Thymus zygis Essential Oil: Phytochemical Characterization, Bioactivity Evaluation and Synergistic Effect with Antibiotics against Staphylococcus aureus
Abstract
1. Introduction
2. Results
2.1. T. zygis EO Chemical Composition
2.2. T. zygis EO Antioxidant Activity
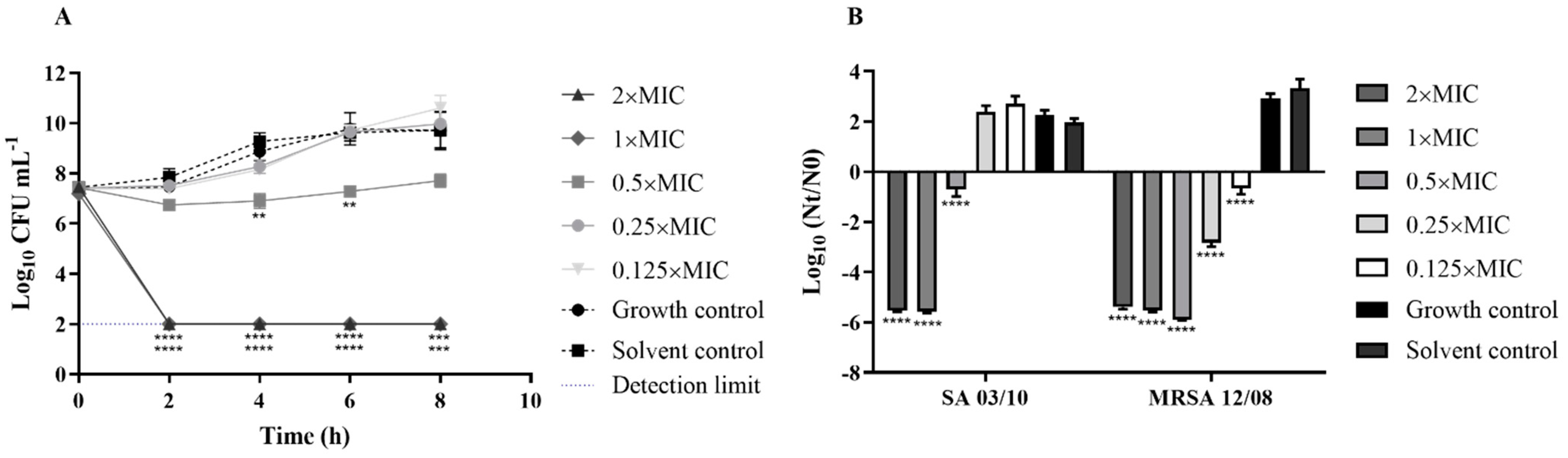
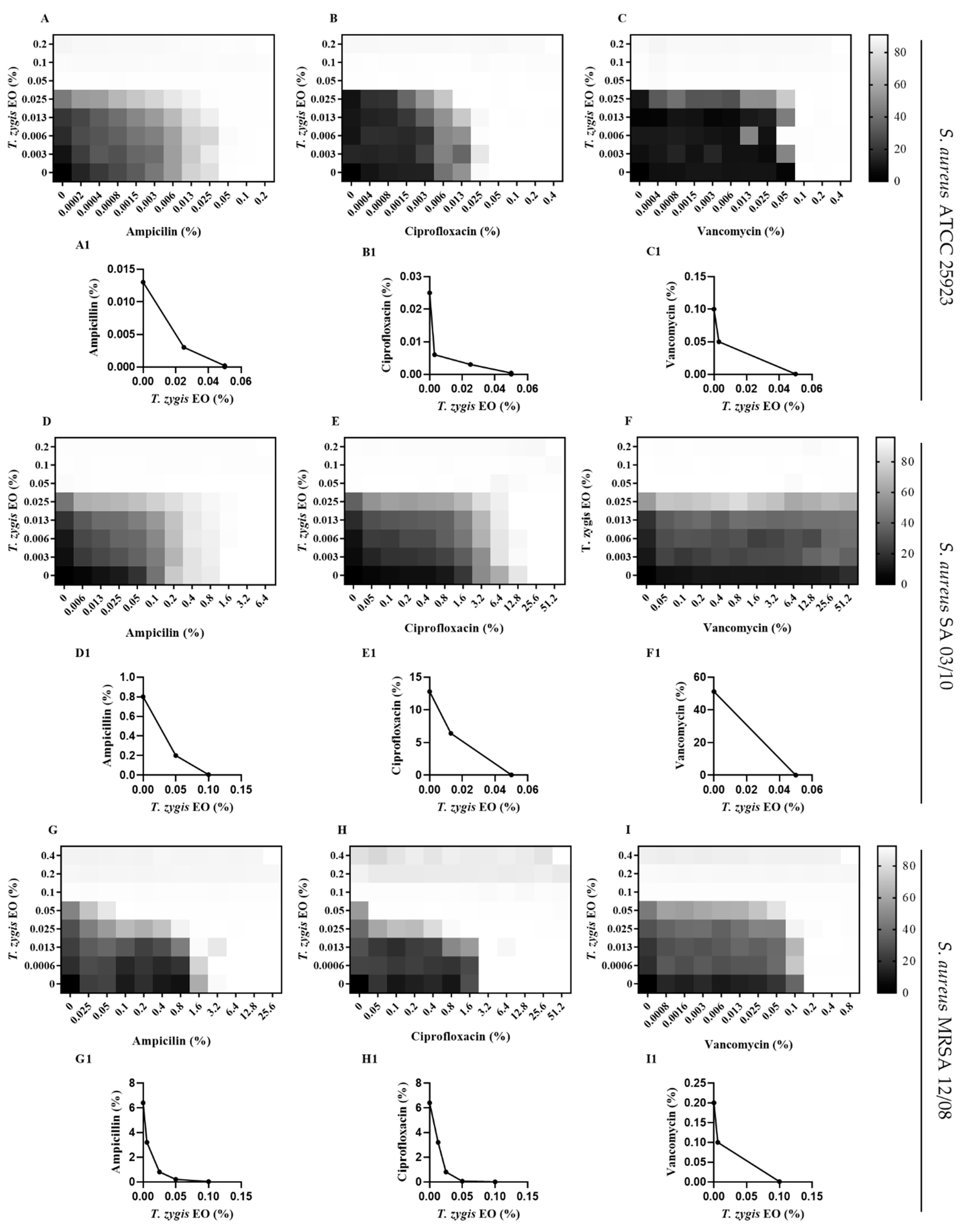
2.3. T. zygis EO Antibacterial Activity
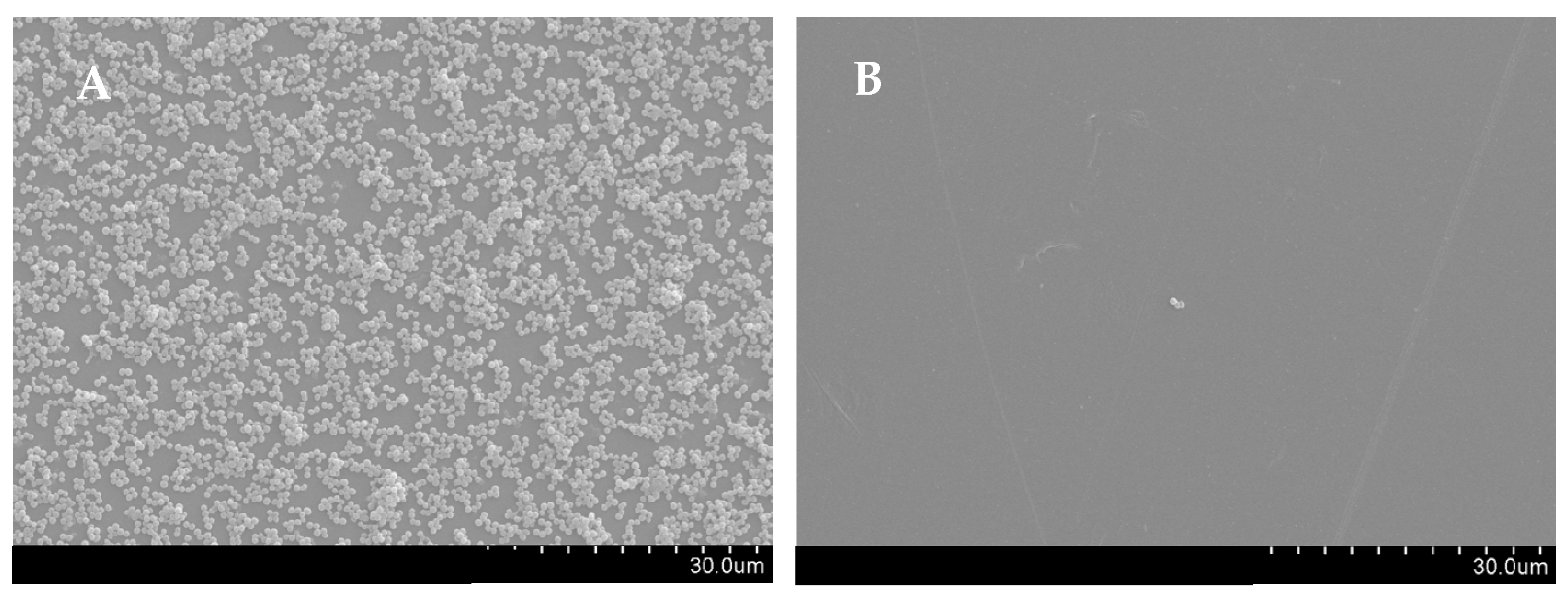
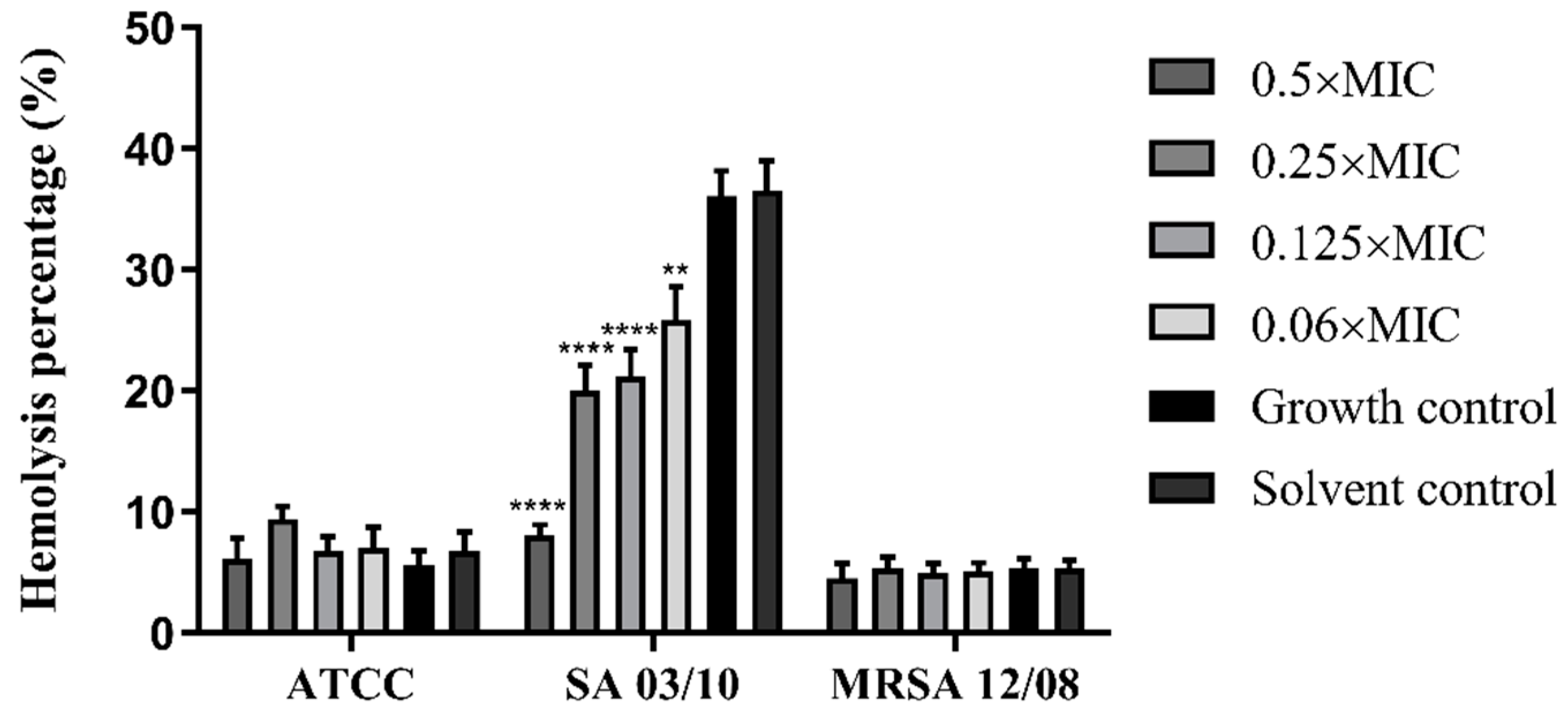
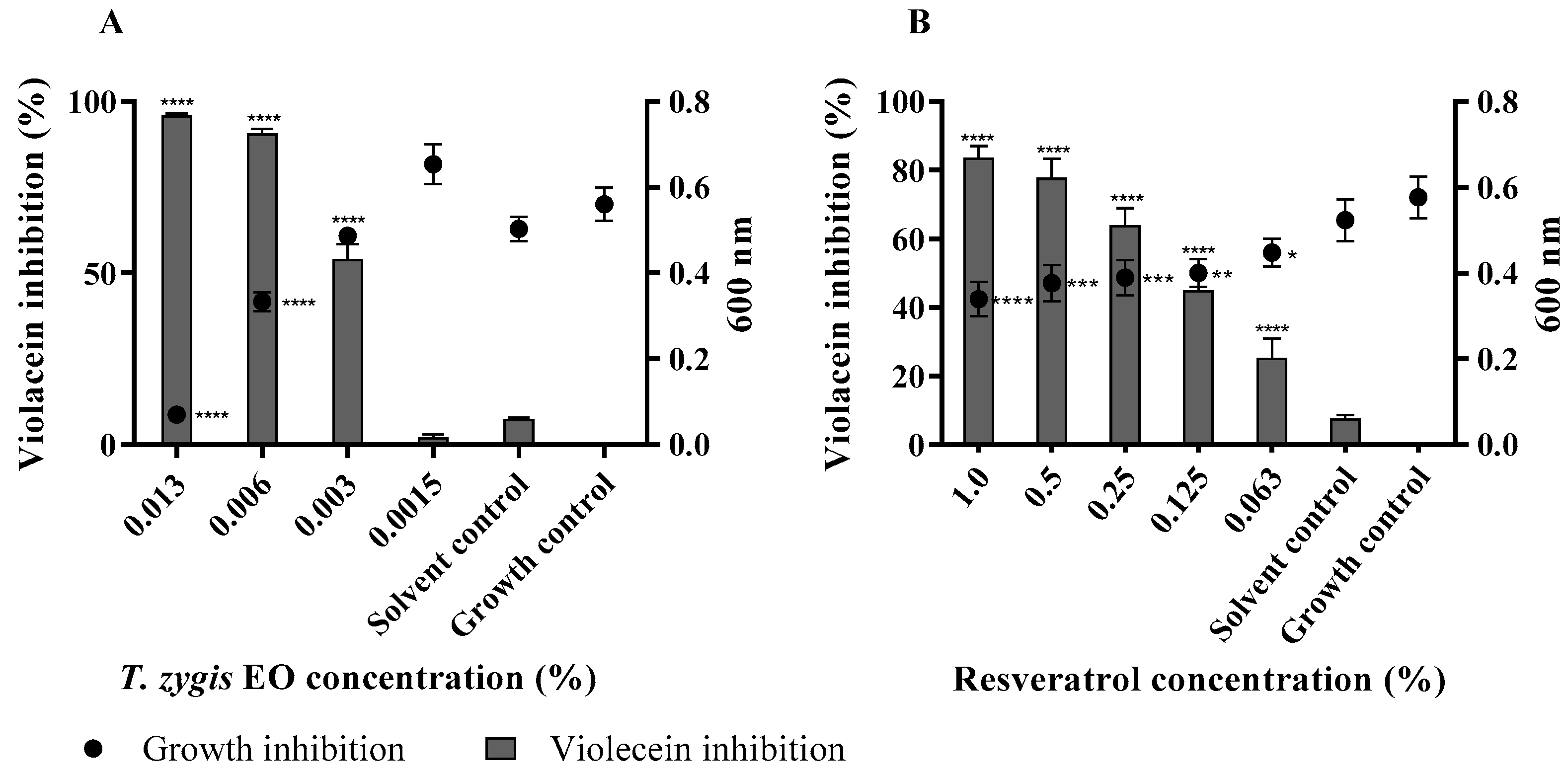
2.4. T. zygis EO Anti-Virulence Activity
3. Discussion
4. Materials and Methods
4.1. Essential Oil and Bacterial Strains
4.2. GC-MS Analysis of the T. zygis Essential Oil
4.3. Antioxidant Activity
4.4. Antimicrobial Activity
4.4.1. Disc-Diffusion Method and Vapour-Phase Antimicrobial Activity Determination
4.4.2. Determination of the Minimum Inhibitory Concentration (MIC)
4.4.3. Time–Kill Curves
4.4.4. Antibiofilm Activity of T. zygis EO
Biofilm Formation
Biofilm Dispersion
4.4.5. Inhibition of Quorum Sensing
4.4.6. Scanning Electron Microscopy (SEM)
4.4.7. Checkerboard Assay
4.4.8. Effect of the T. zygis EO on the Haemolytic Capacity of S. aureus
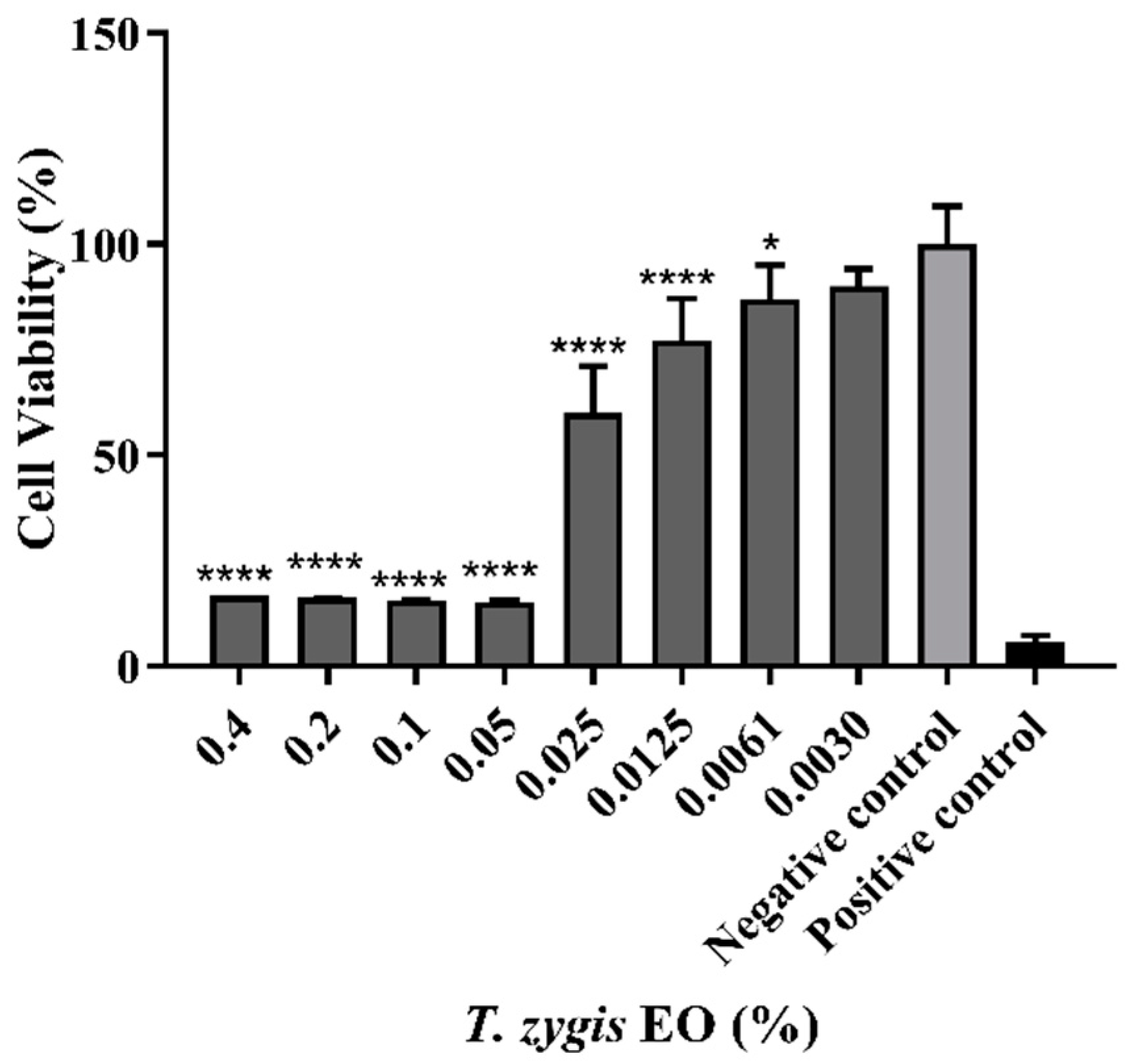
4.5. Evaluation of T. zygis EO Biocompatibility
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, S.C.; Liu, F.; Zhu, K.; Shen, J.Z. Natural products that target virulence factors in antibiotic-resistant Staphylococcus aureus. J. Agric. Food Chem. 2019, 67, 13195–13211. [Google Scholar] [CrossRef] [PubMed]
- Owen, L.; Laird, K. Synchronous application of antibiotics and essential oils: Dual mechanisms of action as a potential solution to antibiotic resistance. Crit. Rev. Microbiol. 2018, 44, 414–435. [Google Scholar] [CrossRef] [PubMed]
- Langeveld, W.T.; Veldhuizen, E.J.A.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, J.H.; Kim, S.I.; Cho, M.H.; Lee, J. Anti-biofilm, anti-hemolysis, and anti-virulence activities of black pepper, cananga, myrrh oils, and nerolidol against Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2014, 98, 9447–9457. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.G.; Park, J.G.; Lee, J. Supercritical fluid extracts of Moringa oleifera and their unsaturated fatty acid components inhibit biofilm formation by Staphylococcus aureus. Food Control 2017, 80, 74–82. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Dong, J.; Wei, J.; Wang, Y.; Dai, X.; Wang, X.; Luo, M.; Tan, W.; Deng, X.; et al. Inhibition of α-toxin production by subinhibitory concentrations of naringenin controls Staphylococcus aureus pneumonia. Fitoterapia 2013, 86, 92–99. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcus aureus toxins. Curr. Opin. Microbiol. 2014, 17, 32–37. [Google Scholar] [CrossRef]
