Synergistic Potentiation of Antimicrobial and Antibiofilm Activities of Penicillin and Bacitracin by Octyl Gallate, a Food-Grade Antioxidant, in Staphylococcus epidermidis
Abstract
1. Introduction
2. Results
2.1. Antimicrobial Synergy of OG and Antibiotics against S. epidermidis
2.2. Permeabilization of Cell Walls by OG in S. epidermidis
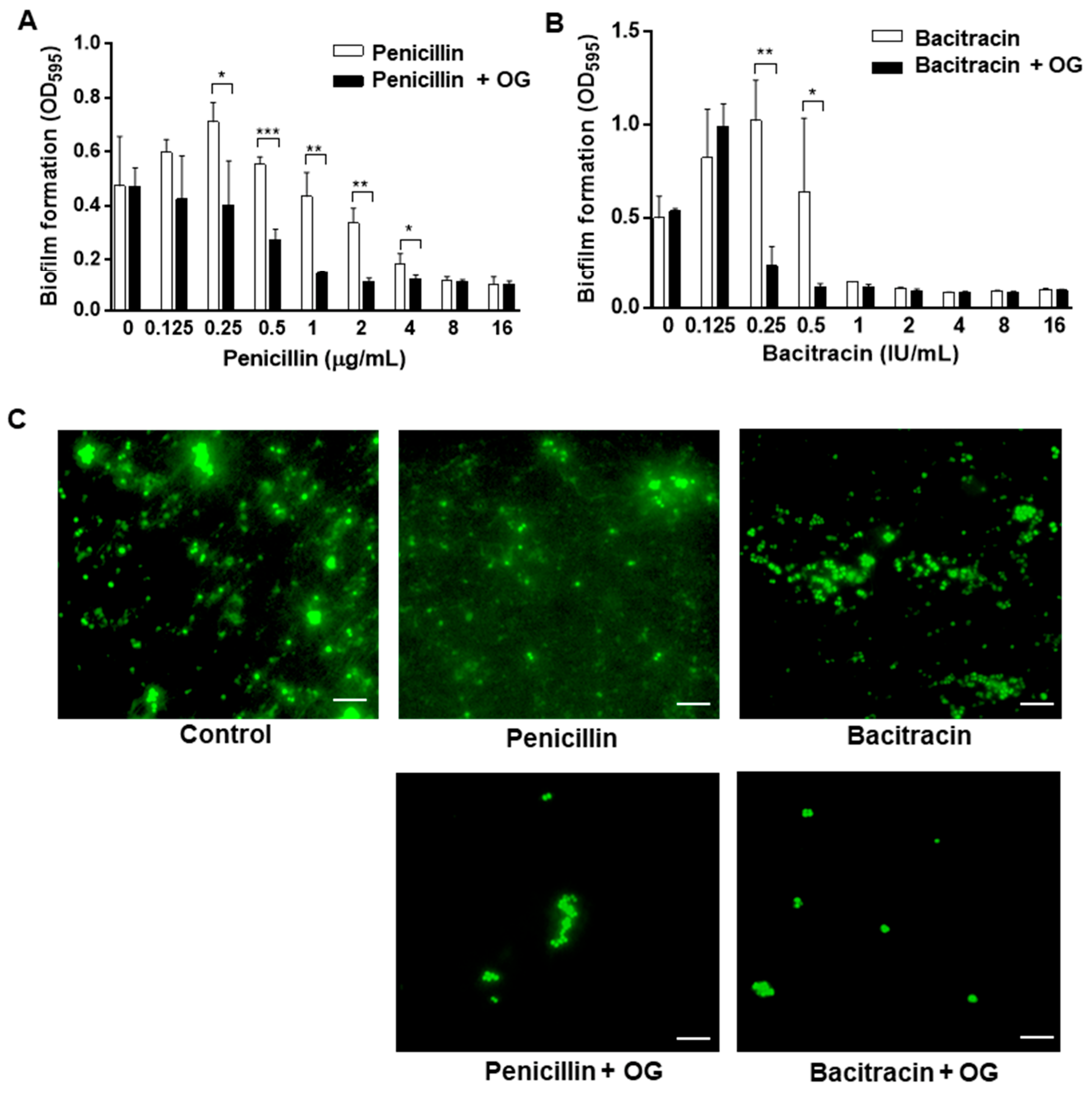
2.3. Antibiofilm Synergies of OG and Antibiotics against S. epidermidis
3. Discussion
4. Materials and Methods
4.1. Bacterial Strain and Culture
4.2. Antimicrobial Susceptibility Tests
4.3. Determination of Antimicrobial Synergy
4.4. Biofilm Assay
4.5. Fluorescence Microscopic Analysis of Biofilms
4.6. Cell Wall Permeability Assay
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Potapova, I. Functional Imaging in Diagnostic of Orthopedic Implant-Associated Infections. Diagnostics 2013, 3, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Severn, M.M.; Horswill, A.R. Staphylococcus epidermidis and its dual lifestyle in skin health and infection. Nat. Rev. Microbiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Uçkay, I.; Pittet, D.; Vaudaux, P.; Sax, H.; Lew, D.; Waldvogel, F. Foreign body infections due to Staphylococcus epidermidis. Ann. Med. 2009, 41, 109–119. [Google Scholar] [CrossRef]
- Cogen, A.L.; Nizet, V.; Gallo, R.L. Skin microbiota: A source of disease or defence? Br. J. Dermatol. 2008, 158, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Anjum, F. Staphylococcus epidermidis. In StatPearls; StatPearls Publishing Copyright© 2022; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Diekema, D.J.; Pfaller, M.A.; Schmitz, F.J.; Smayevsky, J.; Bell, J.; Jones, R.N.; Beach, M. Survey of infections due to Staphylococcus species: Frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin. Infect. Dis. 2001, 32 (Suppl. 2), S114–S132. [Google Scholar] [CrossRef]
- Chabi, R.; Momtaz, H. Virulence factors and antibiotic resistance properties of the Staphylococcus epidermidis strains isolated from hospital infections in Ahvaz, Iran. Trop. Med. Health 2019, 47, 56. [Google Scholar] [CrossRef]
