Changes in Use of Blood Cultures in a COVID-19-Dedicated Tertiary Hospital
Abstract
1. Introduction
2. Results
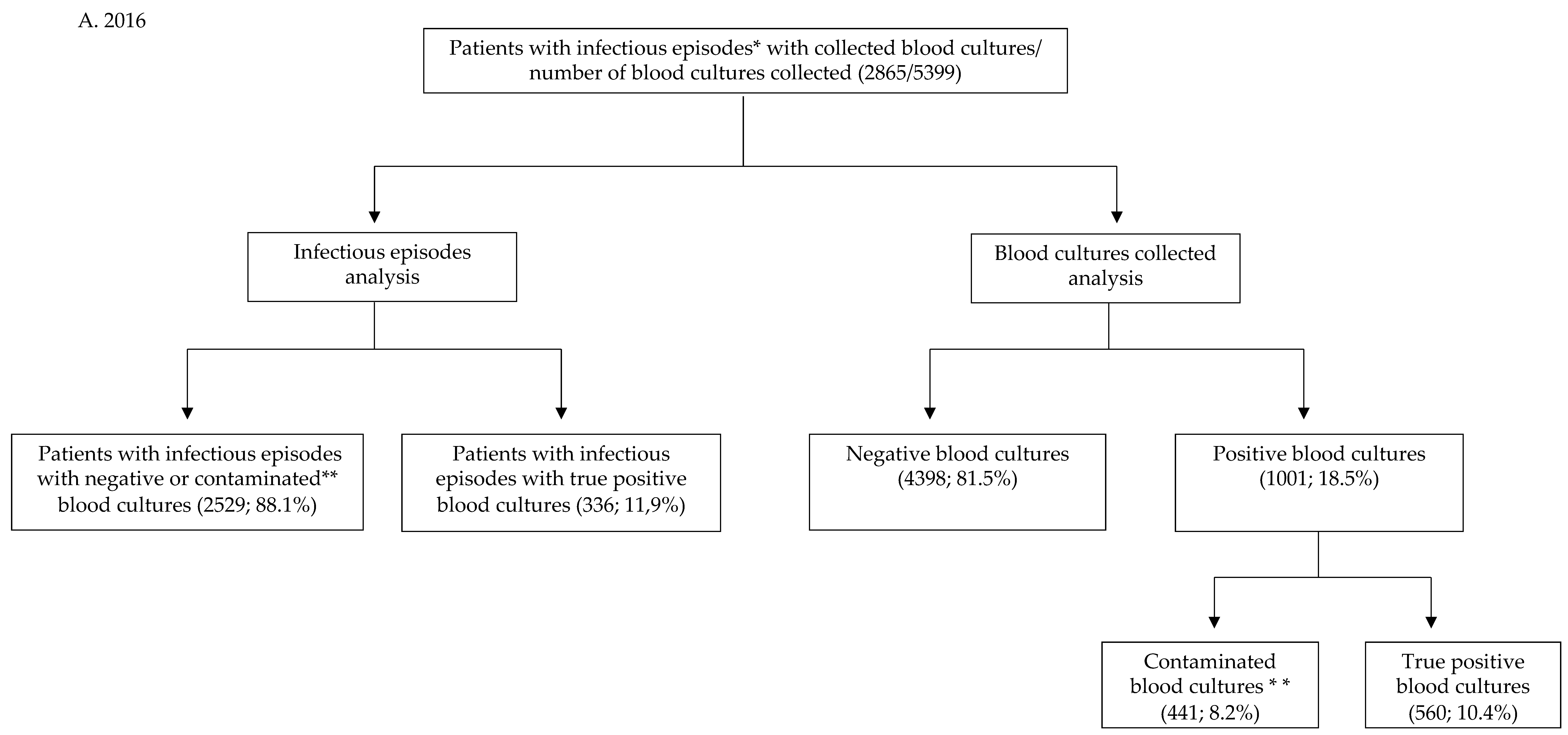
2.1. Statistics in the COVID-19 Group
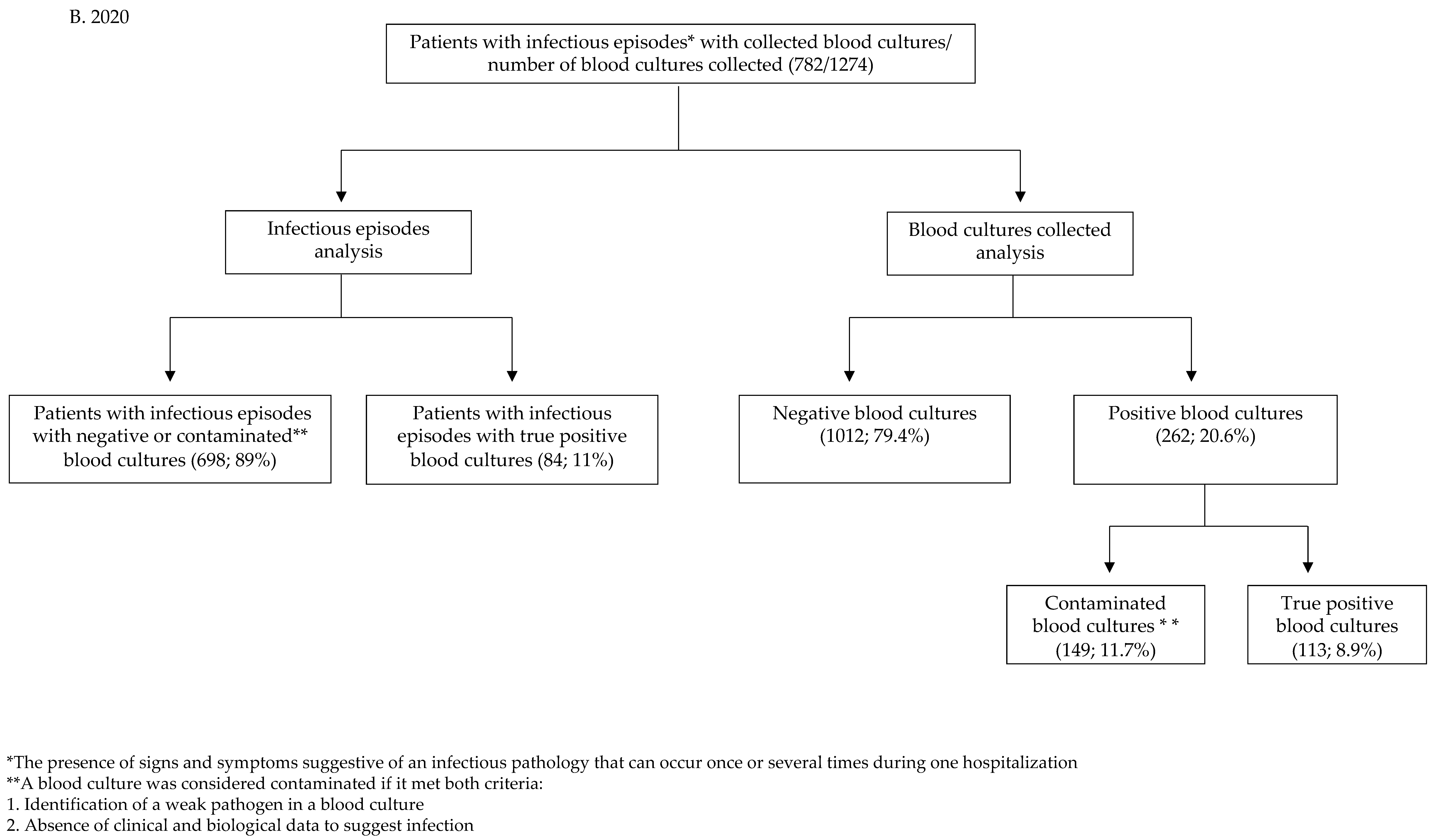
2.2. Statistics in the Pre-COVID-19 Group
3. Discussion
Study Limitations
4. Materials and Methods
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America for the Treatment of Methicillin-Resistant Staphylococcus Aureus Infections in Adults and Children. Clin. Infect. Dis. 2011, 52, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, N.I.; Wolfe, R.E.; Wright, S.B.; Moore, R.; Bates, D.W. Who Needs a Blood Culture? A Prospectively Derived and Validated Prediction Rule. J. Emerg. Med. 2008, 35, 255–264. [Google Scholar] [CrossRef]
- Otani, T.; Ichiba, T.; Seo, K.; Naito, H. Blood Cultures Should Be Collected for Acute Cholangitis Regardless of Severity. J. Infect. Chemother. 2022, 28, 181–186. [Google Scholar] [CrossRef]
- Baron, E.J.; Weinstein, M.P.; Dunne, W.M.; Yagupsky, P.; Welch, D.F.; Wilson, D.M. Blood Cultures IV Cumitech Cumulative Techniques and Procedures in Clinical; Baron, E.J., Ed.; ASM Press: Washington, DC, USA, 2005; pp. 2–32. [Google Scholar]
- Scheer, C.S.; Fuchs, C.; Gründling, M.; Vollmer, M.; Bast, J.; Bohnert, J.A.; Zimmermann, K.; Hahnenkamp, K.; Rehberg, S.; Kuhn, S.O. Impact of Antibiotic Administration on Blood Culture Positivity at the Beginning of Sepsis: A Prospective Clinical Cohort Study. Clin. Microbiol. Infect. 2019, 25, 326–331. [Google Scholar] [CrossRef]
- Lamy, B.; Dargère, S.; Arendrup, M.C.; Parienti, J.J.; Tattevin, P. How to Optimize the Use of Blood Cultures for the Diagnosis of Bloodstream Infections? A State-of-the Art. Front. Microbiol. 2016, 7, 697. [Google Scholar] [CrossRef]
- Weinstein, M.P.; Reller, L.B.; Murphy, J.R.; Lichtenstein, K.A. The Clinical Significance of Positive Blood Cultures: A Comprehensive Analysis of 500 Episodes of Bacteremia and Fungemia in Adults. I. Laboratory and Epidemiologic Observations. Rev. Infect. Dis. 1983, 5, 35–53. [Google Scholar] [CrossRef]
- Hall, K.K.; Lyman, J.A. Updated Review of Blood Culture Contamination. Clin. Microbiol. Rev. 2006, 19, 788–802. [Google Scholar] [CrossRef]