- Vandenesch, F.; Lina, G.; Henry, T. Staphylococcus aureus hemolysins, bi-component leukocidins, and cytolytic peptides: A redundant arsenal of membrane-damaging virulence factors? Front. Cell. Infect. Microbiol. 2012, 2, 12. [Google Scholar] [CrossRef]
- Singh, V.; Phukan, U.J. Interaction of host and Staphylococcus aureus protease-system regulates virulence and pathogenicity. Med. Microbiol. Immunol. 2019, 208, 585–607. [Google Scholar] [CrossRef]
- Quave, C.L.; Horswill, A.R. Flipping the switch: Tools for detecting small molecule inhibitors of staphylococcal virulence. Front. Microbiol. 2014, 5, 706. [Google Scholar] [CrossRef]
- Korem, M.; Gov, Y.; Shirron, N.; Shuster, A.; Rosenberg, M. Alcohol increases hemolysis by staphylococci. FEMS Microbiol. Lett. 2007, 269, 153–159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO’s First global Report on Antibiotic Resistance Reveals Serious, Worldwide Threat to Public Health. Available online: https://www.who.int/news/item/30-04-2014-who-s-first-global-report-on-antibiotic-resistance-reveals-serious-worldwide-threat-to-public-health. (accessed on 9 December 2021).
- Ju, J.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Synergistic interactions of plant essential oils with antimicrobial agents: A new antimicrobial therapy. Crit. Rev. Food Sci. Nutr. 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Ucha, J.C.; Martínez-Guitián, M.; Lasarte-Monterrubio, C.; Conde-Pérez, K.; Arca-Suárez, J.; Álvarez-Fraga, L.; Pérez, A.; Crecente-Campo, J.; Alonso, M.J.; Bou, G.; et al. Syzygium aromaticum (clove) and Thymus zygis (thyme) essential oils increase susceptibility to colistin in the nosocomial pathogens Acinetobacter baumannii and Klebsiella pneumoniae. Biomed. Pharmacother. 2020, 130, 110606. [Google Scholar] [CrossRef] [PubMed]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Trifan, A.; Luca, S.V.; Greige-Gerges, H.; Miron, A.; Gille, E.; Aprotosoaie, A.C. Recent advances in tackling microbial multidrug resistance with essential oils: Combinatorial and nano-based strategies. Crit. Rev. Microbiol. 2020, 46, 338–357. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; Sanches-Silva, A.; de Melo, N.R. Essential oils for food application: Natural substances with established biological activities. Food Bioprocess. Technol. 2018, 11, 43–71. [Google Scholar] [CrossRef]