- Lee, J.Y.H.; Monk, I.R.; Gonçalves da Silva, A.; Seemann, T.; Chua, K.Y.L.; Kearns, A.; Hill, R.; Woodford, N.; Bartels, M.D.; Strommenger, B.; et al. Global spread of three multidrug-resistant lineages of Staphylococcus epidermidis. Nat. Microbiol. 2018, 3, 1175–1185. [Google Scholar] [CrossRef]
- Theuretzbacher, U.; Outterson, K.; Engel, A.; Karlén, A. The global preclinical antibacterial pipeline. Nat. Rev. Microbiol. 2020, 18, 275–285. [Google Scholar] [CrossRef]
- Wright, G.D. Antibiotic Adjuvants: Rescuing Antibiotics from Resistance. Trends Microbiol. 2016, 24, 862–871. [Google Scholar] [CrossRef]
- Wright, G.D. Resisting resistance: New chemical strategies for battling superbugs. Chem. Biol. 2000, 7, R127–R132. [Google Scholar] [CrossRef]
- Pages, J.M.; Amaral, L. Mechanisms of drug efflux and strategies to combat them: Challenging the efflux pump of Gram-negative bacteria. Biochim. Biophys. Acta 2009, 1794, 826–833. [Google Scholar] [CrossRef]
- Nguyen, R.; Khanna, N.R.; Safadi, A.O.; Sun, Y. Bacitracin Topical. In StatPearls; StatPearls Publishing Copyright© 2022; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022. [Google Scholar]
- Heal, C.F.; Banks, J.L.; Lepper, P.D.; Kontopantelis, E.; van Driel, M.L. Topical antibiotics for preventing surgical site infection in wounds healing by primary intention. Cochrane Database Syst. Rev. 2016, 11, Cd011426. [Google Scholar] [CrossRef]
- Kim, J.C.; Jeon, B. Novel adjuvant strategy to potentiate bacitracin against MDR MRSA. J. Antimicrob. Chemother. 2016, 71, 1260–1263. [Google Scholar] [CrossRef][Green Version]
- Oh, E.; Bae, J.; Kumar, A.; Choi, H.J.; Jeon, B. Antioxidant-based synergistic eradication of methicillin-resistant Staphylococcus aureus (MRSA) biofilms with bacitracin. Int. J. Antimicrob. Agents 2018, 52, 96–99. [Google Scholar] [CrossRef]
- Tamang, M.D.; Bae, J.; Park, M.; Jeon, B. Potentiation of β-Lactams against Methicillin-Resistant Staphylococcus aureus (MRSA) Using Octyl Gallate, a Food-Grade Antioxidant. Antibiotics 2022, 11, 266. [Google Scholar] [CrossRef]
- FDA. Food Additive Status List. 2022. Available online: https://www.fda.gov/food/food-additives-petitions/food-additive-status-list#ftnO (accessed on 7 November 2022).
