Abstract
Honey is a natural food consisting mainly of sugars, enzymes, amino acids, organic acids, vitamins, minerals and aromatic substances. In addition to specific organoleptic properties, honey also has other components that contribute to its nutritional and health value. Proteins, vitamins, minerals, organic acids and phenolic compounds, the most variable components of honey, are predominantly responsible for its strong bioactive effect. Honeydew honey is a less known type of honey with outstanding antimicrobial and antioxidant properties that also demonstrates prebiotic effects and can promote the growth of probiotic bacteria. Foodborne illnesses can be prevented by using probiotic strains in combination with prebiotics. The aim of this study was for the first time to determine potential synergistic antimicrobial effect of fir (Abies alba Mill.) honeydew honey (HS) and probiotic bacteria Lactiplantibacillus plantarum on Salmonella enterica serotype Typhimurium, a primary cause of foodborne illnesses. The effect of three different samples of fir honeydew honey on the growth of L. plantarum in de Man, Rogosa and Sharpe (MRS) medium and the potential synergistic effect of HSs and L. plantarum on the growth of S. Typhimurium in the Brain Heart Infusion (BHI) medium were examined. The results indicate that concentrations of 1 and 5% of all three HS samples stimulate the growth and metabolic activity of L. plantarum, while a concentration of 10% inhibits the growth of L. plantarum. The concentration of 5% of all three HS and L. plantarum combined inhibits the growth of S. Typhimurium in BHI broth. Fir honeydew honey showed potential prebiotic properties and antimicrobial activity, both of which can synergistically enhance the probiotic efficacy of L. plantarum against S. Typhimurium To conclude, the combination of fir honeydew honey and L. plantarum represents a successful combination against S. Typhimurium and additional experiments are necessary regarding the mechanisms of their combined effect.
1. Introduction
Honey is an animal and plant-based product traditionally used for its healing and antimicrobial properties. Its health properties are dependent on the geographic origin and type of honey (botanical origin). One of the highest quality honeys, although it is still a relatively unknown type of honey, is honeydew honey—honey that honeybees (Apis mellifera) produce from honeydew secreted by aphids and some scale insects as they feed on plant sap. A higher content of proteins, minerals and phenolic compounds in honeydew honeys compared to nectar honeys significantly contributes to their remarkable biological activities including strong antimicrobial action [1].
Studies on the antibacterial activity of honeydew honeys reported superior antibacterial efficacy compared to nectar honeys, even stronger than manuka honey [2,3,4,5]. The antibacterial activity of honeydew honeys could be largely attributed to hydrogen peroxide formation [2,3,4]. However, results of recent research indicated that although hydrogen peroxide plays an important role in the inhibition of bacterial growth, phenolics and their interaction with hydrogen peroxide might be the key factors for the antibacterial activity of honeydew honeys [6].
Several studies demonstrated the prebiotic effect of honey on probiotic bacteria, which as part of the body’s natural microbiota plays an important role in maintaining health and preventing diseases by various pathogenic microorganisms in the gut [7,8]. In recent times, there has been a renewal of interest in the use of probiotics as biotherapeutic agents as scientific evidence continue to accumulate on the properties, functionality and beneficial effects of probiotic bacteria on humans. The investigation of a new probiotics is driven by the growing demand for probiotic functional food and beverages as well as dietary supplements due to growing consumer awareness regarding the concept of preventive health care. [9,10].
Various probiotic strains have been identified over the years and the most known probiotic bacteria is Lactiplantibacillus plantarum (former Lactobacillus plantarum). This probiotic strain stimulates the digestive system and fights off disease-causing bacteria by maintaining the intestinal barrier and probiotic-mediated immunomodulation [11]. Probiotic supplements are used to treat or prevent specific health problems, such as seasonal allergies and irritable bowel syndrome (IBS) [12]. The action mechanism of probiotics is not yet fully clarified; however, it is expressed by three main mechanisms: Inhibition of the growth of undesirable microorganisms in the intestinal tract by producing antibacterial substances and bacteriocins and competition for nutrients and bonding site; modification of bacterial metabolism and stimulation of the immune system.
Additionally, previous in vitro studies suggested that honey, due to its chemical composition, could play a prebiotic role and favor the growth of lactobacilli and bifidobacteria [7,13,14,15].
On the other side, there are various strains of pathogenic bacteria that colonize the human digestive system. A well-known strain is Salmonella enterica serotype Typhimurium (S. Typhimurium). It is a non-typhoid serotype of S. enterica which is the primary cause of foodborne illnesses. S. Typhimurium causes acute inflammatory diarrhea that can progress to invasive systemic disease in susceptible patients leading to hospitalizations and deaths [16,17]. These diseases can be prevented by using probiotic strains in combination with prebiotics.
Lack of data on the prebiotic potential of honeydew honey motivated us to test the antimicrobial and prebiotic activity of fir (Abies alba Mill.) honeydew honey and to determine whether there is a synergistic antimicrobial effect of this honey and probiotic bacteria L. plantarum on S. Typhimurium.