- Cutillas, A.B.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Thyme essential oils from Spain: Aromatic profile ascertained by GC–MS, and their antioxidant, anti-lipoxygenase and antimicrobial activities. J. Food Drug Anal. 2018, 26, 529–544. [Google Scholar] [CrossRef]
- Gonçalves, M.J.; Cruz, M.T.; Cavaleiro, C.; Lopes, M.C.; Salgueiro, L. Chemical, antifungal and cytotoxic evaluation of the essential oil of Thymus zygis subsp. sylvestris. Ind. Crops Prod. 2010, 32, 70–75. [Google Scholar] [CrossRef]
- Lagha, R.; Abdallah, F.B.; AL-Sarhan, B.O.; Al-Sodany, Y. Antibacterial and biofilm inhibitory activity of medicinal plant essential oils against Escherichia coli isolated from UTI patients. Molecules 2019, 24, 1161. [Google Scholar] [CrossRef]
- Debonne, E.; Vermeulen, A.; Van Bockstaele, F.; Soljic, I.; Eeckhout, M.; Devlieghere, F. Growth/no-growth models of in-vitro growth of Penicillium paneum as a function of thyme essential oil, pH, aw, temperature. Food Microbiol. 2019, 83, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, V.W.; Clausen, C.A. Antifungal effect of essential oils on southern yellow pine. Int. Biodeterior. Biodegrad. 2007, 59, 302–306. [Google Scholar] [CrossRef][Green Version]
- Santoyo, S.; Jaime, L.; García-Risco, M.R.; Lopez-Hazas, M.; Reglero, G. Supercritical fluid extraction as an alternative process to obtain antiviral agents from thyme species. Ind. Crops Prod. 2014, 52, 475–480. [Google Scholar] [CrossRef]
- Machado, M.; Dinis, A.M.; Salgueiro, L.; Cavaleiro, C.; Custódio, J.B.A.; Do Céu Sousa, M. Anti-Giardia activity of phenolic-rich essential oils: Effects of Thymbra capitata, Origanum virens, Thymus zygis subsp. sylvestris, and Lippia graveolens on trophozoites growth, viability, adherence, and ultrastructure. Parasitol. Res. 2010, 106, 1205–1215. [Google Scholar]
- Sangha, J.S.; Astatkie, T.; Cutler, G.C. Ovicidal, larvicidal, and behavioural effects of some plant essential oils on diamondback moth (Lepidoptera: Plutellidae). Can. Entomol. 2017, 149, 639–648. [Google Scholar] [CrossRef]
