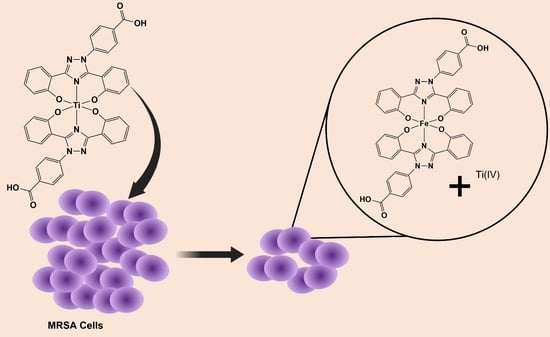
Exploring Titanium(IV) Complexes as Potential Antimicrobial Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sources of Metal Complexes
2.3. Instrumentation
2.4. MRC-5 Cell Viability Study of Dose–Response Treatment with Different Compounds by the MTT Assay
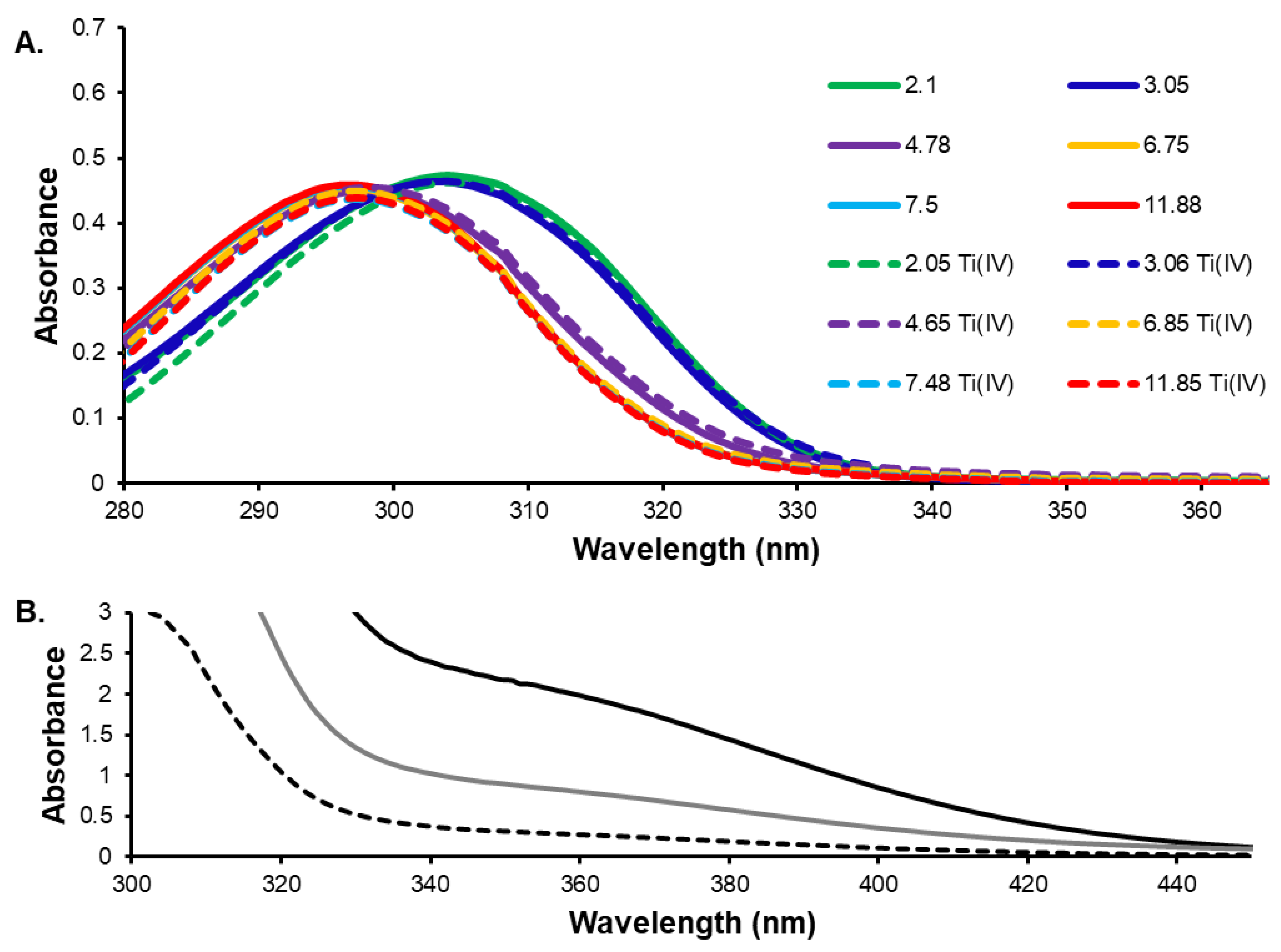
2.5. Solid-State and pH and Concentration Dependent Characterization of Ti(IV) Salicylate Complexation
2.6. Compound Solution Preparation by the CO-ADD
2.7. CO-ADD Antibacterial Assays
2.8. CO-ADD Antifungal Assays
2.9. CO-ADD Cytotoxicity Assays
2.10. CO-ADD Hemolysis Assays
3. Results
3.1. MRC-5 Cell Viability Study of Dose–Response Treatment with Different Compounds by the MTT Assay
3.2. Assesment of the Solution Behavior of the Ti(IV) Compounds
3.3. CO-ADD Screening Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunsaker, E.W.; Franz, K.J. Copper potentiates azole antifungal activity in a way that does not involve complex formation. Dalton Trans. 2019, 48, 9654–9662. [Google Scholar] [CrossRef] [PubMed]
- Zaengle-Barone, J.M.; Jackson, A.C.; Besse, D.M.; Becken, B.; Arshad, M.; Seed, P.C.; Franz, K.J. Copper Influences the Antibacterial Outcomes of a β-Lactamase-Activated Prochelator against Drug-Resistant Bacteria. ACS Infect. Dis. 2018, 4, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.C.; Zaengle-Barone, J.M.; Puccio, E.A.; Franz, K.J. A Cephalosporin Prochelator Inhibits New Delhi Metallo-β-lactamase 1 without Removing Zinc. ACS Infect. Dis. 2020, 6, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, A.; Yan, K.-C.; Mangel, D.N.; Shang, Y.; Steinbrueck, A.; Han, H.H.; Brewster, J.T.; Hu, X.-L.; Snelson, D.W.; Lynch, V.M.; et al. A Fluorescent Pro-Chelator Active Against Antibiotic Resistant Bacteria. ChemRxiv, 2020; in press. [Google Scholar] [CrossRef]
- Mjos, K.D.; Orvig, C. Metallodrugs in medicinal inorganic chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Bazzoli, F.; Delchier, J.-C.; Celiñski, K.; Giguère, M.; Rivière, M.; Mégraud, F. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: A randomised, open-label, non-inferiority, phase 3 trial. Lancet 2011, 377, 905–913. [Google Scholar] [CrossRef]
- De Boer, W.A.; Van Etten, R.J.; Van De Wouw, B.A.; Schneeberger, P.M.; Van Oijen, A.H.; Jansen, J.B. Bismuth-based quadruple therapy for Helicobacter pylori—A single triple capsule plus lansoprazole. Aliment. Pharmacol. Ther. 2000, 14, 85–89. [Google Scholar] [CrossRef]
- Barillo, D.J.; Barillo, A.R.; Korn, S.; Lam, K.; Attar, P.S. The antimicrobial spectrum of Xeroform®. Burns 2017, 43, 1189–1194. [Google Scholar] [CrossRef]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef]