2. Results
2.1. Honey Sample Analyses
Results of honey sample analyses are shown in Table 1. Microscopic analysis for morphometry of pollen grains confirmed that all the tested honey samples belonged to fir honeydew honey [18]. Their water content was within the limits according to the Codex Alimentarius and to the EU Draft 96/0114 (CNS) (≤21%) [19]. The ash content of HSs was in the range from 0.59 to 0.62%, the electrical conductivity values ranged from 1.17 to 1.22 mS/cm (Table 1), which is in accordance with legislation [19]. All three HSs had the same content of glucose (23.15 g/100 g to 24.08 g/100 g) and fructose (31.255 g/100 g to 32.27 g/100 g). We did not detect sucrose in any of the tested samples. There was no significant difference in total phenol content (225–231 mgGAE/100 g)

Table 1.
Characterization of fir (Abies alba Mill.) honeydew samples (HSs).
2.2. Antibacterial Effect of Fir Honeydew Honey Samples
Minimum inhibitory concentrations (MIC) and minimum bactericidal concentrations (MBC) against S. Typhimurium were 125–150 mg/mL and HS 1 showed a slightly weaker effect compared to the other two samples. The MIC concentrations against L. plantarum were 400 mg/mL and no bactericidal effect was detected (Table 2).

Table 2.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of fir honeydew honey (Abies alba Mill.; HS 1–3) against Salmonella enterica serotype Typhimurium and Lactiplantibacillus plantarum.
2.3. Effect of Honeydew Samples on the Growth of Lactiplantibacillus plantarum in de Man, Rogosa and Sharpe Broth
In the experiment, the growth of L. plantarum in de Man, Rogosa and Sharpe (MRS) broth with three HS at concentrations of 1%, 5% and 10% was monitored after 24 h incubation. As the control, growth of bacteria in MRS broth without honeydew honey and in MRS broth with 10% glucose was determined. The results are shown on Figure 1. A comparison with the control sample (without HS) showed that L. plantarum grew better with the addition of 1% and 5% HS. All three HS stimulated the growth of L. plantarum equally well and there was no significant difference between them. In contrast, the addition of 10% HS to MRS broth resulted in an inhibition of L. plantarum growth by 2 logarithmic units relative to the control. Therefore, we can conclude that lower concentrations of HS will stimulate the growth of L. plantarum while higher concentrations (above 10%) inhibit the growth of lactobacilli. At the same time, the addition of 10% glucose to MRS broth did not affect the growth of lactobacilli in MRS broth. The addition of 5% HS to MRS broth did not result in bacterial growth after 24 h incubation and additional cultivation on Mueller Hinton (MH) agar and MRS agar indicating that no other bacteria were present in the HSs as contamination.
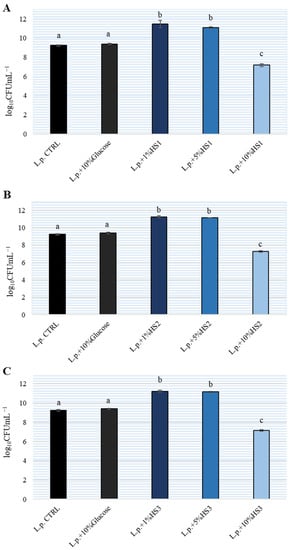
Figure 1.
Growth of Lactiplantibacillus plantarum (L.p.) in de Man, Rogosa and Sharpe (MRS) broth with different honeydew honey samples (HS) (A–C) at different concentrations. The experiment was repeated three times in duplicate and the mean ± SD is shown. Different letters denote significant differences (p < 0.05) determined by nonparametric Wilcoxon rank-sum test.
To monitor the metabolic activity of L. plantarum after 24 h of incubation, the pH of MRS broth was determined (Figure 2). Bacterial-free MRS broth and MRS broth with 10% HSs had a pH of 6.2 ± 3. After 24 h of incubation of L. plantarum without HS, the pH was lowered (around pH4, 5 ± 2). Incubation with different concentrations of HS led to a significant decrease (pH3, 6 ± 2) in pH relative to L. plantarum growth without HS. No differences were observed between the three HSs samples examined. The addition of glucose to the MRS broth did not affect the further lowering of the pH. Therefore, unlike glucose supplementation, HS supplementation enhanced the metabolic activity of lactobacilli in MRS broth.
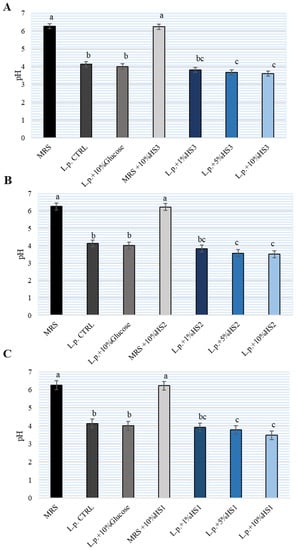
Figure 2.
pH value of de Man, Rogosa and Sharpe (MRS) broth after cultivation of Lactiplantibacillus plantarum (L.p.) with the addition of different concentrations of different honeydew honey samples (HS) (A–C). The experiment was repeated two times and the mean value ± SD were shown. Different letters denote significant differences (p < 0.05) determined by nonparametric Wilcoxon rank-sum test.