- Park, C.G.; Jang, M.; Yoon, K.A.; Kim, J. Insecticidal and acetylcholinesterase inhibitory activities of Lamiaceae plant essential oils and their major components against Drosophila suzukii (Diptera: Drosophilidae). Ind. Crops Prod. 2016, 89, 507–513. [Google Scholar] [CrossRef]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Roudashti, S.; Zeighami, H.; Mirshahabi, H.; Bahari, S.; Soltani, A.; Haghi, F. Synergistic activity of sub-inhibitory concentrations of curcumin with ceftazidime and ciprofloxacin against Pseudomonas aeruginosa quorum sensing related genes and virulence traits. World J. Microbiol. Biotechnol. 2017, 33, 50. [Google Scholar] [CrossRef] [PubMed]
- Algburi, A.; Comito, N.; Kashtanov, D.; Dicks, L.M.T.; Chikindas, M.L. Control of biofilm formation: Antibiotics and beyond. Appl. Environ. Microbiol. 2017, 83, e02508-16. [Google Scholar] [CrossRef] [PubMed]
- Perricone, M.; Arace, E.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Bioactivity of essential oils: A review on their interaction with food components. Front. Microbiol. 2015, 6, 1–7. [Google Scholar] [CrossRef]
- Idrees, M.; Sawant, S.; Karodia, N.; Rahman, A. Staphylococcus aureus biofilm: Morphology, genetics, pathogenesis and treatment strategies Int. J. Environ. Res. Public Health 2021, 18, 7602. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.; Bessa, L.J.; Martins, M.R.; Arantes, S.; Teixeira, A.P.S.; Mendes, Â.; Da Costa, P.M.; Belo, A.D.F. Chemical composition, antibacterial, antibiofilm and synergistic properties of essential oils from Eucalyptus globulus LABILL. and seven Mediterranean aromatic plants. Chem. Biodivers. 2017, 14, e1700006. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.J.D.; Deans, S.G. Chemical composition, antimicrobial and in vitro antioxidant properties of Monarda citriodora var. citriodora, Myristica fragrans, Origanum vulgare ssp. hirtum, Pelargonium sp. and Thymus zygis oils. J. Essent. Oil Res. 2004, 16, 145–150. [Google Scholar] [CrossRef]
- Pina-Vaz, C.; Rodrigues, A.G.; Pinto, E.; Costa-de-Oliveira, S.; Tavares, C.; Salgueiro, L.; Cavaleiro, C.; Gonçalves, M.; Martinez-de-Oliveira, J. Antifungal activity of Thymus oils and their major compounds. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Ballester-Costa, C.; Sendra, E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical composition and in vitro antibacterial properties of essential oils of four Thymus species from organic growth. Ind. Crops Prod. 2013, 50, 304–311. [Google Scholar] [CrossRef]
- Sánchez-Hidalgo, M.; Montalbán-López, M.; Cebrián, R.; Valdivia, E.; Martínez-Bueno, M.; Maqueda, M. AS-48 bacteriocin: Close to perfection. Cell. Mol. Life Sci. 2011, 68, 2845–2857. [Google Scholar] [CrossRef]
