Metabolomic Profiling of the Responses of Planktonic and Biofilm Vibrio cholerae to Silver Nanoparticles
Abstract
:1. Introduction
2. Results
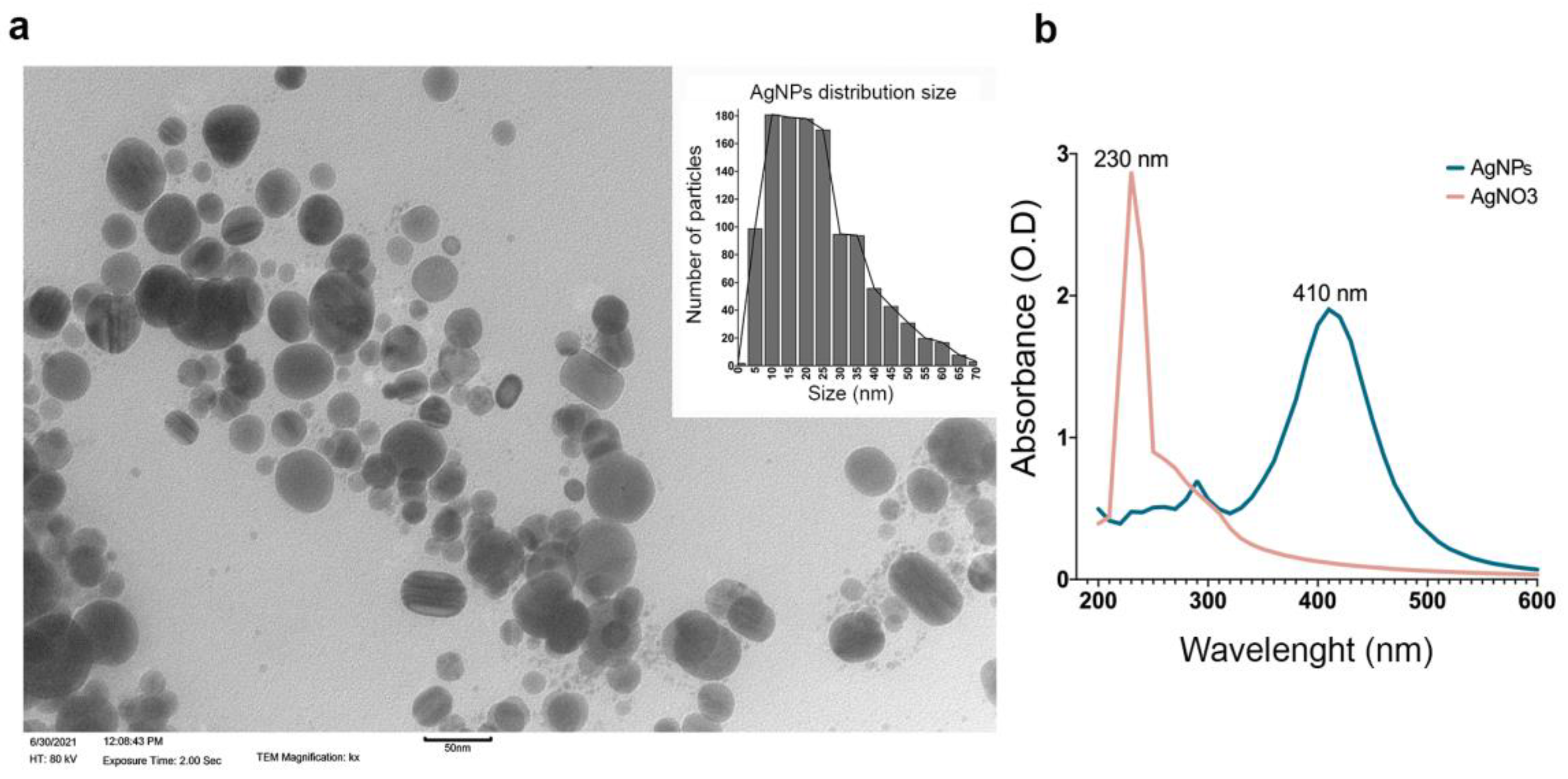
2.1. AgNP Characterization
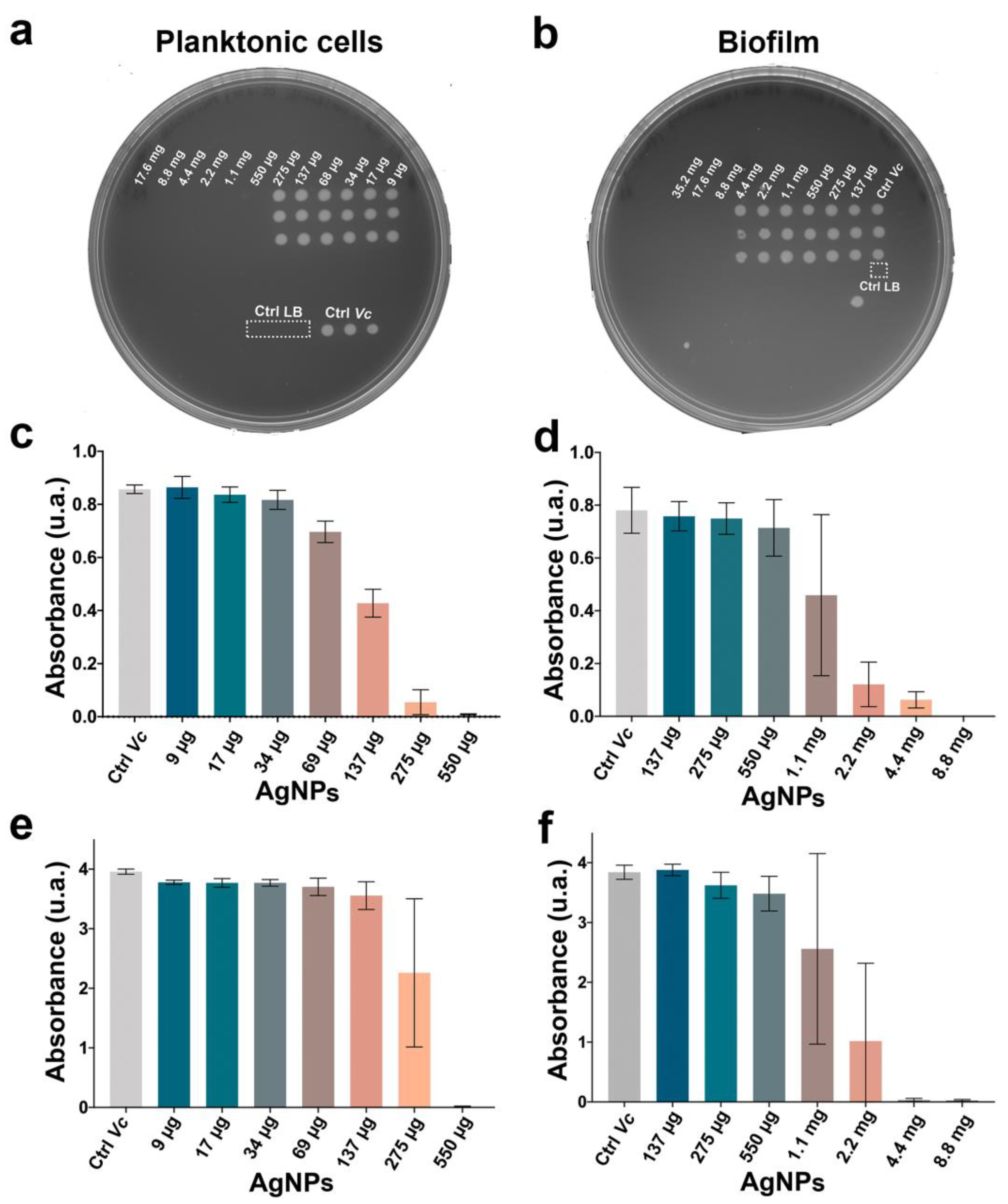
2.2. The AgNPs Showed a Different Antimicrobial