- Barras, F.; Aussel, L.; Ezraty, B. Silver and Antibiotic, New Facts to an Old Story. Antibiotics 2018, 7, 79. [Google Scholar] [CrossRef]
- Ketikidis, I.; Banti, C.N.; Kourkoumelis, N.; Tsiafoulis, C.G.; Papachristodoulou, C.; Kalampounias, A.G.; Hadjikakou, S.K. Conjugation of Penicillin-G with Silver(I) Ions Expands Its Antimicrobial Activity against Gram Negative Bacteria. Antibiotics 2020, 9, 25. [Google Scholar] [CrossRef]
- Sierra, M.A.; Casarrubios, L.; de la Torre, M.C. Bio-Organometallic Derivatives of Antibacterial Drugs. Chemistry 2019, 25, 7232–7242. [Google Scholar] [CrossRef] [PubMed]
- Patra, M.; Gasser, G. The medicinal chemistry of ferrocene and its derivatives. Nat. Rev. Chem. 2017, 1, 0066. [Google Scholar] [CrossRef]
- Frei, A. Metal Complexes, an Untapped Source of Antibiotic Potential? Antibiotics 2020, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Blaskovich, M.A.T.; Zuegg, J.; Elliott, A.G.; Cooper, M.A. Helping Chemists Discover New Antibiotics. ACS Infect. Dis. 2015, 1, 285–287. [Google Scholar] [CrossRef]
- Loza-Rosas, S.A.; Saxena, M.; Delgado, Y.; Gaur, K.; Pandrala, M.; Tinoco, A.D. A ubiquitous metal, difficult to track: Towards an understanding of the regulation of titanium (IV) in humans. Metallomics 2017, 9, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; Loza Rosas, S.; Gaur, K.; Sharma, S.; Perez Otero, S.C.; Tinoco, A.D. Exploring titanium (IV) chemical proximity to iron (III) to elucidate a function for Ti (IV) in the human body. Coord. Chem. Rev. 2018, 363, 109–125. [Google Scholar] [CrossRef]
- Zierden, M.R.; Valentine, A.M. Contemplating a role for titanium in organisms. Metallomics 2016, 8, 9–16. [Google Scholar] [CrossRef]
- Cini, M.; Bradshaw, T.D.; Woodward, S. Using titanium complexes to defeat cancer: The view from the shoulders of titans. Chem. Soc. Rev. 2017, 46, 1040–1051. [Google Scholar] [CrossRef]
- Schur, J.; Manna, C.M.; Deally, A.; Koster, R.W.; Tacke, M.; Tshuva, E.Y.; Ott, I. A comparative chemical-biological evaluation of titanium(iv) complexes with a salan or cyclopentadienyl ligand. Chem. Commun. 2013, 49, 4785–4787. [Google Scholar] [CrossRef] [PubMed]
- Elie, B.T.; Fernandez-Gallardo, J.; Curado, N.; Cornejo, M.A.; Ramos, J.W.; Contel, M. Bimetallic titanocene-gold phosphane complexes inhibit invasion, metastasis, and angiogenesis-associated signaling molecules in renal cancer. Eur. J. Med. Chem. 2019, 161, 310–322. [Google Scholar] [CrossRef]