- Rota, C.; Herrera, A.; Martınez, R.M.; Sotomayor, J.A.; Jordán, M.J. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control 2008, 19, 681–687. [Google Scholar] [CrossRef]
- Andrés, M.F.; González-coloma, A.; Muñoz, R.; De la Peña, F.; Julio, L.F.; Burillo, J. Nematicidal potential of hydrolates from the semi industrial vapor-pressure extraction of Spanish aromatic plants. Environ. Sci. Pollut. Res. 2018, 25, 29834–29840. [Google Scholar] [CrossRef]
- Marinković, J.; Ćulafić, D.M.; Nikolić, B.; Đukanović, S.; Marković, T.; Tasić, G.; Ćirić, A.; Marković, D. Antimicrobial potential of irrigants based on essential oils of Cymbopogon martinii and Thymus zygis towards in vitro multispecies biofilm cultured in ex vivo root canals. Arch. Oral Biol. 2020, 117, 104842. [Google Scholar] [CrossRef]
- Solarte, A.L.; Astorga, R.J.; De Aguiar, F.C.; De Frutos, C.; Barrero-Domínguez, B.; Huerta, B. Susceptibility Ddistribution to essential oils of Salmonella enterica strains involved in animal and public health and comparison of the Typhimurium and Enteritidis Serotypes. J. Med. Food 2018, 21, 946–950. [Google Scholar] [CrossRef]
- Youdim, K.A.; Deans, S.G.; Finlayson, H.J. The antioxidant properties of thyme (Thymus zygis L.) essential oil: An inhibitor of lipid peroxidation and a free radical scavenger. J. Essent. Oil Res. 2002, 14, 210–215. [Google Scholar] [CrossRef]
- Carrasco, A.; Tomas, V.; Tudela, J.; Miguel, M.G. Comparative study of GC-MS characterization, antioxidant activity and hyaluronidase inhibition of different species of Lavandula and Thymus essential oils. Flavour Fragr. J. 2015, 31, 57–69. [Google Scholar] [CrossRef]
- Jordán, M.J.; Martínez, R.M.; Martínez, C.; Moñino, I.; Sotomayor, J.A. Polyphenolic extract and essential oil quality of Thymus zygis ssp. gracilis shrubs cultivated under different watering levels. Ind. Crops Prod. 2009, 29, 145–153. [Google Scholar] [CrossRef]
- Ballester-Costa, C.; Sendra, E.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Assessment of antioxidant and antibacterial properties on meat homogenates of essential oils obtained from four Thymus species achieved from organic growth. Foods 2017, 6, 59. [Google Scholar] [CrossRef] [PubMed]
- Escobar, A.; Pérez, M.; Romanelli, G.; Blustein, G. Thymol bioactivity: A review focusing on practical applications. Arab. J. Chem. 2020, 13, 9243–9269. [Google Scholar] [CrossRef]