Effect Depending on the Lifeform of Vibrio cholerae
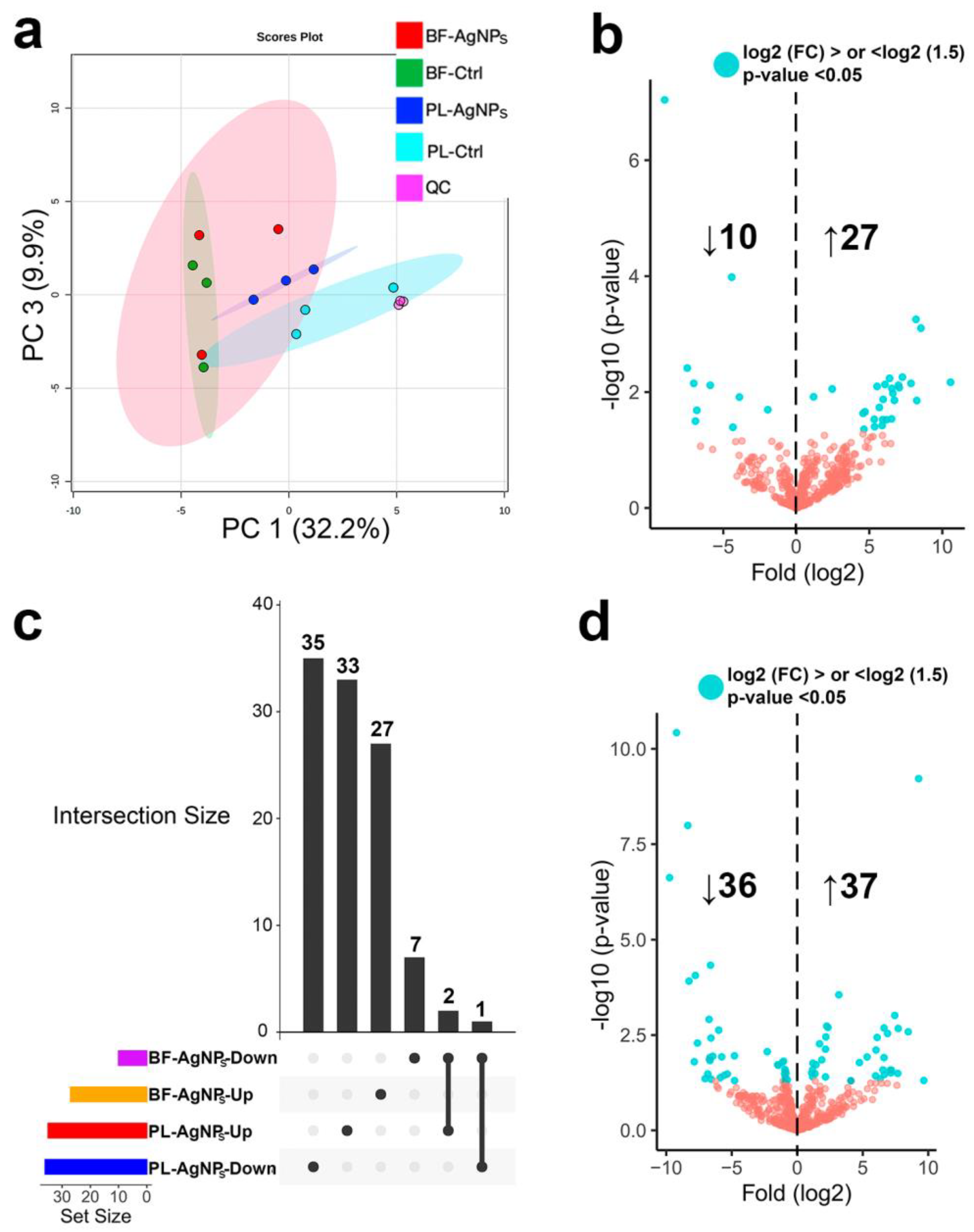
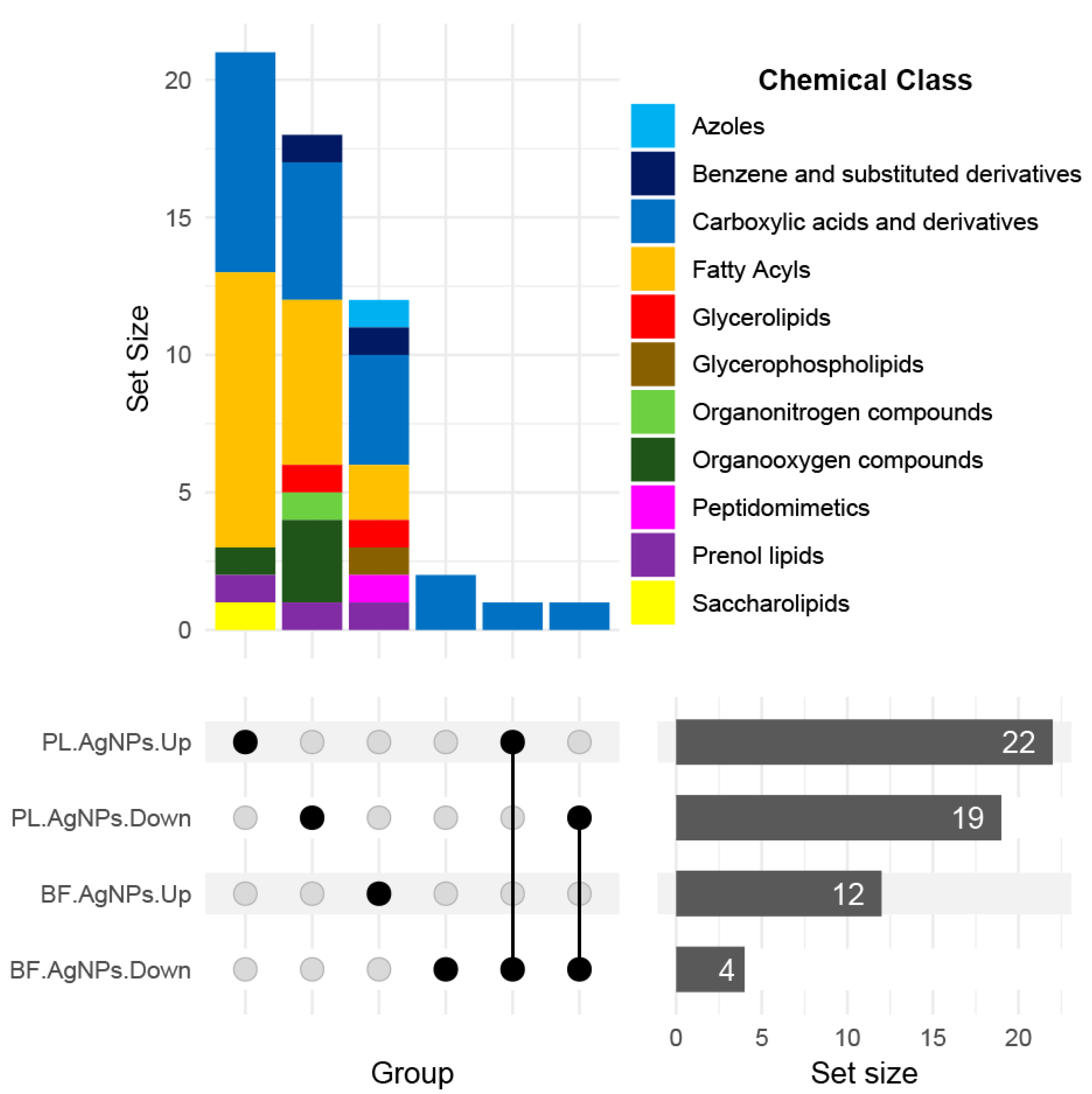
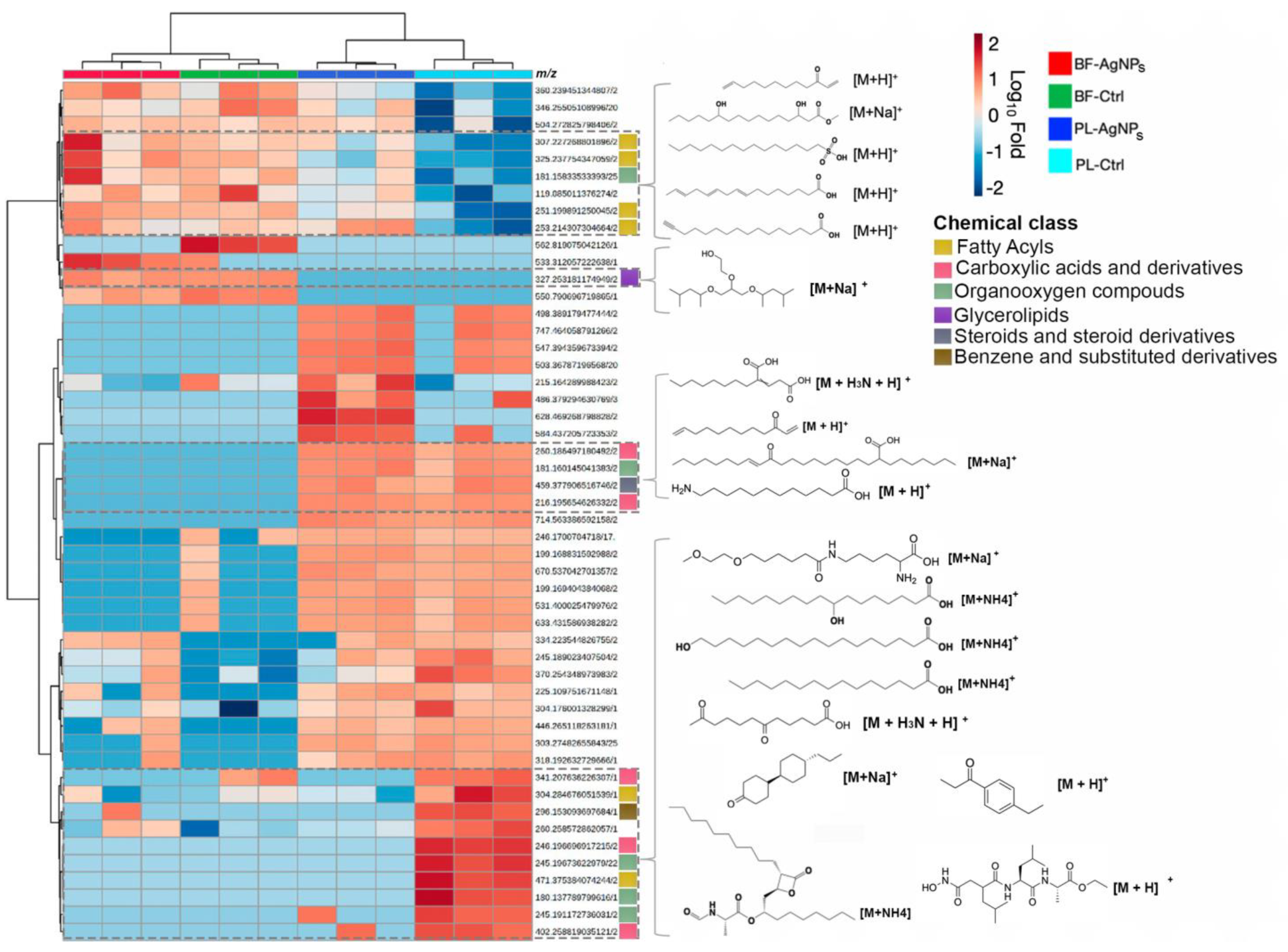
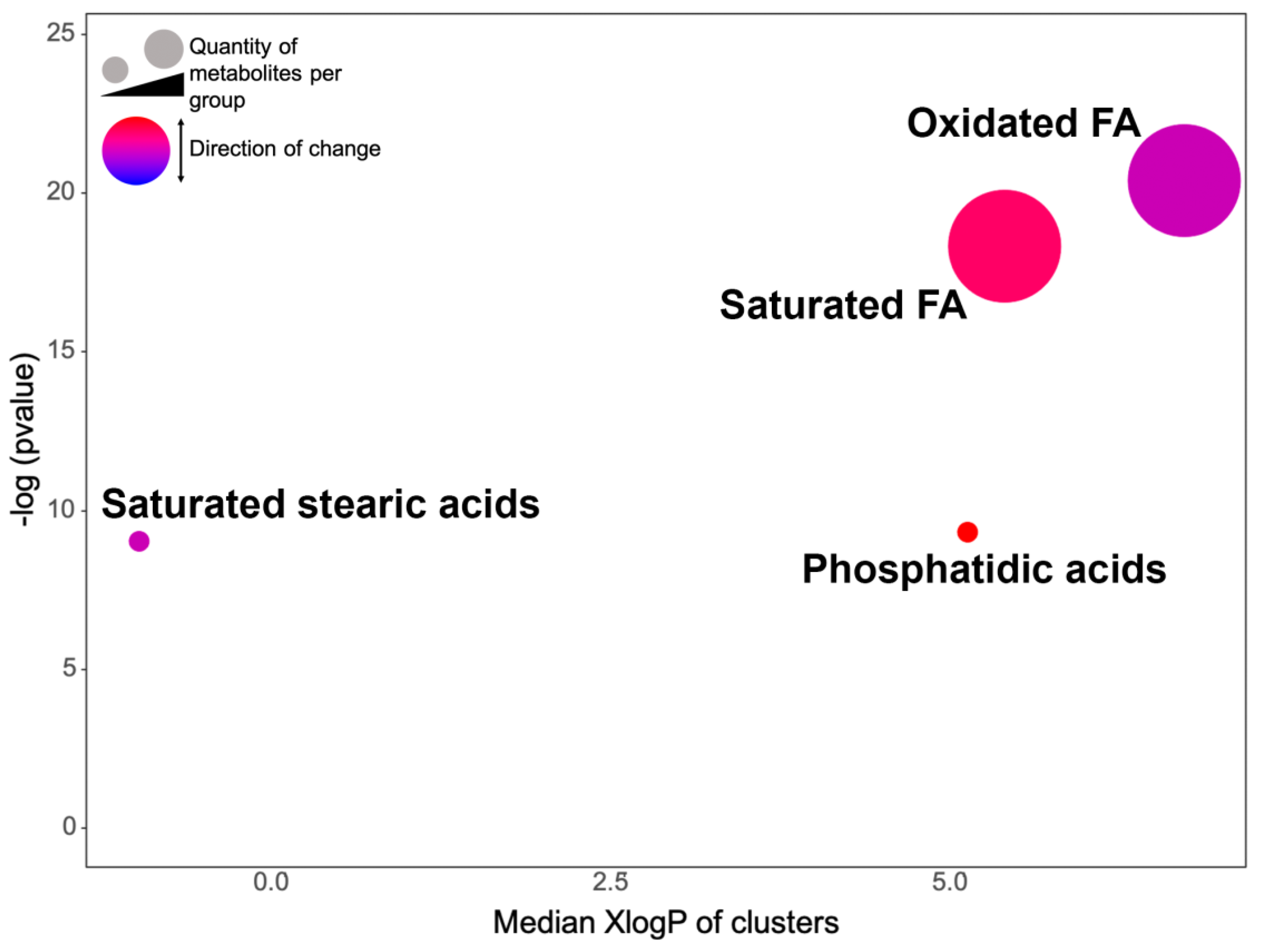
2.3. AgNPs Differentially Modify the Metabolome of Vibrio cholerae According to its Phenotype
3. Discussion
4. Materials and Methods
4.1. AgNPs Synthesis and Characterization
4.2. Vibrio cholerae Growth and AgNPs Exposure
4.3. Vibrio cholerae Planktonic Cells Antimicrobial Test
4.4. V. cholerae Biofilm Antimicrobial Test
4.5. Untargeted Metabolomics
4.5.1. Microbial Growth
4.5.2. Metabolites Extraction
4.5.3. LC-MS2 Data Acquisition
4.5.4. LC-MS2 Data Processing and Analysis
4.5.5. Data Availability
4.5.6. Metabolomics Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clemens, J.D.; Nair, G.B.; Ahmed, T.; Qadri, F.; Holmgren, J. Cholera. Lancet 2017, 390, 1539–1549. [Google Scholar] [CrossRef]
- Fanous, M.; King, K.C. Cholerae. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470232/?report=reader (accessed on 8 March 2021).
- World Health Organization. Cholera. Available online: https://www.who.int/news-room/fact-sheets/detail/cholera (accessed on 23 May 2021).