- Schilling, T.; Keppler, K.B.; Heim, M.E.; Niebch, G.; Dietzfelbinger, H.; Rastetter, J.; Hanauske, A.-R. Clinical phase I and pharmacokinetic trial of the new titanium complex budotitane. Investig. New Drugs 1996, 13, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Lummen, G.; Sperling, H.; Luboldt, H.; Otto, T.; Rubben, H. Phase II trial of titanocene dichloride in advanced renal-cell carcinoma. Cancer Chemother. Pharmacol. 1998, 42, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sharma, R.K.; Gaur, K.; Cátala Torres, J.F.; Loza-Rosas, S.A.; Torres, A.; Saxena, M.; Julin, M.; Tinoco, A.D. Fueling a Hot Debate on the Application of TiO2 Nanoparticles in Sunscreen. Materials 2019, 12, 2317. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.K.; Lyon, D.Y.; Alvarez, P.J. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006, 40, 3527–3532. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.L.; Leung, W.K.; Yao, N.; Cao, S. Reactivity and antimicrobial properties of nanostructured titanium dioxide. Catal. Today 2009, 143, 218–224. [Google Scholar] [CrossRef]
- Schwietert, C.W.; McCue, J.P. Coordination compounds in medicinal chemistry. Coord. Chem. Rev. 1999, 184, 67–89. [Google Scholar] [CrossRef]
- Kubo, M.; Onodera, R.; Shibasaki-Kitakawa, N.; Tsumoto, K.; Yonemoto, T. Kinetics of ultrasonic disinfection of Escherichia coli in the presence of titanium dioxide particles. Biotechnol. Prog. 2005, 21, 897–901. [Google Scholar] [CrossRef] [PubMed]
- George, S.A.; Raj, M.S.; Solomon, D.; Roselin, P. A Comparative Study of the Anti-Fungal Activity of Zinc Oxide and Titanium Dioxide Nano and Bulk Particles with Anti-Fungals against Fungi Isolated from Infected Skin and Dandruff Flakes. Res. Rev. J. Microbiol. Biotechnol. 2014, 3, 23–30. [Google Scholar]
- Ahmad, N.S.; Abdullah, N.; Yasin, F.M. Antifungal Activity of Titanium Dioxide Nanoparticles against Candida albicans. Bioresources 2019, 14, 8866–8878. [Google Scholar]
- Kermani, S.A.; Salari, S.; Almani, P.G.N. Comparison of antifungal and cytotoxicity activities of titanium dioxide and zinc oxide nanoparticles with amphotericin B against different Candida species: In vitro evaluation. J. Clin. Lab. Anal. 2021, 35, e23577. [Google Scholar] [CrossRef]
- Duffy, B.; Schwietert, C.; France, A.; Mann, N.; Culbertson, K.; Harmon, B.; McCue, J.P. Transition metals as protease inhibitors. Biol. Trace Elem. Res. 1998, 64, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, S.; Schwietert, C.W.; McCue, J.P. Biological roles of titanium. Biol. Trace Elem. Res. 2000, 78, 205–217. [Google Scholar] [CrossRef]