2.4. Combined Effect of Honeydew Samples and L. plantarum on S. Typhimurium Growth in MH Broth
To investigate the potential combined effect of HSs and lactobacilli on S. Typhimurium growth, MH broth and subinhibitory concentration of 5% of HS were used. The results are shown on Figure 3. The ratio of L. plantarum and S. Typhimurium was 100:1. After 24 h, we determined more than 1010 CFU/mL S. Typhimurium in MH broth (control). The addition of L. plantarum inhibited the increase in Salmonella by approximately 2 logarithmic units, while the addition of 5% HSs inhibited the increase by slightly more than two logarithms. The combination of L. plantarum and 5% HS inhibited the growth of S. Typhimurium by 5 logarithmic units. There was no statistically significant difference between the tested HSs regarding inhibition of Salmonella growth. The addition of glucose to the HM broth did not affect the growth of S. Typhimurium. The combination of 10% glucose and L. plantarum did not show a combined effect on the growth of S. Typhimurium.
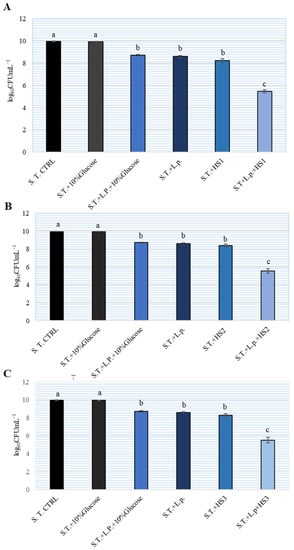
Figure 3.
Combined inhibitory effect of different honeydew samples (HS) (A–C) and Lactiplantibacillus plantarum (L.p.) on the number of Salmonella enterica serotype Typhimurium (S.T.) in Brain Heart Infusion (BHI) broth. The experiment was repeated two times and the mean values ± SD were shown. Different letters denote significant differences between groups (p < 0.05) determined by nonparametric Wilcoxon rank-sum test.
At the same time, the number of lactobacilli was determined in the tested samples. The results are shown on Figure 4. In MH broth and MH broth with the addition of 10% sucrose, we determined an increase of 0.5 logarithm so L. plantarum would not multiply successfully in MH broth. The addition of HSs significantly affected the ability of L. plantarum to multiply and the number of bacteria increased by 2 logarithmic units. In the sample which, in addition to lactobacilli, contained S. Typhimurium and HSs, the same increase by 2 logarithmic units was determined. Therefore, the addition of honey to MH broth positively affected the growth of L. plantarum.
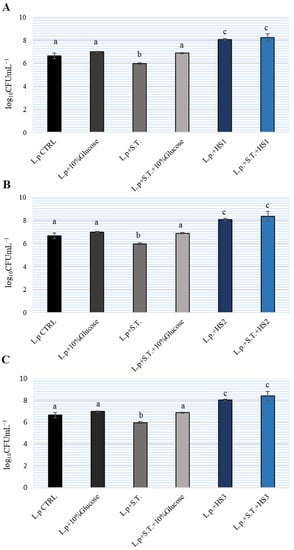
Figure 4.
The number of Lactiplantibacillus plantarum (L.p.) during co-cultivation in Brain Heart Infusion (BHI) broth with different honeydew samples (HS) (A–C) and Salmonella enterica serotype Typhimurium (S.T.). The experiment was repeated two times and the mean values ± SD were shown. Different letters denote significant differences between groups (p < 0.05) determined by nonparametric Wilcoxon rank-sum test.
3. Discussion
Honeydew honey is a type of honey produced from honeysuckle; a sweet substance secreted by aphids after processing plant juice. In addition to its attractive sensorial characteristics (dark brownish color and sweet flavor with pleasant, slightly resinous aftertaste and aroma), honeydew honey is valued due to its pronounced antibacterial potential. In our previous study with samples of fir (Abies alba Mill.) honeydew honey collected from Gorski kotar (Croatia), we proved an antibacterial effect against resistant strains of Acinetobacter baumannii and methicillin-resistant Staphylococcus aureus (MRSA) and the bactericidal effect was concentration dependent [20]. Majtan et al. have proven the bactericidal effect of Slovak honeydew honey from the floral source of Abies spp. against multidrug-resistant Stenotrophomonas maltophilia [21]. Pérez Martin et al. showed that Spanish honeydew honey from different floral origins had the capacity to inhibit Gram-positive bacteria Micrococcus luteus and Staphylococcus aureus [22]. The antimicrobial activity of Turkish honeydew honey was tested on 12 bacteria and two yeasts. The honey samples showed the highest antimicrobial activity against Escherichia coli O157:H7, S. aureus and Listeria monocytogenes [23].
In addition to the antimicrobial effect, honeydew honey has a pronounced and antioxidant effect mostly due to biologically active compounds like phenolics. Most phenolic compounds proven in honey such as, chrysin, quercetin, pinocembrin, caffeic acid and apigenin possess potential biological activity [24,25,26]. Total phenol content in our samples is consistent with the results of previous research [26,27]. Generally, larger amounts of phenolic compounds in honeydew honeys than in flower honeys have been reported in the literature [1]. Besides phenolics, which are mainly flavonoids in honeydew honeys, catalase, peroxidase, carotenoids and non-peroxide components are also responsible for its antioxidant characteristics [28].
Furthermore, due to the oligosaccharide content, honey is also recognized as a potential prebiotic. It has been proven that honey oligosaccharides can promote the growth of lactobacilli and bifidobacteria [29,30]. A comparative study involving honey oligosaccharides has demonstrated a definite prebiotic potential, which however was not as prominent as fructooligosaccharide (FOS) [31]. One of the possible superiorities of honeydew honey as a prebiotic over nectar honey may be due to the significantly higher average content of oligosaccharide melezitose. However, honeydew honey is still one of the rarer and relatively unknown types of honey, and research on its functional potential should be further explored [32,33,34].