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Tipper, D.J.; Strominger, J.L. Mechanism of action of penicillins: A proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc. Natl. Acad. Sci. USA 1965, 54, 1133–1141. [Google Scholar] [CrossRef]
- Stone, K.J.; Strominger, J.L. Mechanism of action of bacitracin: Complexation with metal ion and C 55 -isoprenyl pyrophosphate. Proc. Natl. Acad. Sci. USA 1971, 68, 3223–3227. [Google Scholar] [CrossRef]
- Oliveira, W.F.; Silva, P.M.S.; Silva, R.C.S.; Silva, G.M.M.; Machado, G.; Coelho, L.; Correia, M.T.S. Staphylococcus aureus and Staphylococcus epidermidis infections on implants. J. Hosp. Infect. 2018, 98, 111–117. [Google Scholar] [CrossRef]
- Dadi, N.C.T.; Radochová, B.; Vargová, J.; Bujdáková, H. Impact of Healthcare-Associated Infections Connected to Medical Devices-An Update. Microorganisms 2021, 9, 2332. [Google Scholar] [CrossRef]
- Brown, M.M.; Horswill, A.R. Staphylococcus epidermidis—Skin friend or foe? PLoS Pathog. 2020, 16, e1009026. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Mojica, N.; Jiang, W.; Cosgrove, S.E.; Septimus, E.; Morgan, D.J.; Laxminarayan, R. Trends in Methicillin-Resistant Staphylococcus aureus Hospitalizations in the United States, 2010–2014. Clin. Infect. Dis. 2017, 65, 1921–1923. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.H.; Yu, C.M.; Yu, V.L.; Chow, J.W. Synergy assessed by checkerboard a critical analysis. Diagn. Microbiol. Infect. Dis. 1993, 16, 343–349. [Google Scholar] [CrossRef]
- Rand, K.H.; Houck, H.J.; Brown, P.; Bennett, D. Reproducibility of the microdilution checkerboard method for antibiotic synergy. Antimicrob. Agents Chemother. 1993, 37, 613–615. [Google Scholar] [CrossRef]
- Park, M.; Kim, J.; Horn, L.; Haan, J.; Strickland, A.; Lappi, V.; Boxrud, D.; Hedberg, C.; Ryu, S.; Jeon, B. Sugar Modification of Wall Teichoic Acids Determines Serotype-Dependent Strong Biofilm Production in Listeria monocytogenes. Microbiol. Spectr. 2022, 10, e02769-22. [Google Scholar] [CrossRef]


| OG (µg/mL) | Penicillin | Bacitracin | ||
|---|---|---|---|---|
| MIC a (µg/mL) | MBC b (µg/mL) | MIC a (IU/mL) | MBC b (IU/mL) | |
| 0 | 4 | 8 | 4 | 8 |
| 2 | 2 | 8 | 2 | 4 |
| 4 | 1 | 4 | 2 | 4 |
| 8 | 0.5 | 1 | 1 | 2 |
| OG (µg/mL) | FIC Index | FBC Index | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Penicillin (µg/mL) | Bacitracin (IU/mL) | Penicillin (µg/mL) | Bacitracin (IU/mL) | |||||||||||||
| 0.5 | 1 | 2 | 4 | 0.5 | 1 | 2 | 4 | 0.5 | 1 | 2 | 4 | 0.5 | 1 | 2 | 4 | |
| 2 | 0.750 | 0.625 | 0.563 | 0.500 | 1.000 | 0.750 | 0.563 | 0.500 | 1.250 | 1.250 | 1.125 | 1.063 | 1.000 | 1.000 | 1.000 | 0.563 |
| 4 | 0.500 | 0.375 | 0.313 | 0.250 | 1.000 | 0.750 | 0.563 | 0.500 | 0.750 | 0.750 | 0.625 | 0.563 | 1.000 | 1.000 | 1.000 | 0.563 |
| 8 | 0.375 | 0.250 | 0.188 | 0.125 | 0.750 | 0.500 | 0.313 | 0.250 | 0.500 | 0.500 | 0.375 | 0.313 | 1.000 | 1.000 | 1.000 | 0.531 |
| 16 | 0.266 | 0.141 | 0.078 | 0.016 | 0.508 | 0.258 | 0.070 | 0.008 | 0.313 | 0.313 | 0.188 | 0.125 | 0.313 | 0.313 | 0.313 | 0.094 |
| 32 | 0.250 | 0.125 | 0.063 | 0.016 | 0.500 | 0.250 | 0.063 | 0.008 | 0.281 | 0.281 | 0.156 | 0.094 | 0.281 | 0.281 | 0.281 | 0.063 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santativongchai, P.; Tulayakul, P.; Ji, Y.; Jeon, B. Synergistic Potentiation of Antimicrobial and Antibiofilm Activities of Penicillin and Bacitracin by Octyl Gallate, a Food-Grade Antioxidant, in Staphylococcus epidermidis. Antibiotics 2022, 11, 1775. https://doi.org/10.3390/antibiotics11121775
Santativongchai P, Tulayakul P, Ji Y, Jeon B. Synergistic Potentiation of Antimicrobial and Antibiofilm Activities of Penicillin and Bacitracin by Octyl Gallate, a Food-Grade Antioxidant, in Staphylococcus epidermidis. Antibiotics. 2022; 11(12):1775. https://doi.org/10.3390/antibiotics11121775
Chicago/Turabian StyleSantativongchai, Pitchaya, Phitsanu Tulayakul, Yinduo Ji, and Byeonghwa Jeon. 2022. "Synergistic Potentiation of Antimicrobial and Antibiofilm Activities of Penicillin and Bacitracin by Octyl Gallate, a Food-Grade Antioxidant, in Staphylococcus epidermidis" Antibiotics 11, no. 12: 1775. https://doi.org/10.3390/antibiotics11121775
APA StyleSantativongchai, P., Tulayakul, P., Ji, Y., & Jeon, B. (2022). Synergistic Potentiation of Antimicrobial and Antibiofilm Activities of Penicillin and Bacitracin by Octyl Gallate, a Food-Grade Antioxidant, in Staphylococcus epidermidis. Antibiotics, 11(12), 1775. https://doi.org/10.3390/antibiotics11121775