- Van de Velde, G.M.H. The oxalato complexes of titanium (IV)—I: Mononuclear Ti(OH)2(C2O4)22− in solution. J. Inorg. Nucl. Chem. 1977, 39, 1357–1362. [Google Scholar] [CrossRef]
- Schwietert, C.W.; Yaghoubi, S.; Gerber, N.C.; McSharry, J.J.; McCue, J.P. Dietary titanium and infant growth. Biol. Trace Elem. Res. 2001, 83, 149–167. [Google Scholar] [CrossRef]
- Loza-Rosas, S.A.; Vázquez-Salgado, A.M.; Rivero, K.I.; Negrón, L.J.; Delgado, Y.; Benjamín-Rivera, J.A.; Vázquez-Maldonado, A.L.; Parks, T.B.; Munet-Colón, C.; Tinoco, A.D. Expanding the therapeutic potential of the iron chelator deferasirox in the development of aqueous stable Ti(IV) anticancer complexes. Inorg. Chem. 2017, 56, 7788–7802. [Google Scholar] [CrossRef]
- Tinoco, A.D.; Thomas, H.R.; Incarvito, C.D.; Saghatelian, A.; Valentine, A.M. Cytotoxicity of a Ti (IV) compound is independent of serum proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 5016–5021. [Google Scholar] [CrossRef]
- Tinoco, A.D.; Eames, E.V.; Incarvito, C.D.; Valentine, A.M. Hydrolytic Metal with a Hydrophobic Periphery: Titanium (IV) Complexes of Naphthalene-2,3-diolate and Interactions with Serum Albumin. Inorg. Chem. 2008, 47, 8380–8390. [Google Scholar] [CrossRef]
- Dey, R.; Mukharjee, K.; Langer, V.; Roychowdhury, P. A titanium salicylate, Na4[Ti(C7H4O3)3]2·11H2O. Acta Crystallogr. Sect. E Struct. Rep. Online 2005, 61, m1495–m1497. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Deng, Y.F.; Jiang, Y.Q.; Wan, H.L.; Ng, S.W. The first structural examples of tricitratotitanate [Ti(H2cit)3]2− dianions. Dalton Trans. 2003, 13, 2636–2638. [Google Scholar] [CrossRef]
- Fernandez-Vega, L.; Loza Rosas, S.A.; Maser-Figueroa, A.N.; Rodriguez, I.; Alvarez-Carillo, V.; Morales-Rodriguez, S.; Zayas-Ortiz, A.; Mendez-Fernandez, A.P.; Vazquez-Maldonado, A.L.; Ebenki, V.B.; et al. A potential copper (II) contribution to the anticancer transmetallation mechanism of Ti(Deferasirox)2. 2022; Unpublished work. [Google Scholar]
- Tinoco, A.D.; Valentine, A.M. Ti (IV) Binds to Human Serum Transferrin More Tightly Than Does Fe (III). J. Am. Chem. Soc. 2005, 127, 11218–11219. [Google Scholar] [CrossRef]
- Tinoco, A.D.; Saxena, M.; Sharma, S.; Noinaj, N.; Delgado, Y.; Gonzalez, E.P.Q.; Conklin, S.E.; Zambrana, N.; Loza-Rosas, S.A.; Parks, T.B. Unusual synergism of transferrin and citrate in the regulation of Ti (IV) speciation, transport, and toxicity. J. Am. Chem. Soc. 2016, 138, 5659–5665. [Google Scholar] [CrossRef]