Potentially probiotic microorganisms are extensively studied and are used in a wide range of applications such as prevention of food poisoning, treatment of certain gastrointestinal disorders, food preservation, etc. [35,36]. Lactic acid bacteria, predominantly lactobacilli, are the most frequently mentioned potentially probiotic bacteria. These bacteria have been shown to interfere with pathogenic bacteria by different mechanisms, like lowering the pH and production of antimicrobial compounds such as lactic acid, hydrogen peroxide and bacteriocin-like substances [37]. Lactic acid bacteria can inhibit the adhesion of pathogenic bacteria on intestinal epithelial cells, and consequently reduce pathogen colonization and prevent infection [38,39].
In our previous research, we isolated the L. plantarum strain B from homemade sheep’s cheese [39]. This strain showed metabolic activity and lowered the pH of the BHI medium with the addition of bile salts. Furthermore, during cocultivation with S. Typhimurium, L. plantarum strain B significantly inhibited Salmonella growth and it was shown that the mechanism of action was probably related to the lowering of pH of the media. Other authors also point out that one of the main mechanisms of action of probiotic microorganisms is lowering the pH, which is especially important in the case of S. Typhimurium, which is extremely sensitive to low pH [40,41]. Kajiwara et al. showed effects of honey on lactic and acetic acid production by intestinal Bifidobacterium spp. in a manner like those of FOS, galactooligosaccharide and inulin [42]. All of the honey types (wild and commercial, 5%) supported the growth and acid production in skim milk [43].
Additionally, the tested L. plantarum strain showed good adhesion properties on human enterocyte cell line Caco-2 and inhibited the adhesion of S. Typhimurium during cocultivation or after pretreatment. Another important property of probiotic bacteria is the ability to aggregate, and the L. plantarum strain B showed high auto-aggregation properties (≥80%) and coaggregated with S. Typhimurium (≥30%) [44]. Therefore, in addition to lowering the pH and aggregation property, this bacterium appears to use other mechanisms that need to be further studied. Since both honeydew honey and the tested L. plantarum strain showed good antibacterial properties, we wanted to examine their potential combined or synergistic effect. Table 2 indicates that it has showed an antibacterial effect on both tested bacteria, with the MIC being several times lower for S. Typhimurium (about 125 mg/mL). To test whether the HS tested had a potential prebiotic effect on L. plantarum strain B, different concentrations were tested (Figure 1) and the application of 1% and 5% HS stimulated the growth of L. plantarum. The addition of 10% glucose did not have any effect on the growth of L. plantarum. To claim that HSs possess a prebiotic effect the effect of the combination of glucose and fructose should be examined. In addition to glucose, the effect of sucrose was also examined and had no effect on lactobacilli growth (data not shown). From this we can speculate that simple carbohydrates are not the only ones responsible for stimulating the growth of L. plantarum. The favorable effect of honey on the growth of lactobacilli has been previously reported by several authors (Mohan et al., Shamala et al., Jiang et al.) [7,15,45]. It seems that phenolics and oligosaccharides have a synergistic effect on human intestinal microbes, which was also speculated by Jiang et al. who determined the positive impact of these compounds from buckwheat honey on the growth of Bifidobacteria [45]. Further research is needed to examine the effect of oligosaccharides present in this fir honeydew honey on the growth stimulation of L. plantarum.
To investigate the potentially combined inhibitory effect of HS and L. plantarum on S. Typhimurium, bacteria were grown in BHI broth. To study the combined effect, we chose a 5% solution of HS and the ratio of S. Typhimurium and L. plantarum was 1:100. In previous tests, we determined that this ratio of bacteria showed the best inhibition (data not shown). From the results we can see that a single application of HSs or L. plantarum equally inhibited the growth of S. Typhimurium, while their combination proved to be the most effective. To the best of the authors’ knowledge, this is the first report of a combined antimicrobial effect of honeydew honey and probiotic bacteria L. plantarum on S. Typhimurium.
In the same experiment, we monitored the number of lactobacilli that multiply very slowly in this medium. The addition of HSs resulted in an increase in the number of lactobacilli by 2 logarithmic units and a decrease in the pH of the sample (around pH5, 3 ± 2). From the above, we could conclude that the reason for the decrease in S. Typhimurium in the samples lies in the fact that lactobacilli are metabolically active and that their number in the samples increased, which increased the chance of coaggregation. In this way the bacteria are in close contact and better exhibit their antimicrobial effect. Interestingly, the addition of 10% glucose did not show the same effect and did not lead to a significant increase in the number of lactobacilli in the samples, nor did it inhibit the increase in Salmonella. Therefore, we can say that other active components that manifest their effect are obviously present in honeydew samples.
4. Materials and Methods
4.1. The Honeydew Honey Samples
The fir (Abies alba Mill.) honeydew samples (HS) were purchased from Gorski d.o.o., Fužine, Croatia. They were obtained during summer 2015 from the mountain region Gorski kotar (western part of Croatia) defined by Universal Transverse Mercator (UTM) system coordinates as follows: Sample 1 (HS 1), location 1: Lič; Potkoš (45°17′59″ N, 14°44′12″ W), sample 2 (HS 2), location 2: Crni lug; Lazac (45°22′02″ N, 14°43′10″ W) and sample 3 (HS 3), location 3: Fužine; Vrelo (45°19′34″ N, 14°42′16″ W). HS were stored at 4 °C in hermetically closed glass jars until analysis. For microbiological analyses, HS samples were diluted in MRS or BHI broth and pasteurized at 70 °C for 15 min [46].