- Bekeris, L.G.; Tworek, J.A.; Walsh, M.K.; Valenstein, P.N. Trends in Blood Culture Contamination A College of American Pathologists Q-Tracks Study of 356 Institutions. Arch. Pathol. Lab. Med. 2005, 129, 1222–1225. [Google Scholar] [CrossRef]
- Bates, D.W.; Cook, E.F.; Lee Goldman, S.; Lee, T.H. Predicting Bacteremia in Hospitalized Patients A Prospectively Validated Model. Ann. Intern. Med. 1990, 113, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.; Wiklund, A.E.; Pålsson, A.S.; Melander, E.Z.; Wullt, M.; Cronqvist, J.; Walder, M.; Sturegård, E. Reducing Blood Culture Contamination by a Simple Informational Intervention. J. Clin. Microbiol. 2010, 48, 4552–4558. [Google Scholar] [CrossRef] [PubMed]
- Dawson, S. Blood Culture Contaminants. J. Hosp.Infect. 2014, 87, 1–10. [Google Scholar] [CrossRef]
- Miller, J.M.; Binnicker, M.J.; Campbell, S.; Carroll, K.C.; Chapin, K.C.; Gilligan, P.H.; Gonzalez, M.D.; Jerris, R.C.; Kehl, S.C.; Patel, R.; et al. A Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2018 Update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin. Infect. Dis. 2018, 67, e1–e94. [Google Scholar] [CrossRef] [PubMed]
- Bentley, J.; Thakore, S.; Muir, L.; Baird, A.; Lee, J. A Change of Culture: Reducing Blood Culture Contamination Rates in an Emergency Department. BMJ Qual. Improv. Rep. 2016, 5, u206760.w2754. [Google Scholar] [CrossRef]
- Halstead, D.C.; Sautter, R.L.; Snyder, J.W.; Crist, A.E.; Nachamkin, I. Reducing Blood Culture Contamination Rates: Experiences of Four Hospital Systems. Infect. Dis. Ther. 2020, 9, 389–401. [Google Scholar] [CrossRef]
- Sezgin, F.M.; Babaoglu, U.T. Blood Culture Results at a Research and Training Hospital and the Importance of Training. Niger J. Clin. Pract. 2019, 22, 1693–1697. [Google Scholar] [CrossRef]
- Self, W.H.; Speroff, T.; Grijalva, C.G.; McNaughton, C.D.; Ashburn, J.; Liu, D.; Arbogast, P.G.; Russ, S.; Storrow, A.B.; Talbot, T.R. Reducing Blood Culture Contamination in the Emergency Department: An Interrupted Time Series Quality Improvement Study. Acad. Emerg. Med. 2013, 20, 89–97. [Google Scholar] [CrossRef]
- Gander, R.M.; Byrd, L.; DeCrescenzo, M.; Hirany, S.; Bowen, M.; Baughman, J. Impact of Blood Cultures Drawn by Phlebotomy on Contamination Rates and Health Care Costs in a Hospital Emergency Department. J. Clin. Microbiol. 2009, 47, 1021–1024. [Google Scholar] [CrossRef]