- Van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Toney, J.H.; Marks, T.J. Hydrolysis chemistry of the metallocene dichlorides M(η5-C5H5)2Cl2, M = titanium, vanadium, or zirconium. Aqueous kinetics, equilibria, and mechanistic implications for a new class of antitumor agents. J. Am. Chem. Soc. 1985, 107, 947–953. [Google Scholar] [CrossRef]
- Hall, M.D.; Telma, K.A.; Chang, K.-E.; Lee, T.D.; Madigan, J.P.; Lloyd, J.R.; Goldlust, I.S.; Hoeschele, J.D.; Gottesman, M.M. Say no to DMSO: Dimethylsulfoxide inactivates cisplatin, carboplatin, and other platinum complexes. Cancer Res. 2014, 74, 3913–3922. [Google Scholar] [CrossRef] [PubMed]
- Plumb, J.A.; Milroy, R.; Kaye, S.B. Effects of the pH dependence of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer Res. 1989, 49, 4435–4440. [Google Scholar] [PubMed]
- Tinoco, A.D.; Incarvito, C.D.; Valentine, A.M. Calorimetric, Spectroscopic, and Model Studies Provide Insight into the Transport of Ti (IV) by Human Serum Transferrin. J. Am. Chem. Soc. 2007, 129, 3444–3454. [Google Scholar] [CrossRef] [PubMed]
- Parks, T.B.; Cruz, Y.M.; Tinoco, A.D. Applying the Fe (III) Binding Property of a Chemical Transferrin Mimetic to Ti(IV) Anticancer Drug Design. Inorg. Chem. 2014, 53, 1743–1749. [Google Scholar] [CrossRef]
- Collins, J.M.; Uppal, R.; Incarvito, C.D.; Valentine, A.M. Titanium (IV) Citrate Speciation and Structure under Environmentally and Biologically Relevant Conditions. Inorg. Chem. 2005, 44, 3431–3440. [Google Scholar] [CrossRef]
- Panagiotidis, P.; Kefalas, E.T.; Raptopoulou, C.P.; Terzis, A.; Mavromoustakos, T.; Salifoglou, A. Delving into the complex picture of Ti (IV)-citrate speciation in aqueous media: Synthetic, structural, and electrochemical considerations in mononuclear Ti(IV) complexes containing variably deprotonated citrate ligands. Inorg. Chim. Acta 2008, 361, 2210–2224. [Google Scholar] [CrossRef]
- Tinoco, A.D.; Eames, E.V.; Valentine, A.M. Reconsideration of serum Ti (IV) transport: Albumin and transferrin trafficking of Ti(IV) and its complexes. J. Am. Chem. Soc. 2008, 130, 2262–2270. [Google Scholar] [CrossRef]
- Gritsenko, V.A.; Ajsuvakova, O.P.; Tinkov, A.A.; Bezryadin, S.G.; Gatiatulina, E.R.; Ivanova, V.Y.; Chevela, V.V.; Nikonorov, A.A. The effect of the Ti(IV)-citrate complex on Staphylococcus aureus growth and biofilm formation. Arch. Biol. Sci. 2015, 67, 981–992. [Google Scholar] [CrossRef]
- Van De Velde, G.; Harkema, S.; Gellings, P.J. The crystal and molecular structure of ammonium titanyl oxalate. Inorg. Chim. Acta 1974, 11, 243–252. [Google Scholar] [CrossRef][Green Version]