- Teschler, J.K.; Zamorano-Sánchez, D.; Utada, A.S.; Warner, C.J.A.; Wong, G.C.L.; Linington, R.G.; Yildiz, F.H. Living in the Matrix: Assembly and Control of Vibrio Cholerae Biofilms. Nat. Rev. Microbiol. 2015, 13, 255–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallego-Hernandez, A.L.; DePas, W.H.; Park, J.H.; Teschler, J.K.; Hartmann, R.; Jeckel, H.; Drescher, K.; Beyhan, S.; Newman, D.K.; Yildiz, F.H. Upregulation of Virulence Genes Promotes Vibrio Cholerae Biofilm Hyperinfectivity. Proc. Natl. Acad. Sci. USA 2020, 117, 11010–11017. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus Biofilm: An Emerging Battleground in Microbial Communities. Antimicrob. Resist. Infect. Control 2019, 3, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, B.; Verma, J.; Kumar, P.; Ghosh, A.; Ramamurthy, T. Antibiotic Resistance in Vibrio Cholerae: Understanding the Ecology of Resistance Genes and Mechanisms. Vaccine 2020, 38, A83–A92. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.C.; Toole, G.A.O. Mechanisms of Biofilm Resistance to Antimicrobial Agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Davies, D. Understanding Biofilm Resistance to Antibacterial Agents. Nat Rev Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef]
- Ventola, C.L. The Antibiotic Resistance Crisis Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277. [Google Scholar]
- Li, L.; Su, Y.; Peng, B.; Peng, X.; Li, H. Metabolic Mechanism of Colistin Resistance and Its Reverting in Vibrio Alginolyticus. Environ. Microbiol. 2020, 22, 4295–4313. [Google Scholar] [CrossRef]
- Mlynarcik, P.; Kolar, M. Molecular Mechanisms of Polymyxin Resistance and Detection of Mcr Genes. Biomed. Pap. 2019, 163, 28–38. [Google Scholar] [CrossRef] [Green Version]
- Rozhin, A.; Batasheva, S.; Kruychkova, M.; Cherednichenko, Y.; Rozhina, E.; Fakhrullin, R. Biogenic Silver Nanoparticles: Synthesis and Application as Antibacterial and Antifungal Agents. Micromachines 2021, 12, 1480. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal Nanoparticles: Understanding the Mechanisms behind Antibacterial Activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Kailasa, S.K.; Park, T.J.; Rohit, J.V.; Koduru, J.R. Antimicrobial Activity of Silver Nanoparticles; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128165041. [Google Scholar]
- Martinez-Gutierrez, F.; Boegli, L.; Agostinho, A.; Sánchez, E.M.; Bach, H.; Ruiz, F.; James, G. Anti-Biofilm Activity of Silver Nanoparticles against Different Microorganisms. Biofouling 2013, 29, 651–660. [Google Scholar] [CrossRef]
- Cheeseman, S.; Christofferson, A.J.; Kariuki, R.; Cozzolino, D.; Daeneke, T.; Crawford, R.J.; Truong, V.K.; Chapman, J.; Elbourne, A. Antimicrobial Metal Nanomaterials: From Passive to Stimuli-Activated Applications. Adv. Sci. 2020, 7, 1902913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Health Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Vazquez-Muñoz, R.; Meza-Villezcas, A.; Fournier, P.G.J.; Soria-Castro, E.; Juarez-Moreno, K.; Gallego-Hernández, A.L.; Bogdanchikova, N.; Vazquez-Duhalt, R.; Huerta-Saquero, A. Enhancement of Antibiotics Antimicrobial Activity Due to the Silver Nanoparticles Impact on the Cell Membrane. PLoS ONE 2019, 14, e0224904. [Google Scholar] [CrossRef] [Green Version]
- Jaime-Acuña, O.E.; Meza-Villezcas, A.; Vasquez-Peña, M.; Raymond-Herrera, O.; Villavicencio-García, H.; Petranovskii, V.; Vazquez-Duhalt, R.; Huerta-Saquero, A. Synthesis and Complete Antimicrobial Characterization of CEOBACTER, an Ag-Based Nanocomposite. PLoS ONE 2016, 11, e0166205. [Google Scholar] [CrossRef] [Green Version]
- Meza-Villezcas, A.; Gallego-Hernández, A.L.; Yildiz, F.H.; Jaime-Acuña, O.E.; Raymond-Herrera, O.; Huerta-Saquero, A. Effect of Antimicrobial Nanocomposites on Vibrio Cholerae Lifestyles: Pellicle Biofilm, Planktonic and Surface-Attached Biofilm. PLoS ONE 2019, 14, e0217869. [Google Scholar] [CrossRef]
- Bertini, I.; Hu, X.; Luchinat, C. Global Metabolomics Characterization of Bacteria: Pre-Analytical Treatments and Profiling. Metabolomics 2014, 10, 241–249. [Google Scholar] [CrossRef]
- Dayalan, S.; Xia, J.; Spicer, R.A.; Salek, R.; Roessner, U. Metabolome Analysis. In Encyclopedia of Bioinformatics and Computational Biology: ABC of Bioinformatics; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1–3, pp. 396–409. ISBN 9780128114322. [Google Scholar]