- Rupp, M.E.; Cavalieri, R.J.; Marolf, C.; Lyden, E. Reduction in Blood Culture Contamination Through Use of Initial Specimen Diversion Device. Clin. Infect. Dis. 2017, 65, 201–205. [Google Scholar] [CrossRef]
- Menni, C.; Valdes, A.M.; Polidori, L.; Antonelli, M.; Penamakuri, S.; Nogal, A.; Louca, P.; May, A.; Figueiredo, J.C.; Hu, C.; et al. Symptom Prevalence, Duration, and Risk of Hospital Admission in Individuals Infected with SARS-CoV-2 during Periods of Omicron and Delta Variant Dominance: A Prospective Observational Study from the ZOE COVID Study. Lancet 2022, 399, 1618–1624. [Google Scholar] [CrossRef] [PubMed]
- Estenssoro, E.; Loudet, C.I.; Dubin, A.; Kanoore Edul, V.S.; Plotnikow, G.; Andrian, M.; Romero, I.; Sagardía, J.; Bezzi, M.; Mandich, V.; et al. Clinical Characteristics, Respiratory Management, and Determinants of Oxygenation in COVID-19 ARDS: A Prospective Cohort Study. J. Crit. Care. 2022, 71, 154021. [Google Scholar] [CrossRef] [PubMed]
- Trougakos, I.P.; Stamatelopoulos, K.; Terpos, E.; Tsitsilonis, O.E.; Aivalioti, E.; Paraskevis, D.; Kastritis, E.; Pavlakis, G.N.; Dimopoulos, M.A. Insights to SARS-CoV-2 Life Cycle, Pathophysiology, and Rationalized Treatments That Target COVID-19 Clinical Complications. J. Biomed. Sci. 2021, 28, 9. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Kollef, M.H.; Timsit, J.F. Bacterial and Fungal Superinfections in Critically Ill Patients with COVID-19. Intensive Care Med. 2020, 46, 2071–2074. [Google Scholar] [CrossRef]
- Ohki, R.; Fukui, Y.; Morishita, N.; Iwata, K. Increase of Blood Culture Contamination during COVID-19 Pandemic. A Retrospective Descriptive Study. Am. J. Infect. Control 2021, 49, 1359–1361. [Google Scholar] [CrossRef]
- Haedo, M.F.; Melendi, S.E.; Mauri, M.L.; Ujeda, C.; Leis, R. Usefulness of blood cultures in COVID-19 pneumonia. Medicina 2020, 80, 44–47. [Google Scholar]
- Russo, E.; Bolondi, G.; Gamberini, E.; Santonastaso, D.P.; Circelli, A.; Spiga, M.; Sambri, V.; Agnoletti, V. Increased Blood Culture Contamination Rate during COVID-19 Outbreak in Intensive Care Unit: A Brief Report from a Single-Centre. J. Intensive Care Soc. 2021, 23, 500–502. [Google Scholar] [CrossRef]