4.2. The Honeydew Honey Analyses
The melissopalynological analysis followed the methods recommended by the International Commission for Bee Botany (now known as International Commission on Plant Pollinator Relations; ICPPR) [47]. Microscopic analysis for morphometry of pollen grains and honeydew elements such as hyphae, fungal spores, mycelium or unicellular algae was performed on a Hund H500 (Helmut Hund GmbH, Wetzlar, Germany) light microscope with attached digital camera (model Dino-Eye AM423U; Dino-Lite, AnMo Electronics Corp., Hsinchu, Taiwan) and coupled to an analysis system (DinoCapture 2.0 v. 1.4.9; Dino-Lite). Water content was determined by refractometry, measuring the refractive index, using standard model Abeé refractometer (Carl Zeiss, Jena, Germany) at 20 °C. Water content (%) was obtained from the Chataway table [48]. Electrical conductivity was measured in a solution of 20 g honey sample in low-conductivity water system at 20 °C using a conductometer (HI-8733; Hanna Instruments, Woonsocket, RI, USA), while the ash content was calculated according to the results of electrical conductivity [13]. To determine concentrations of sugars (glucose, fructose, sucrose) in HS, high performance liquid chromatography combined with RI detector (HPLC-RID) (Knauer, Berlin, Germany) was used [48].
The Folin–Ciocalteu method was used to determine the concentration of total phenols [49]. Gallic acid (Sigma Aldrich, Darmstadt, Germany) was used as a standard to produce the calibration curve.
Each HS (1 g) was diluted in 10 mL distilled water and filtered through Whatman No. 1 paper. This solution (0.5 mL) was then mixed with 2.5 mL of 0.2 N Folin–Ciocalteu reagent (Sigma Aldrich, Darmstadt, Germany) for 5 min and 2 mL of 75 g/L sodium carbonate (Na2CO3) (Sigma Aldrich, Darmstadt, Germany) was then added. After incubation at room temperature for 2 h, the absorbance of the reaction mixture was measured at 760 nm against a methanol blank (Eppendorf Biofotometar, Hamburg, Germany). The mean of three readings was used and the total phenolic content was expressed in mg of gallic acid equivalents (GAE)/100 g of honey.
4.3. Bacterial Strains and Growth Conditions
L. plantarum isolates strain B from homemade sheep’s cheese were kindly provided by Prof. Jadranka Frece from the Faculty of Food Technology and Biotechnology, University of Zagreb, Croatia [44,50]. S. enterica serotype Typhimurium ATCC 14,028 were obtained from the culture collection of the Department of Microbiology and Parasitology, Faculty of Medicine, University of Rijeka. All of the tested bacteria were kept in 30% glycerol broth at –80 °C. L. plantarum was grown in MRS broth (Biolife Italiana, Milan, Italy) in microaerophilic atmosphere (5% CO2) for 48 h at 37 °C. S. Typhimurium was cultivated in nutrient broth (Biolife Italiana, Milan, Italy) and the number of bacteria was determined by plate counting on Salmonella Shigella (SS) agar (Biolife Italiana, Milan, Italy) or Mueller Hinton agar (MHA) (Biolife Italiana, Milan, Italy). The number of bacteria in the suspension was determined photometrically at λ = 600 nm and the absorbance (A) was set to 0.3, corresponding to a concentration of 108 CFU/mL.
4.4. Antibacterial Activity Assay
MIC and MBC of the HSs were determined using a standard microdilution technique in Muller Hinton Broth (MHB). Each honey sample was dissolved in MHB to prepare stock solutions of 0.8 g/mL. Furthermore, twofold serial dilutions in MHB were prepared from stock solutions of each honey sample to give final concentrations ranging from 0.0125 to 0.4 g/mL. A volume of 100 µL of each diluted sample was mixed with equal volume of bacterial suspension. Positive (broth and inoculum) and negative (simple broth) growth controls were prepared. The plates were incubated for 24 h at 37 °C and 120 rpm (Unimax 1010; Heidolph Instruments GmbH&CO., KG, Schwabach, Germany). MIC values were taken as the lowest concentration of honey sample that produced no visible bacterial growth compared to the control wells after 24 h of incubation at 37 °C. MBC is determined by inoculating the samples used for MIC determinations onto MHA and incubating further for 18–24 h. MBC was defined as the lowest concentration of honey sample that killed ≥99% of bacteria. Meropenem for S. Typhimurium and gentamicin for L. plantarum served as positive controls of growth inhibition. The final antibiotic concentrations used in the assays ranged between 0.0015 and 3.84 mg/L for both antibiotics. The results were interpreted following EUCAST recommendations [51].
4.5. Effect of Honeydew Samples on the Growth of L. plantarum in MRS Broth
The suspension of lactobacilli (106 CFU/mL) in MRS broth was inoculated using different concentrations of honeys (1, 5 and 10%). After incubation for 24 h at 37 °C and 120 rpm (Unimax 1010; Heidolph Instruments GmbH & CO., KG, Schwabach, Germany), the number of lactobacilli was determined by plating ten-fold dilutions on MRS agar. The ability of the isolates to reduce the pH of the medium was tested (Mettler Toledo pH Meter).