- Jiménez-Gómez, I.; Valdés-Muñoz, G.; Moreno-Ulloa, A.; Pérez-Llano, Y.; Moreno-Perlín, T.; Silva-Jiménez, H.; Barreto-Curiel, F.; Sánchez-Carbente, M.D.R.; Folch-Mallol, J.L.; Gunde-Cimerman, N.; et al. Surviving in the Brine: A Multi-Omics Approach for Understanding the Physiology of the Halophile Fungus Aspergillus Sydowii at Saturated NaCl Concentration. Front. Microbiol. 2022, 13, 840408. [Google Scholar] [CrossRef]
- Workentine, M.L.; Harrison, J.J.; Weljie, A.M.; Tran, V.A.; Stenroos, P.U.; Tremaroli, V.; Vogel, H.J.; Ceri, H.; Turner, R.J. Phenotypic and Metabolic Profiling of Colony Morphology Variants Evolved from Pseudomonas Fluorescens Biofilms. Environ. Microbiol. 2010, 12, 1565–1577. [Google Scholar] [CrossRef] [PubMed]
- Booth, S.C.; Workentine, M.L.; Wen, J.; Shaykhutdinov, R.; Vogel, H.J.; Ceri, H.; Turner, R.J.; Weljie, A.M. Differences in Metabolism between the Biofilm and Planktonic Response to Metal Stress. J. Proteome Res. 2011, 10, 3190–3199. [Google Scholar] [CrossRef]
- Vazquez-Muñoz, R.; Arellano-Jimenez, M.J.; Lopez, F.D.; Lopez-Ribot, J.L. Protocol Optimization for a Fast, Simple and Economical Chemical Reduction Synthesis of Antimicrobial Silver Nanoparticles in Non-Specialized Facilities. BMC Res. Notes 2019, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mock, J.J.; Barbic, M.; Smith, D.R.; Schultz, D.A.; Schultz, S. Shape Effects in Plasmon Resonance of Individual Colloidal Silver Nanoparticles. J. Chem. Phys. 2002, 116, 6755–6759. [Google Scholar] [CrossRef]
- Feunang, Y.D.; Eisner, R.; Knox, C.; Chepelev, L.; Hastings, J.; Owen, G.; Fahy, E.; Steinbeck, C.; Subramanian, S.; Bolton, E.; et al. ClassyFire: Automated Chemical Classification with a Comprehensive, Computable Taxonomy. J. Cheminform. 2016, 8, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dührkop, K.; Nothias, L.F.; Fleischauer, M.; Reher, R.; Ludwig, M.; Hoffmann, M.A.; Petras, D.; Gerwick, W.H.; Rousu, J.; Dorrestein, P.C.; et al. Systematic Classification of Unknown Metabolites Using High-Resolution Fragmentation Mass Spectra. Nat. Biotechnol. 2021, 39, 462–471. [Google Scholar] [CrossRef]
- Olsen, I. Biofilm-Specific Antibiotic Tolerance and Resistance. Eur. J. Clin. Microbiol. 2015, 34, 877–886. [Google Scholar] [CrossRef]
- Mamun, M.M.; Sorinolu, A.J.; Munir, M.; Vejerano, E.P. Nanoantibiotics: Functions and Properties at the Nanoscale to Combat Antibiotic Resistance. Front. Chem. 2021, 9, 1–23. [Google Scholar] [CrossRef]
- Chen, X.; Schluesener, H.J. Nanosilver: A Nanoproduct in Medical Application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1323. [Google Scholar] [CrossRef] [Green Version]
- Lewis, K.I.M. Minireview Riddle of Biofilm Resistance. Antimicrob. Agent. Chemother. 2001, 45, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Macia, M.D.; Rojo-Molinero, E.; Oliver, A. Antimicrobial Susceptibility Testing in Biofilm-Growing Bacteria. Clin. Microbiol. Infect. 2014, 20, 981–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, P.S. Antimicrobial Tolerance in Biofilms. Microbiol. Spectr. 2015, 3, 269–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.J.; Benitez, J.A. Vibrio Cholerae Biofilms and Cholera Pathogenesis. PLoS Negl. Trop. Dis. 2016, 10, e0004330. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.; Lata, M.; Singh, R.; Deo, N.; Venkatesan, K.; Bisht, D. Cytosolic Proteome Profiling of Aminoglycosides Resistant Mycobacterium Tuberculosis Clinical Isolates Using MALDI-TOF/MS. Front. Microbiol. 2016, 7, 1816. [Google Scholar] [CrossRef] [Green Version]
- Papenfort, K.; Förstner, K.U.; Cong, J.P.; Sharma, C.M.; Bassler, B.L. Differential RNA-Seq of Vibrio Cholerae Identifies the VqmR Small RNA as a Regulator of Biofilm Formation. Proc. Natl. Acad. Sci. USA 2015, 112, E766–E775. [Google Scholar] [CrossRef] [Green Version]
- Kumar, B.; Sharma, D.; Sharma, P.; Katoch, V.M.; Venkatesan, K.; Bisht, D. Proteomic Analysis of Mycobacterium Tuberculosis Isolates Resistant to Kanamycin and Amikacin. J. Proteom. 2013, 94, 68–77. [Google Scholar] [CrossRef]
- Nikaido, H. Molecular Basis of Bacterial Outer Membrane Permeability Revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [Google Scholar] [CrossRef] [Green Version]
- Magnet, S.; Courvalin, P.; Lambert, T. Resistance-Nodulation-Cell Division-Type Efflux Pump Involved in Aminoglycoside Resistance in Acinetobacter Baumannii Strain BM4454. Antimicrob. Agent. Chemother. 2001, 45, 3375–3380. [Google Scholar] [CrossRef] [Green Version]