- Ghabraie, M.; Vu, K.D.; Tata, L.; Salmieri, S.; Lacroix, M. Antimicrobial effect of essential oils in combinations against five bacteria and their effect on sensorial quality of ground meat. LWT Food Sci. Technol. 2016, 66, 332–339. [Google Scholar] [CrossRef]
- Abdallah, F.B.; Lagha, R.; Gaber, A. Biofilm inhibition and eradication properties of medicinal plant essential oils against methicillin-resistant Staphylococcus aureus clinical isolates. Pharmaceuticals 2020, 13, 369. [Google Scholar] [CrossRef]
- Brochot, A.; Guilbot, A.; Haddioui, L.; Roques, C. Antibacterial, antifungal, and antiviral effects of three essential oil blends. Microbiol. Open 2017, 6, e00459. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.G.; Hu, F.; Wei, Z.J. Antibacterial activity and mechanism of ginger essential oil against Escherichia coli and Staphylococcus aureus. Molecules 2020, 25, 3955. [Google Scholar] [CrossRef]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. Evid. Complement. Altern. Med. 2014, 2014, 651593. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, Approvated standard, CLSI Document M100-S31, 31st ed.; CLSI: Wayne, PA, USA, 2021; Volume 31. [Google Scholar]
- Palaniappan, K.; Holley, R.A. Use of natural antimicrobials to increase antibiotic susceptibility of drug resistant bacteria. Int. J. Food Microbiol. 2010, 140, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Zhang, Z.H.; Zeng, X.A.; Gong, D.M.; Wang, M.S. Combination of microbiological, spectroscopic and molecular docking techniques to study the antibacterial mechanism of thymol against Staphylococcus aureus: Membrane damage and genomic DNA binding. Anal. Bioanal. Chem. 2017, 409, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhao, X.; Yan, H.; Meng, R.; Zhang, Y.; Li, W.; Liu, Z.; Guo, N. Effect of tea tree oil on Staphylococcus aureus growth and enterotoxin production. Food Control 2016, 62, 257–263. [Google Scholar] [CrossRef]
- Qiu, J.; Li, H.; Su, H.; Dong, J.; Luo, M.; Wang, J.; Leng, B.; Deng, Y.; Liu, J.; Deng, X. Chemical composition of fennel essential oil and its impact on Staphylococcus aureus exotoxin production. World J. Microbiol. Biotechnol. 2012, 28, 1399–1405. [Google Scholar] [CrossRef]
- Coimbra, A.T.; Luís, Â.F.S.; Batista, M.T.; Ferreira, S.M.P.; Duarte, A.P.C. Phytochemical characterization, bioactivities evaluation and synergistic effect of Arbutus unedo and Crataegus monogyna extracts with amphotericin B. Curr. Microbiol. 2020, 77, 2143–2154. [Google Scholar] [CrossRef]