4.6. Effect of Co-Cultivation of L. plantarum and Honeydew Samples on S. Typhimurium Growth
To investigate the potentially synergistic effect of L. plantarum and HSs on S. Typhimurium growth, the concentration of HSs used was 5%. A bacterial suspension (S. Typhimurium) at a concentration of 104 CFU/mL and a suspension of lactobacilli at a concentration of 106 CFU/mL in BHI broth were prepared. A bacterial suspension of S. Typhimurium without the addition of HS and lactobacilli was used as the control. The individual effect of HS and the combined effect of HS and lactobacilli on S. Typhimurium growth were tested. In addition, the effect of glucose (10%) was tested individually and in combination with lactobacilli. The number of S. Typhimurium was monitored by plating tenfold dilutions of samples on Salmonella Shigella (SS) agar. The pH of the samples was also monitored at the end of the experiments.
4.7. The Number of L. plantarum during Co-Cultivation
The number of lactobacilli was monitored during the previously described experiment (Section 4.6). The number of lactobacilli was determined by plating ten-fold dilutions on MRS agar.
4.8. Statistical Analysis
Statistical analysis was preformed using analytic software Statistica 13.5.0.17. (TIBCO, Palo Alto, CA, USA). Statistical significance was tested on a significance level of p < 0.05 by nonparametric Wilcoxon rank-sum test and verified with Mann–Whitney U test. Letters on top of columns present statistical significances. Results were graphically displayed using Microsoft Excel.
5. Conclusions
The combined combination of fir honeydew honey and probiotic bacteria Lactiplantibacillus plantarum can be more beneficial in inhibiting S. Typhimurium growth than their individual application. Fir honeydew honey shows potential prebiotic properties, with antimicrobial activity, both of which can enhance the probiotic efficacy of L. plantarum against S. Typhimurium.
Author Contributions
Conceptualization, D.V. and I.G.; methodology, G.C., S.M., I.B.K., A.L. and T.P.; investigation, G.C., A.L. and S.M.; resources, I.G.; writing—original draft preparation, S.M., I.B.K., D.V. and I.G.; writing—review and editing, D.V., I.B.K. and I.G.; supervision, I.G. and D.V.; project administration, I.G. All authors have read and agreed to the published version of the manuscript.
Funding
The research described here was funded by a grant from the University of Rijeka (uniri-biomed-18-171). We gratefully acknowledge the project “Analysis of rare unifloral honeys in Croatia”, funded by the Paying Agency for Agriculture, Fisheries and Rural Development (PAAFRD). We are also grateful for the project “Research infrastructure for campus-based laboratories at the University of Rijeka”, co-funded from the European Regional Development Fund (ERDF).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tischer Seraglio, S.K.; Silva, B.; Bergamo, G.; Brugnerotto, P.; Vlademiro Gonzaga, L.; Roseane, F.; Oliveira Costa, A.C. An overview of physicochemical characteristics and health-promoting properties of honeydew honey. Food Res. Int. 2019, 119, 44–66. [Google Scholar] [CrossRef] [PubMed]
- Anthimidou, E.; Mossialos, D. Antibacterial activity of Greek and Cypriot honeys against Staphylococcus aureus and Pseudomonas aeruginosa in comparison to manuka honey. J. Med. Food 2013, 16, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Kafantaris, I.; Tsadila, C.; Nikolaidis, M.; Tsavea, E.; Dimitrou, T.G.; Iliopoulos, I.; Amoutzias, G.D.; Mossialos, D. Transcriptomic analysis of Pseudomonas aeruginosa response to pine honey via RNA sequncing indicates multiple mechanisms of antibacterial activity. Foods 2021, 10, 936. [Google Scholar] [CrossRef] [PubMed]
- Godocikova, J.; Bugarova, V.; Kast, C.; Majtan, V.; Majtan, J. Antibacterial potential of swiss honeys and characterisation of their bee.derived bioactive compounds. J. Sci. Food Agric. 2020, 100, 335–342. [Google Scholar] [CrossRef]
- Gobin, I.; Crnković, G.; Magdalenić, M.; Begić, G.; Babić, A.; Lušić, D.; Vučković, D. Antibacterial potential of Croatian honey aganst antibiotic resistant pathogenic bacteria. Med. Glas. 2018, 15, 139–144. [Google Scholar]
- Bucekova, M.; Buriova, M.; Pekarik, L.; Majtan, V.; Majtan, J. Phytochemicals-mediated production of hydrogen peroxide is crucial for high antibacterial activity of honeydew honey. Sci. Rep. 2018, 8, 9061. [Google Scholar] [CrossRef]
- Mohan, A.; Quek, S.Y.; Guiterrez-Maddox, N.; Gao, Y.; Shu, Q. Effect of honey in improving the gut microbial balance. Food Qual. Saf. 2017, 2, 107–115. [Google Scholar] [CrossRef]
- Ulrich Landry, B.K.; Moumita, S.; Jayabalan, R.; Zambou Ngoufack, F. Honey, probiotics and prebiotics: Review. Res. J. Pharm. Biol. Chem. 2016, 7, 2428–2438. [Google Scholar]
- Zoričić, P. Functional characteristics of selected probiotic strains of Lactobacillus plantarum [In Croatian]. Graduation Thesis, University of Rijeka, Rijeka, Croatia, 2012. [Google Scholar]
- Shokryazdan, P.; Sieo, C.C.; Kalavathy, R.; Liang, J.B.; Alitheen, N.B.; Jahromi, M.F.; Ho, Y.W. Probiotic potential of Lactobacillus strains with antimicrobial activity against some human pathogenic strains. BioMed Res. Int. 2014, 2014, 927268. [Google Scholar] [CrossRef]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Therap. Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef]
- Harvard Medical School. Health Benefits of Taking Probiotics. Harv. Health Lett. 2020. Available online: https://www.health.harvard.edu/vitamins-and-supplements/health-benefits-of-taking-probiotics (accessed on 13 April 2020).