- Haddad, M.; Brisse, F. Sels alcalins des complexes oxalato titanates (IV); 2, La structure cristalline de l’oxalato titanate (IV) de potassium hydrate K2[TiO(CO2O4)2].2.25 H2O. Can. Miner. 1978, 16, 379–385. [Google Scholar]
- Fester, A.; Bensch, W.; Trömel, M. Dipotassium bis(oxalato)oxotitanate(IV) dihydrate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1994, 50, 850–852. [Google Scholar] [CrossRef]
- Masato, K.; Makoto, K.; Koji, T.; Valery, P. Application of Water-Soluble Titanium Complexes as Precursors for Synthesis of Titanium-Containing Oxides via Aqueous Solution Processes. Bull. Chem. Soc. Jpn. 2010, 83, 1285–1308. [Google Scholar] [CrossRef]
- Gaur, K.; Pérez Otero, S.C.; Benjamín-Rivera, J.A.; Rodríguez, I.; Loza-Rosas, S.A.; Vázquez Salgado, A.M.; Akam, E.A.; Hernández-Matias, L.; Sharma, R.K.; Alicea, N.; et al. Iron Chelator Transmetalative Approach to Inhibit Human Ribonucleotide Reductase. JACS Au 2021, 1, 865–878. [Google Scholar] [CrossRef]
- Fu, Y.-L.; Xu, Z.-W.; Ren, J.-L.; Ng, S.W. Bis(dimethylammonium)mer-tris(salicylato-κ2O,O′) titanate(IV). Acta Crystallogr. Sect. E Struct. Rep. Online 2005, 61, m1730–m1732. [Google Scholar] [CrossRef]
- Gigant, K.; Rammal, A.; Henry, M. Synthesis and Molecular Structures of Some New Titanium (IV) Aryloxides. J. Am. Chem. Soc. 2001, 123, 11632–11637. [Google Scholar] [CrossRef]
- Borgias, B.A.; Cooper, S.R.; Koh, Y.B.; Raymond, K.N. Synthetic, structural, and physical studies of titanium complexes of catechol and 3,5-di-tert-butylcatechol. Inorg. Chem. 1984, 23, 1009–1016. [Google Scholar] [CrossRef]
- Faraji, M.; Farajtabar, A.; Gharib, F.; Ghasemnejad-Borsa, H. Deprotonation of salicylic acid and 5-nitrosalicylic acid in aqueous solutions of ethanol. J. Serb. Chem. Soc. 2011, 76, 1455–1463. [Google Scholar] [CrossRef]
- Perrin, D.D. Stability of Metal Complexes with Salicylic Acid and Related Substances. Nature 1958, 182, 741–742. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W., Jr. The 2019 Cosmetic Ingredient Review Expert Panel: Safety Assessment of Titanium Complexes as Used in Cosmetics; Personal Care Products Council: Washington, DC, USA, 2019. [Google Scholar]
- Yasuyuki, M.; Kunihiro, K.; Kurissery, S.; Kanavillil, N.; Sato, Y.; Kikuchi, Y. Antibacterial properties of nine pure metals: A laboratory study using Staphylococcus aureus and Escherichia coli. Biofouling 2010, 26, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Ullah, K.; Khan, S.A.; Mannan, A.; Khan, R.; Murtaza, G.; Yameen, M.A. Enhancing the Antibacterial Activity of Erythromycin with Titanium Dioxide Nanoparticles against MRSA. Curr. Pharm. Biotechnol. 2020, 21, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.L.; Tin, A.S.; Lim, W.W.; Lim, J.; Kurup, A.; Ling, M.L.; Tan, A.L.; Ong, B.C. Efficacy of titanium dioxide compounds in preventing environmental contamination by meticillin resistant Staphylococcus aureus (MRSA). Int. J. Infect. Control. 2013, 9, 1–9. [Google Scholar] [CrossRef]