- Luís, Â.; Duarte, A.P.; Pereira, L.; Domingues, F. Chemical profiling and evaluation of antioxidant and anti-microbial properties of selected commercial essential oils: A comparative study. Medicines 2017, 4, 36. [Google Scholar] [CrossRef]
- Duarte, A.; Luís, Â.; Oleastro, M.; Domingues, F.C. Antioxidant properties of coriander essential oil and linalool and their potential to control Campylobacter spp. Food Control 2016, 61, 115–122. [Google Scholar] [CrossRef]
- Ferreira, S.; Domingues, F. The antimicrobial action of resveratrol against Listeria monocytogenes in food-based models and its antibiofilm properties. J. Sci. Food Agric. 2016, 96, 4531–4535. [Google Scholar] [CrossRef]
- Stepanović, S.; Ćirković, I.; Ranin, L.; Švabić-Vlahović, M. Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Lett. Appl. Microbiol. 2004, 38, 428–432. [Google Scholar] [CrossRef]
- Duarte, A.; Alves, A.C.; Ferreira, S.; Silva, F.; Domingues, F.C. Resveratrol inclusion complexes: Antibacterial and anti-biofilm activity against Campylobacter spp. and Arcobacter butzleri. Food Res. Int. 2015, 77, 244–250. [Google Scholar] [CrossRef]
- Asensio, C.M.; Quiroga, P.R.; Al-Gburi, A.; Huang, Q.; Grosso, N.R. Rheological behavior, antimicrobial and Qquorum sensig inhibition study of an argentinean oregano essential oil nanoemulsion. Front. Nutr. 2020, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Luís, Â.; Silva, F.; Sousa, S.; Duarte, A.P.; Domingues, F. Antistaphylococcal and biofilm inhibitory activities of gallic, caffeic, and chlorogenic acids. Biofouling 2014, 30, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Ferreira, S.; Duarte, A.; Mendona, D.I.; Domingues, F.C. Antifungal activity of Coriandrum sativum essential oil, its mode of action against Candida species and potential synergism with amphotericin B. Phytomedicine 2011, 19, 42–47. [Google Scholar] [CrossRef] [PubMed]

| Compounds | Retention Time | Kovats Index | % |
|---|---|---|---|
| α-Thujene | 10.75 | 929 | 0.73 |
| α-Pinene | 10.98 | 936 | 1.01 |
| Camphene | 11.50 | 950 | 1.19 |
| β-Myrcene | 13.14 | 989 | 1.29 |
| α-Terpinene | 13.98 | 1017 | 1.38 |
| p-Cymene | 14.31 | 1024 | 10.58 |
| Limonene | 14.42 | 1030 | 0.56 |
| γ-Terpinene | 15.50 | 1060 | 8.04 |
| Trans-Sabinene hydrate | 15.71 | 1098 | 1.14 |
| β-Linalool | 16.85 | 1099 | 3.77 |
| Camphor | 18.16 | 1143 | 1.10 |