- Ustunol, Z.; Ghandi, H. Growth and viability of commercial Bifidobacterium spp. in honey-sweetened skim milk. J. Food Prot. 2001, 64, 1775–1779. [Google Scholar] [CrossRef] [PubMed]
- Shruti, S.; Sreeja, V.; Jashbhai, P.B. Development of synbiotic lassi containing honey: Studies on probiotic viability, product characteristics and shelf life. Ind. J. Dairy Sci. 2016, 69, 148–153. [Google Scholar]
- Shmala, T.R.; Shri Jyothi, Y.; Saibaba, P. Stimulatory effect of honey on multiplication of lactic acid bacteria under in vitro and in vivo conditions. Lett. Appl. Microbiol. 2001, 30, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.M.; Molyneux, E.M.; Walsh, A.L.; Cheesbrough, J.S.; Molyneux, M.E.; Hart, C.A. Nontyphoidal Salmonella infections of children in tropical Africa. J. Pediatr. Infect. Dis. 2000, 19, 1189–1196. [Google Scholar] [CrossRef]
- Vojdani, J.D.; Beuchat, L.R.; Tauxe, R.V. Juice-associated outbreaks of human illness in the Unites States 1995 through 2005. J. Food Prot. 2008, 71, 356–364. [Google Scholar] [CrossRef]
- Von Der Ohe, W.; Oddo, L.; Piana, M.; Morlot, M.; Martin, P. Harmonized methods of melissopalinology. Apidologie 2004, 35, 18–25. [Google Scholar] [CrossRef]
- Bogdanov, S.; Lüllmann, C.; Martin, P.; von der Ohe, W.; Russmann, H.; Vorwohl, G.; Persano Oddo, L.; Sabatini, A.G.; Marcazzan, G.L.; Piro, R.; et al. Honey quality and international regulatory standardas: Review by the International Honey Commission. Bee World 1999, 80, 61–69. [Google Scholar] [CrossRef]
- Broznić, D.; Malenica Staver, M.; Kraljević Pavelić, S.; Gobin, I. Evaluation of the antioxidant capacity, antimicrobial and antiproliferative potential of fir (Abies alba Mill.) honeydew honeycollected from Gorski kotar (Croatia). Food Tech. Biotech. 2018, 56, 533–545. [Google Scholar] [CrossRef]
- Majtan, J.; Majtanova, L.; Bohova, J.; Majtan, V. Honeydew honey as a potent antibacterial agent in eradication of multi-drug resistant Stenotrophomonas maltophilia isolates from cancer patients. Phytother. Res. 2011, 25, 584–587. [Google Scholar] [CrossRef]
- Perez Martin, R.A.; Hortiguela, L.V.; Lorenzo, P.; Cortina, M.D.R.; de Lorenzo, C. In vitro antioxidant and antimicrobial activities of Spanish honeys. Int. J. Food Prop. 2008, 11, 727–737. [Google Scholar] [CrossRef]
- Sagdic, O.; Silici, S.; Ekici, L. Evaluation of the phenolic content, antiradical, antioxidant, and antimicrobial activity of different floral sources of honey. Int. J. Food Prop. 2013, 16, 658–666. [Google Scholar] [CrossRef]
- Spilioti, E.; Jaakkola, M.; Tolonen, T.; Lipponen, M.; Virtanen, V.; Chinou, I.; Kassi, E.; Karabournioti, S.; Moutsatsou, P. Phenolic acid composition, antiatherogenic and anticancer potential of honeys derived from various regions in Greece. PLoS ONE 2014, 9, e94860. [Google Scholar] [CrossRef] [PubMed]
- Bertoncelj, J.; Golob, T.; Kropf, U.; Korošec, M. Characterisation of Slovenian honeys on the basis of sensory and physicochemical analysis with a chemometric approach. Int. J. Food Sci. Tech. 2011, 46, 1661–1671. [Google Scholar] [CrossRef]
- Bertoncelj, J.; Doberšek, U.; Korošec, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Kuš, P.M.; Jerković, I.; Marijanović, Z.; Tuberoso, C.I.G. Screening of Polish fir honeydew honey using GC/MS, HPLC-DAD, and physical-chemical parameters: Benzene derivatives and terpenes as chemical markers. Chem. Biodivers. 2017, 14, e1700179. [Google Scholar] [CrossRef] [PubMed]
- Bertoncelj, J.; Polak, T.; Korpf, U.; Korošec, M.; Golob, T. LC-DAD/ESI-MS analysis of flavonoids and abscisic acid with chemometric approach for the classification of Slovenian honey. Food Chem. 2011, 127, 296–302. [Google Scholar] [CrossRef]
- Macedo, L.N.; Luchese, R.H.; Guerra, A.F.; Barbarosa, C.G. Prebiotic effect of honey on growth and viability of Bifidobacterium spp. and Lactobacillus spp. in milk. Cienc. Tecnnol. 2008, 28, 935–942. [Google Scholar]
- Popa, D.; Ustunol, Z. Influence of sucrose, high fructose corn syrup and honey from different floral sources on growth and acid production by lactic acid bacteria and bifidobacteria. Int. J. Dairy Technol. 2011, 64, 247–253. [Google Scholar] [CrossRef]