- Yu, D.; Ininbergs, K.; Hedman, K.; Giske, C.G.; Strålin, K.; Özenci, V. Low Prevalence of Bloodstream Infection and High Blood Culture Contamination Rates in Patients with COVID-19. PLoS ONE 2020, 15, e0242533. [Google Scholar] [CrossRef]
- Mormeneo Bayo, S.; Palacián Ruíz, M.P.; Moreno Hijazo, M.; Villuendas Usón, M.C. Bacteremia during COVID-19 Pandemic in a Tertiary Hospital in Spain. Enferm. Infecc. Microbiol. Clin. 2021, 40, 183–186. [Google Scholar] [CrossRef]
- Sepulveda, J.; Westblade, L.F.; Whittier, S.; Satlin, M.J.; Greendyke, W.G.; Aaron, J.G.; Zucker, J.; Dietz, D.; Sobieszczyk, M.; Choi, J.J.; et al. Bacteremia and Blood Culture Utilization during COVID-19 Surge in New York City. J. Clin. Microbiol. 2020, 58, e00875-20. [Google Scholar] [CrossRef]
| Number of Isolates | Difference 2020 vs. 2016 | ||
|---|---|---|---|
| Species | 2016 | 2020 | |
| Escherichia coli | 95 (28.3%) | 4 (4.8%) | p < 0.0001 |
| Staphylococcus aureus | 71 (21.1%) | 9 (10.7%) | p = 0.029 |
| Klebsiella pneumoniae | 35 (10.4%) | 16 (19%) | p = 0.031 |
| Enterobacterales (other) | 24 (7.1%) | 3 (3.6%) | p = 0.241 |
| Enterococcus spp. | 20 (5.9%) | 6 (7.1%) | p = 0.682 |
| Streptococcus pneumoniae | 16 (4.8%) | 1 (1.2%) | p = 0.136 |
| Acinetobacter baumannii | 15 (4.5%) | 22 (26.2%) | p < 0.0001 |
| Pseudomonas aeruginosa | 9 (2.7%) | 6 (7.1%) | p = 0.052 |
| Candida spp. | 9 (2.7%) | 5 (5.9%) | p = 0.145 |
| Stenotrophomonas maltophilia | 1 (0.3%) | 2 (2.4%) | p = 0.042 |
| Others | 41 (12.2%) | 10 (11.9%) | |
| TOTAL | 336 | 84 | |
| Parameter | 2016 (n; %) | 2020 (n; %) | Difference 2020 vs. 2016 |
|---|---|---|---|
| Infectious episodes for which blood cultures were collected | 2865 | 782 | |
Infectious episodes per medical unit type
| 2526 (88.2%) 339 (11.8%) | 562 (71.9%) 220 (28.1%) | z = 11.22 p < 0.0001 |
| Days of hospitalization | 140,733 | 88,767 | |
| Total number of blood cultures collected | 5399 | 1274 | |
| Blood cultures/1000 patient days | 38.36 | 14.35 | z = 33.36 p < 0.00001 |
Blood cultures collected per medical unit type
| 4850 (89.8%) 549 (10.2%) | 831 (65.2%) 443 (34.8%) | z = −22.13 p < 0.00001 |