- Roy, A.S.; Parveen, A.; Koppalkar, A.R.; Prasad, M.V.N.A. Effect of Nano—Titanium Dioxide with Different Antibiotics against Methicillin-Resistant Staphylococcus aureus. J. Biomater. Nanobiotechnol. 2010, 1, 37–41. [Google Scholar] [CrossRef]
- Sedgwick, A.C.; Yan, K.C.; Mangel, D.N.; Shang, Y.; Steinbrueck, A.; Han, H.H.; Brewster, J.T., 2nd; Hu, X.L.; Snelson, D.W.; Lynch, V.M.; et al. Deferasirox (ExJade): An FDA-Approved AIEgen Platform with Unique Photophysical Properties. J. Am. Chem. Soc. 2021, 143, 1278–1283. [Google Scholar] [CrossRef]
- Masalha, M.; Borovok, I.; Schreiber, R.; Aharonowitz, Y.; Cohen, G. Analysis of transcription of the Staphylococcus aureus aerobic class Ib and anaerobic class III ribonucleotide reductase genes in response to oxygen. J. Bacteriol. 2001, 183, 7260–7272. [Google Scholar] [CrossRef]
- Rabinovitch, I.; Yanku, M.; Yeheskel, A.; Cohen, G.; Borovok, I.; Aharonowitz, Y. Staphylococcus aureus NrdH redoxin is a reductant of the class Ib ribonucleotide reductase. J. Bacteriol. 2010, 192, 4963–4972. [Google Scholar] [CrossRef]
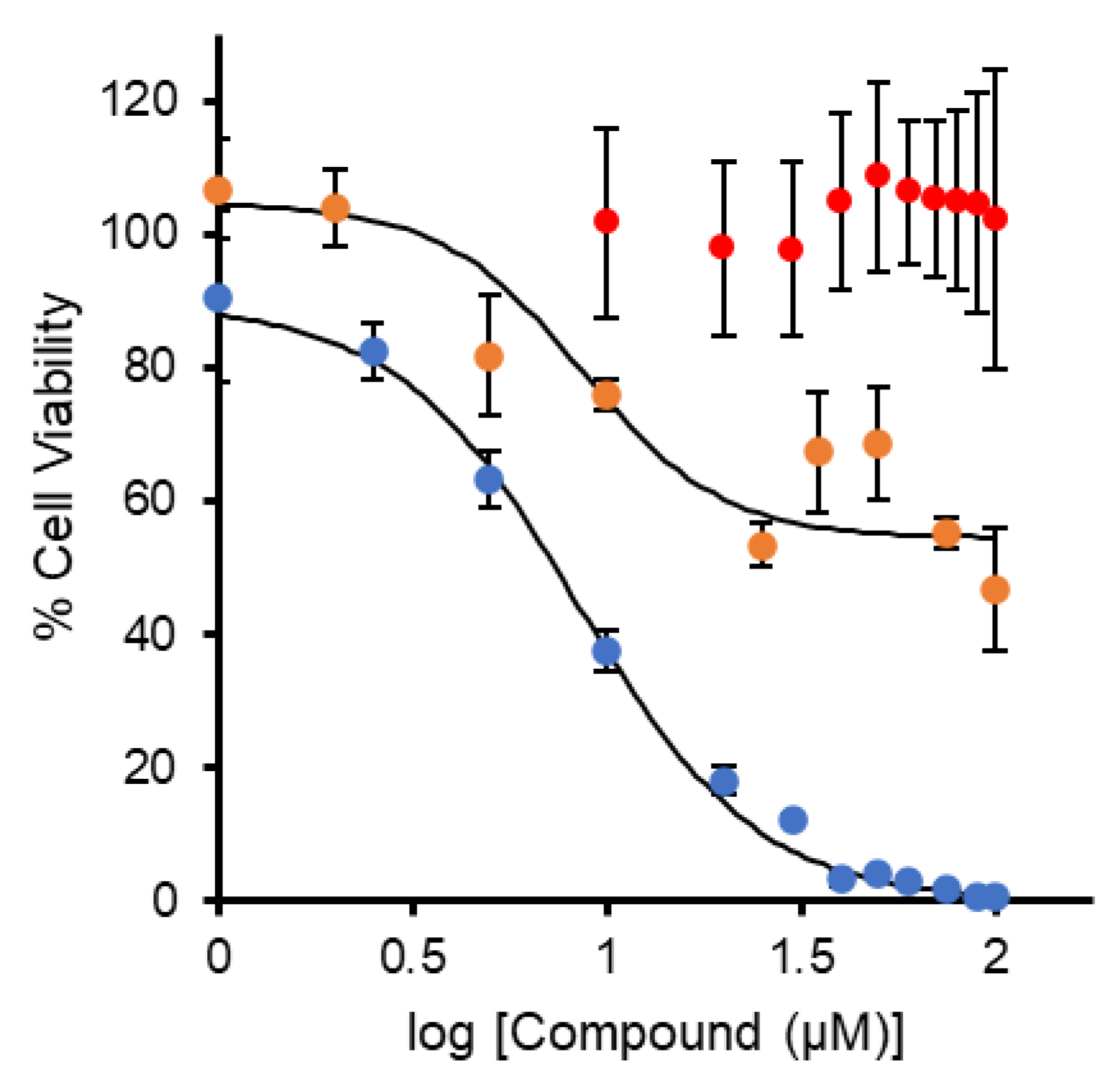
| Compound | IC50 ± SD (µM) | IC50 ± SD (µg/mL) | Ref. |
|---|---|---|---|
| Ti(Deferasirox)2 (10) | 25 ± 1 | 19.8 ± 0.8 | [36] |
| [Cu(Def)(H2O)]2 (15) | 8.40 ± 1.62 | 7.70 ± 1.47 | This work |
| Deferasirox a (4) | Proliferative Behavior | [36] | |
| Ti(BHPT)2 (9) | 2.4 b | 1.5 | [36] |
| BHPT (3) | >>100 | >>25.3 | [36] |
| Ti(HBED) (11) | 42.0 ± 4.4 | 18.5 ± 1.9 | [49] |
| HBED a (5) | >>100 | >>44.3 | [49] |
| K2[Ti(naphthalene-2,3-diolate)3] (8) | >>100 | >>65.5 | This work |
| 2,3-dihydroxynaphthalene (2) | >>100 | >>16.0 | This work |
| Titanocene Dichloride (13) | >>100 | >>24.9 | This work |
| K2[TiO(Oxalate)2] (14) | >>100 | >>35.4 | This work |
| Na2[Ti(Salicylate)3] (12) | 8.60 ± 1.54 c | 5.14 ± 0.92 | This work |
| Salicylic Acid a (6) | >>100 | >>13.8 | This work |
| K2[Ti(Citrate)3] (7) | >>100 | >>73.2 | This work |
| Na3Citrate (1) | >>100 | >>29.4 | This work |
| G+ve | G−ve | Fungi | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus (MRSA) | A. baumannii | E. coli | K. pneumoniae | P. aeruginosa | C. albicans | C. neoformans | ||||||||
| MIC | Dmax | MIC | Dmax | MIC | Dmax | MIC | Dmax | MIC | Dmax | MIC | Dmax | MIC | Dmax | |