| Trans-pinocarveol | 18.31 | 1140 | 0.89 |
| Borneol | 18.90 | 1166 | 3.79 |
| 4-Terpineol | 19.21 | 1177 | 0.46 |
| Thymol | 22.78 | 1290 | 43.17 |
| Carvacrol | 22.99 | 1300 | 13.00 |
| β-Caryophyllene | 26.03 | 1420 | 1.43 |
| Caryophyllene oxide | 30.01 | 1581 | 0.59 |
| DPPH Method | β-Carotene-Bleaching Assay | |||
|---|---|---|---|---|
| Samples | IC50 (%) | AAI | Antioxidant Activity Classification | IC50 (%) |
| T. zygis | 2.00 ± 0.15 | 12.87 ± 3.65 | Very strong | 0.27 |
| Gallic acid | 2.14 ± 0.39 | 22.16 ± 3.53 | Very strong | - |
| Trolox | 3.26 ± 1.21 | 15.02 ± 0.64 | Very strong | - |
| BHT | - | - | - | 0.10 |
| Inhibition Zone (mm) | MIC (%) | ||||
|---|---|---|---|---|---|
| Species | T. zygis (10 µL/Disc) | Tetracycline (20 µg/Disc) | Volatile Compounds (10 µL/Disc) | T. zygis | Tetracycline |
| S. aureus ATCC 25923 | 35.10 ± 4.57 | 31.17 ± 2.73 | 27.54 ± 4.10 | 0.05 | 0.013 |
| S. aureus SA 03/10 | 20.67 ± 1.59 | 8.24 ± 0.49 | 16.26 ± 5.15 | 0.05 | 6.4 |
| S. aureus MRSA 12/08 | 30.93 ± 4.64 | 8.42 ± 0.75 | 16.45 ± 3.63 | 0.1 | 6.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coimbra, A.; Miguel, S.; Ribeiro, M.; Coutinho, P.; Silva, L.; Duarte, A.P.; Ferreira, S. Thymus zygis Essential Oil: Phytochemical Characterization, Bioactivity Evaluation and Synergistic Effect with Antibiotics against Staphylococcus aureus. Antibiotics 2022, 11, 146. https://doi.org/10.3390/antibiotics11020146
Coimbra A, Miguel S, Ribeiro M, Coutinho P, Silva L, Duarte AP, Ferreira S. Thymus zygis Essential Oil: Phytochemical Characterization, Bioactivity Evaluation and Synergistic Effect with Antibiotics against Staphylococcus aureus. Antibiotics. 2022; 11(2):146. https://doi.org/10.3390/antibiotics11020146
Chicago/Turabian StyleCoimbra, Alexandra, Sónia Miguel, Maximiano Ribeiro, Paula Coutinho, Lúcia Silva, Ana Paula Duarte, and Susana Ferreira. 2022. "Thymus zygis Essential Oil: Phytochemical Characterization, Bioactivity Evaluation and Synergistic Effect with Antibiotics against Staphylococcus aureus" Antibiotics 11, no. 2: 146. https://doi.org/10.3390/antibiotics11020146
APA StyleCoimbra, A., Miguel, S., Ribeiro, M., Coutinho, P., Silva, L., Duarte, A. P., & Ferreira, S. (2022). Thymus zygis Essential Oil: Phytochemical Characterization, Bioactivity Evaluation and Synergistic Effect with Antibiotics against Staphylococcus aureus. Antibiotics, 11(2), 146. https://doi.org/10.3390/antibiotics11020146