- Sanz, M.L.; Polemis, N.; Morales, V.; Corzo, N.; Drakoularakou, A.; Gibson, G.R.; Rastall, R.A. In vitro investigation into the potential prebiotic. J. Agric. Food. 2005, 53, 2914–2921. [Google Scholar] [CrossRef]
- Krauze, A.; Zalewski, R.I. Classification of honeys by principal component analysis on the basis of chemical and physical parameters. Z. Für Lebensm.-Unters. Und Forsch. 1991, 192, 19–23. [Google Scholar] [CrossRef]
- Kaškoiene, V.; Venskutonis, P.R. Floral markers in honey of various botanical and geographic origins: A review. Comp. Rev. 2010, 9, 620–634. [Google Scholar]
- Bogdanov, S.; Martin, P. Honey authenticity. Mitt. Aus Lebensm. Und Hyg. 2002, 93, 232–254. [Google Scholar]
- Gibson, G.R.; Fuller, R. Aspects of in vitro and in vivo research approaches directed toward identifying probiotics and prebiotics for human use. J. Nutr. 2000, 130, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Satishi Kumar, R.; Paari, K.A.; Pattukumar, V.; Arul, V. Probiotics and its functionally valubale products-a rewiev. Crit. Rev. Food Sci. Nutr. 2013, 53, 641–658. [Google Scholar] [CrossRef] [PubMed]
- Travers, M.A.; Florent, I.; Kohl, L.; Grellire, P. Probiotics for the control of parasites: An overview. J. Parasitol. Res. 2011, 2011, 610769. [Google Scholar] [CrossRef]
- Alizadeh Behbajani, B.; Noshad, M.; Falah, F. Inhibition of Escherichia coli adhesion to human intestinal Caco-2 cells by probiotic candidate Lactobacillus plantarum strain L15. Microb. Pathog. 2019, 136, 103677. [Google Scholar] [CrossRef]
- Fonesca, H.C.; de Sousa Melo, D.; Lacerda Ramos, C.; Ribeiro Dias, D.; Freitas Schwan, R. Probiotic properties of lactobacilli and their ability to inhibit the adhesion of entheropathogenic bacteria to Caco-2 and HT-29 cells. Probiotics Antimicrob. Protein. 2021, 13, 102–112. [Google Scholar] [CrossRef]
- Lin, J.; Lee, I.S.; Slonczewski, J.L.; Foster, J.W. Comparative analysis of extreme acid survival in Salmonella typhimurium, Shigella flexneri, and Escherichia coli. J. Bacteriol. 1995, 177, 4097–4104. [Google Scholar] [CrossRef]
- Brenneman, K.E.; Willingham, C.; Kong, W.; Curtis, R., 3rd; Roland, K.L. Low-pH rescue of acid-sensitive Salmonella enterica serovar Typhi strains by a rhamnose-regulated arginine decarboxylase system. J. Bacteriol. 2013, 195, 3062–3072. [Google Scholar] [CrossRef][Green Version]
- Kajiwara, S.; Gandhi, H.; Ustunol, Z. Effect of honey on the growth of and acid production by human intestinal Bifidobacterium spp.: An in vitro comparison with commercial oligosaccharides and inulin. J. Food Prot. 2002, 65, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Jan Mei, S.; Mohd Nordin, M.S.; Norrakiah, A.S. Fructooligosaccharides in honey and effects of honey on growth of Bifidobacterium longum BB 536. Int. Food Res. J. 2010, 17, 557–561. [Google Scholar]
- Janković, T.; Frece, J.; Abram, M.; Gobin, I. Aggregation ability of potential probiotic Lactobacillus plantarum strains. Int. J. Sanit. Eng. Res. 2012, 6, 19–24. [Google Scholar]
- Jiang, L.; Xie, M.; Chen, G.; Qiao, J.; Zhang, H.; Zeng, X. Phenolics and carbohydrates in buckwheat honey regulate the human intestinal microbiota. Evid.-Based Complement. Altern. Med. 2020, 2020, 6432942. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, R.; Kaur, A. Synbiotic effect of various prebiotics on in vitro activities of probiotic lactobacilli. Ecol. Food Nutr. 2011, 50, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Bogdanov, S. Harmonised Methods of the International Honey Commission; International Honey Commission Bremen: Bremen, Germany, 2009. [Google Scholar]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Potočnjak, M.; Pušić, P.; Frece, J.; Abram, M.; Janković, T.; Gobin, I. Three new Lactobacillus plantarum strains in the probiotic toolbox against gut pathogen Salmonella enterica serotype Typhimurium. Food Technol. Biotechnol. 2017, 55, 48–54. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters; EUCAST: Växjö, Sweden, 2021. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).