| 1 | >32 | 10.59 | >32 | 18 | >32 | 6.14 | >32 | 14.22 | >32 | 10.27 | >32 | 0.28 | >32 | −8.24 |
| 2 | >32 | 12.55 | >32 | 31.32 | >32 | 6.86 | >32 | 26.77 | >32 | 14.61 | >32 | 16.32 | >32 | −9.97 |
| 3 | >32 | −2.34 | >32 | −1.93 | >32 | −9.07 | >32 | 4.11 | >32 | 10.03 | >32 | 6.23 | >32 | −7.23 |
| 4 | >32 | 44.31 | >32 | 18.95 | >32 | 7.72 | >32 | 20.2 | >32 | 5.82 | >32 | 4.31 | >32 | −25.58 |
| 5 | >32 | 24.28 | >32 | 68.29 | >32 | 51.65 | >32 | 52.86 | >32 | 69.01 | >32 | 3.5 | >32 | −17.49 |
| 6 | >32 | 13.86 | >32 | 17.87 | >32 | −0.01 | >32 | 17.1 | >32 | 9.76 | >32 | 30.95 | >32 | −10.66 |
| 7 | >32 | 38.01 | >32 | 17.94 | >32 | 10.41 | >32 | 15.43 | >32 | 7.99 | >32 | 44.53 | >32 | −7.88 |
| 8 | >32 | 52.5 | >32 | 24.76 | >32 | 2.67 | >32 | 22.94 | >32 | 13.43 | >32 | 57.57 | >32 | −1.45 |
| 9 | >32 | 7.82 | >32 | 1.55 | >32 | −0.56 | >32 | 6.35 | >32 | 10.27 | >32 | 4.27 | >32 | −1.7 |
| 10 | 16 | 98.2 | >32 | 16.28 | >32 | 20.44 | >32 | 34.61 | >32 | 7.57 | >32 | 5.74 | >32 | −5.72 |
| 11 | >32 | 61.46 | >32 | 68.73 | >32 | 48.02 | >32 | 56.3 | >32 | 72.48 | >32 | 10.43 | >32 | −18.86 |
| 12 | >32 | 3.66 | >32 | 9.4 | >32 | 2.6 | >32 | 12.46 | >32 | 9.57 | >32 | 49.3 | >32 | −5.2 |
| 13 | >32 | 51.55 | >32 | 15.78 | >32 | 23.76 | >32 | 16.65 | >32 | 16.01 | >32 | 16.15 | >32 | 6.5 |
| 14 | >32 | 40.5 | >32 | 8.2 | >32 | 11.0 | >32 | 17.2 | >32 | 7.5 | 32 | 77.3 | >32 | ND |
| 15 | >32 | 26.9 | >32 | 7.8 | >32 | −9.5 | >32 | 7.7 | >32 | 19.2 | >32 | 34.0 | >32 | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, I.; Fernández-Vega, L.; Maser-Figueroa, A.N.; Sang, B.; González-Pagán, P.; Tinoco, A.D. Exploring Titanium(IV) Complexes as Potential Antimicrobial Compounds. Antibiotics 2022, 11, 158. https://doi.org/10.3390/antibiotics11020158
Rodríguez I, Fernández-Vega L, Maser-Figueroa AN, Sang B, González-Pagán P, Tinoco AD. Exploring Titanium(IV) Complexes as Potential Antimicrobial Compounds. Antibiotics. 2022; 11(2):158. https://doi.org/10.3390/antibiotics11020158
Chicago/Turabian StyleRodríguez, Israel, Lauren Fernández-Vega, Andrea N. Maser-Figueroa, Branlee Sang, Patricia González-Pagán, and Arthur D. Tinoco. 2022. "Exploring Titanium(IV) Complexes as Potential Antimicrobial Compounds" Antibiotics 11, no. 2: 158. https://doi.org/10.3390/antibiotics11020158
APA StyleRodríguez, I., Fernández-Vega, L., Maser-Figueroa, A. N., Sang, B., González-Pagán, P., & Tinoco, A. D. (2022). Exploring Titanium(IV) Complexes as Potential Antimicrobial Compounds. Antibiotics, 11(2), 158. https://doi.org/10.3390/antibiotics11020158