- Walsh, C. Molecular Mechanisms That Confer Antibacterial Drug Resistance. Nature 2000, 406, 775–781. [Google Scholar] [CrossRef]
- Debik, J.; Sangermani, M.; Wang, F.; Madssen, T.S.; Giskeødegård, G.F. Multivariate Analysis of NMR-Based Metabolomic Data. NMR Biomed. 2022, 35, e4638. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.E.; He, Z.; Redding, A.M.; Joachimiak, M.P.; Keasling, J.D.; Zhou, J.Z.; Arkin, A.P.; Mukhopadhyay, A.; Fields, M.W. Transcriptomic and Proteomic Analyses of Desulfovibrio Vulgaris Biofilms: Carbon and Energy Flow Contribute to the Distinct Biofilm Growth State. BMC Genom. 2012, 13, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, H.J.; Zelaya, A.J.; de León, K.B.; Chakraborty, R.; Elias, D.A.; Hazen, T.C.; Arkin, A.P.; Cunningham, A.B.; Fields, M.W. Impact of Hydrologic Boundaries on Microbial Planktonic and Biofilm Communities in Shallow Terrestrial Subsurface Environments. FEMS Microbiol. Ecol. 2018, 94, fiy191. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Ramalingam, B.; Parandhaman, T.; Das, S.K. Antibacterial Effects of Biosynthesized Silver Nanoparticles on Surface Ultrastructure and Nanomechanical Properties of Gram-Negative Bacteria Viz. Escherichia Coli and Pseudomonas Aeruginosa. ACS Appl. Mater. Interfaces 2016, 8, 4963–4976. [Google Scholar] [CrossRef]
- Park, H.-J.; Park, S.; Roh, J.; Kim, S.; Choi, K.; Yi, J.; Kim, Y.; Yoon, J. Biofilm-Inactivating Activity of Silver Nanoparticles: A Comparison with Silver Ions. J. Ind. Eng. Chem. 2013, 19, 614–619. [Google Scholar] [CrossRef]
- Jung, A.; Jik, Y. “ Nanoantibiotics ”: A New Paradigm for Treating Infectious Diseases Using Nanomaterials in the Antibiotics Resistant Era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef]
- Moreno-Ulloa, A.; la Herrán, H.C.D.-D.; Álvarez-Delgado, C.; Mendoza-Porras, O.; Carballo-Castañeda, R.A.; Donis-Maturano, L.; Villarreal, F. Multi-Omics Study Identifies Novel Signatures of DNA/RNA, Amino Acid, Peptide, and Lipid Metabolism by Simulated Diabetes on Coronary Endothelial Cells. Sci. Rep. 2022, 12, 12027. [Google Scholar] [CrossRef]
- Cao, L.; Guler, M.; Tagirdzhanov, A.; Lee, Y.Y.; Gurevich, A.; Mohimani, H. MolDiscovery: Learning Mass Spectrometry Fragmentation of Small Molecules. Nat. Commun. 2021, 12, 3718. [Google Scholar] [CrossRef]
- Dührkop, K.; Shen, H.; Meusel, M.; Rousu, J.; Böcker, S. Searching Molecular Structure Databases with Tandem Mass Spectra Using CSI:FingerID. Proc. Natl. Acad. Sci. USA 2015, 112, 12580–12585. [Google Scholar] [CrossRef] [Green Version]
- Spoering, A.M.Y.L.; Lewis, K.I.M. Biofilms and Planktonic Cells of Pseudomonas Aeruginosa Have Similar Resistance to Killing by Antimicrobials. J. Bacteriol. 2001, 183, 6746–6751. [Google Scholar] [CrossRef] [PubMed]
- Romeo, T.; Cooper, M.D. Bacterial Biofilms. In Current Topics in Microbiology and Immunology; Romeo, T., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 322, ISBN 978-3-540-75417-6. [Google Scholar]
- Flores-Núñez, V.; Camarena-Pozos, D.; Chávez-González, D.; Hernández-Melgar, A.; Moreno-Ulloa, A.; Partida-Martínez, L. Microbiome-Inspired Phyllospheric Synthetic Communities Increase Microbial Diversity and Productivity of Field-Grown Plants of Agave Tequilana. Research Square 2022. [Google Scholar] [CrossRef]
- Olivon, F.; Grelier, G.; Roussi, F.; Litaudon, M.; Touboul, D. MZmine 2 Data-Preprocessing to Enhance Molecular Networking Reliability. Anal. Chem. 2017, 89, 7836–7840. [Google Scholar] [CrossRef]
- Willforss, J.; Chawade, A.; Levander, F. NormalyzerDE: Online Tool for Improved Normalization of Omics Expression Data and High-Sensitivity Differential Expression Analysis. J. Proteome Res. 2019, 18, 732–740. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Web-Based Inference of Biological Patterns, Functions and Pathways from Metabolomic Data Using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.T.; Gentry, E.C.; McPhail, K.L.; Nothias, L.F.; Nothias-Esposito, M.; Bouslimani, A.; Petras, D.; Gauglitz, J.M.; Sikora, N.; Vargas, F.; et al. Reproducible Molecular Networking of Untargeted Mass Spectrometry Data Using GNPS. Nat. Protoc. 2020, 15, 1954–1991. [Google Scholar] [CrossRef]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A Rapid Tool for Turning Tandem Mass Spectra into Metabolite Structure Information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef] [Green Version]
- