|---|
| Chlorogenic acid isomer b,c | C16H18O9 | 353.0878 | 8.25 | [73,74,75] | 15 ± 2 f |
| Chlorogenic acid a | C16H18O9 | 353.0878 | 9.05 | [50,74] | 105 ± 4 |
| Caffeic acid a | C9H8O4 | 179.0350 | 9.39 | [73,76] | 45 ± 6 |
| Flavone or flavonol c | C25H28O12 | 519.1508 | 9.97 | [63] | 650 ± 33 d |
| p-coumaric acid a | C9H8O3 | 163.0401 | 10.26 | [73,74,76] | 256 ± 23 |
| Ferulic acid a | C10H10O4 | 193.0506 | 10.52 | [73,76] | 8.1 ± 0.6 |
| Dicaffeoylquinic acid isomer b,c | C25H24O12 | 515.1195 | 10.66 | [50,73,74,75] | 97 ± 9 e |
| Dicaffeoylquinic acid isomerb,c | C25H24O12 | 515.1195 | 10.81 | [50,73,74,75] | 97±6e |
| Dicaffeoylquinic acid isomer b,c | C25H24O12 | 515.1195 | 11.00 | [50,73,74,75] | 321 ± 27 e |
| Caffeic acid derivative b,c | – | 487.1563 | 11.39 | [73] | 32.9 ± 0.8 e |
| Luteolin methyl ether crotonylglucoside or luteolin glucoside methyl butanoate c | C26H26O12 | 529.1315 | 11.61 | [63] | 17.1 ± 1.2 j |
| Caffeic acid prenyl ester b,c | C14H16O4 | 247.0976 | 11.83 | [76] | 18.4 ± 1.3 e |
| Tricaffeoylquinic acid b,c | C34H30O15 | 677.1512 | 12.02 | [73] | 98 ± 9 e |
| Dimethyl-dicaffeoylquinic acid b,c | C27H28O12 | 543.1508 | 12.13 | [73] | 40 ± 4 e |
| Dimethyl-dicaffeoylquinic acid b,c | C27H28O12 | 543.1508 | 12.36 | [73] | 46 ± 6 e |
| 3,4-Dimethyl caffeic acid | C11H12O4 | 207.0663 | 12.43 | [76] | 13.6 ± 1.0 e |
| Chlorogenic acid derivative c | – | 353.0878 * | 12.55 | – | 4.2 ± 0.2 f |
| Chlorogenic acid derivative c | – | 353.0878 * | 12.71 | – | 3.3 ± 0.2 f |
| Naringenin a | C15H12O5 | 271.0612 | 12.96 | [73] | 27 ± 2 |
| Hesperetin b,c | C16H14O6 | 301.0718 | 13.04 | [73] | 270 ± 16 i |
| Kaempferol b,c | C15H10O6 | 285.0405 | 13.10 | [73,76] | 17 ± 2j |
| Isorhamnetin b,c | C16H12O7 | 315.0510 | 13.14 | [50,73,76] | 21 ± 3 j |
| Caffeic acid derivative b,c | – | 705.1835 | 13.31 | [73] | 16 ± 2 e |
| NI b,c | – | 301.1495 | 13.47 | [73] | 25 ± 2 d |
| Rhamnetin b,c | C16H12O7 | 315.0510 | 13.56 | [50,73] | 25 ± 3 j |
| 3,4-Dihydroxy 5-prenylcinnamic acid b,c | C14H16O4 | 247.0976 | 13.61 | [50,73,74,75] | 121 ± 8 g |
| NI b,c | – | 331.1613 | 13.72 | [73] | 140 ± 6 d |
| Coumaric acid prenyl ester b,c | C14H16O3 | 231.1027 | 13.99 | [73,76] | 301 ± 11 d |
| 4-Hydroxy-3-prenylcinnamic acid b,c | C14H16O3 | 231.1027 | 13.99 | [50,73,74,75] | 327 ± 24 g |
| Caffeic acid benzyl ester b,c | C16H14O4 | 269.0819 | 14.45 | [73,76] | 3.0 ± 0.3 e |
| Coumaric acid derivative b,c | – | 315.1601 | 14.50 | [73] | 56 ± 7 d |
| Kaempferide derivative b,c | – | 377.1957 | 14.60 | [73] | 36.5 ± 1.3 j |
| Sakuranetin b,c | C16H14O5 | 285.0768 | 14.63 | [73] | 23.3 ± 1.5 i |
| Chrysin b,c | C15H10O4 | 253.0506 | 14.65 | [50,76] | 10.1 ± 0.9 j |
| Pinocembrin b,c | C15H12O4 | 255.0663 | 14.68 | [50,73,76] | 10.1 ± 0.7 i |
| Kaempferide derivative b,c | – | 377.1953 | 14.75 | [73] | 59 ± 3 j |
| Galangin b,c | C15H10O5 | 269.0455 | 14.80 | [50,76] | 7.3 ± 0.5 j |
| Caffeic acid phenethyl ester b,c | C17H16O4 | 283.0976 | 14.83 | [50,73,76] | 7.4 ± 0.2 e |
| Kaempferide b,c | C16H12O6 | 299.0561 | 14.89 | [50,73,76] | 110 ± 11 j |
| Dimethoxyquercetin b,c | C17H14O7 | 329.0667 | 15.01 | [50] | 33.6 ± 0.5 j |
| Dicoumaric prenyl ester b,c | C23H22O6 | 393.1344 | 15.08 | [73,76] | 50 ± 6 d |
| Caffeic acid cinnamyl ester b,c | C18H16O4 | 295.0976 | 15.35 | [76] | 6.5 ± 0.2 d |
| Kaempferide derivative b,c | – | 529.1497 | 15.38 | [73] | 22.4 ± 0.2 j |
| Coumaric acid derivative b,c | – | 315.1600 | 15.44 | [73] | 52 ± 5 d |
| Baccharin b,c | C29H38O11 | 561.2341 | 15.61 | [50,74] | 1.5 ± 0.2 d |
| Artepillin C derivative b,c | – | 329.1780 | 15.72 | [73] | 156 ± 24 d |
| Coumaric acid derivative b,c | – | 559.1628 | 15.78 | [73] | 1.81 ± 0.02 d |
| Artepillin C b,c | C19H24O3 | 299.1653 | 16.39 | [50,73,74,75] | 414 ± 26 d |
| NI c | – | 613.2155 | 16.72 | – | NQ |
| NI c | – | 613.3219 | 17.50 | – | NQ |
| NI c | – | 627.2288 | 17.66 | – | NQ |
| C20 isoprenoid c | C20H30O2 | 301.2226 | 19.14 | [63] | NQ |
| S. aureus | K. pneumoniae | H. influenzae | ||||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | |
| Azithromycin (μg/mL) | 0.5 | >128 | 8 | >128 | 0.25 | >128 |
| Clarithromycin (μg/mL) | 0.25 | 128 | 64 | >128 | 0.5 | >128 |
| Amoxiclav (μg/mL) | 0.13 | 1.0 | 8.0 | 64 | 8 | >128 |
| GoImmune Strong® (mg/mL) | 0.78 | 1.56 | 12.5 | 12.5 | 3.13 | 12.5 |
| Antibiotic | Azithromycin | Clarithromycin | Amoxiclav | |
|---|---|---|---|---|
| Staphylococcus aureus | FICantib. | 0.25 | 0.06 | 0.5 |
| FICComp | 0.06 | 0.03 | 0.5 | |
| FICI | 0.31 | 0.09 | 1.0 | |
| Synergy a,b,c | Synergy a,b,c | Additive a, commutative c; no interaction b | ||
| Klebsiella pneumoniae | FICantib. | 1.0 | 0.5 | 0.5 |
| FICComp | 0.5 | 0.03 | 0.5 | |
| FICI | 1.5 | 0.53 | 1.0 | |
| No interaction b,c | Additive a; no interaction b; synergy c | Additive a, commutative c; no interaction b | ||
| Haemophilus influenzae | FICantib. | 0.12 | 0.25 | 0.13 |
| FICComp | 0.25 | 0.13 | 0.25 | |
| FICI | 0.37 | 0.38 | 0.38 | |
| Synergy a,b,c | Synergy a,b,c | Synergy a,b,c | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramata-Stunda, A.; Petriņa, Z.; Valkovska, V.; Borodušķis, M.; Gibnere, L.; Gurkovska, E.; Nikolajeva, V. Synergistic Effect of Polyphenol-Rich Complex of Plant and Green Propolis Extracts with Antibiotics against Respiratory Infections Causing Bacteria. Antibiotics 2022, 11, 160. https://doi.org/10.3390/antibiotics11020160
Ramata-Stunda A, Petriņa Z, Valkovska V, Borodušķis M, Gibnere L, Gurkovska E, Nikolajeva V. Synergistic Effect of Polyphenol-Rich Complex of Plant and Green Propolis Extracts with Antibiotics against Respiratory Infections Causing Bacteria. Antibiotics. 2022; 11(2):160. https://doi.org/10.3390/antibiotics11020160
Chicago/Turabian StyleRamata-Stunda, Anna, Zaiga Petriņa, Valda Valkovska, Mārtiņs Borodušķis, Līga Gibnere, Eleonora Gurkovska, and Vizma Nikolajeva. 2022. "Synergistic Effect of Polyphenol-Rich Complex of Plant and Green Propolis Extracts with Antibiotics against Respiratory Infections Causing Bacteria" Antibiotics 11, no. 2: 160. https://doi.org/10.3390/antibiotics11020160
APA StyleRamata-Stunda, A., Petriņa, Z., Valkovska, V., Borodušķis, M., Gibnere, L., Gurkovska, E., & Nikolajeva, V. (2022). Synergistic Effect of Polyphenol-Rich Complex of Plant and Green Propolis Extracts with Antibiotics against Respiratory Infections Causing Bacteria. Antibiotics, 11(2), 160. https://doi.org/10.3390/antibiotics11020160