| Blood culture sets/infectious episode | 1.91 ± 1.4 | 1.67 ± 1.16 | t = −4.50 p < 0.0001 |
Blood culture sets per infectious episode and per medical unit type
| 1.92 ± 1.16 1.62 ± 1.01 | 1.48 ± 0.84 2.01 ± 1.26 | t = −10.47, p < 0.0001 t = 5.41, p < 0.0001 |
| Infectious episodes with only one blood culture | 1475 (51.48%) | 489 (62.53%) | z = −5.49 p < 0.0001 |
Infectious episodes with only one blood culture per medical unit type
| 1257 (49.8%) 218 (64.3%) | 378 (67.3%) 111 (50.5%) | z = −7.52 p < 0.0001 z = 3.25 p = 0.0011 |
| Parameter | 2016 | 2020 | Difference 2020 vs. 2016 |
|---|---|---|---|
| Contaminated blood cultures (blood culture contamination rate) | 441 (8.2%) | 149 (11.7%) | z = −3.99 p = 0.00006 |
Contaminants
| 71.7% 7.3% 0.9% 20.1% | 91.3% 1.3% 4.7% 2.7% | p < 0.0001 |
| Infectious episodes with true positive blood cultures (rate of infectious episodes with true positive blood cultures/infectious episodes with blood cultures sampled) | 336 (11.9%) | 84 (11%) | z = 0.71 p = 0.479 |
| Infectious episodes with true positive blood cultures/1000 patient days | 2.39 | 0.95 | z = 7.87 p < 0.00001 |
Infectious episodes with true positive blood cultures/medical unit
| 36 (10.7%) 300 (89.3%) | 53 (63.1%) 31(36.9%) | z = 10.51, p < 0.00001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrei, A.-I.; Popescu, G.-A.; Popoiu, M.A.; Mihai, A.; Tălăpan, D. Changes in Use of Blood Cultures in a COVID-19-Dedicated Tertiary Hospital. Antibiotics 2022, 11, 1694. https://doi.org/10.3390/antibiotics11121694
Andrei A-I, Popescu G-A, Popoiu MA, Mihai A, Tălăpan D. Changes in Use of Blood Cultures in a COVID-19-Dedicated Tertiary Hospital. Antibiotics. 2022; 11(12):1694. https://doi.org/10.3390/antibiotics11121694
Chicago/Turabian StyleAndrei, Alina-Ioana, Gabriel-Adrian Popescu, Mona Argentina Popoiu, Alexandru Mihai, and Daniela Tălăpan. 2022. "Changes in Use of Blood Cultures in a COVID-19-Dedicated Tertiary Hospital" Antibiotics 11, no. 12: 1694. https://doi.org/10.3390/antibiotics11121694
APA StyleAndrei, A.-I., Popescu, G.-A., Popoiu, M. A., Mihai, A., & Tălăpan, D. (2022). Changes in Use of Blood Cultures in a COVID-19-Dedicated Tertiary Hospital. Antibiotics, 11(12), 1694. https://doi.org/10.3390/antibiotics11121694





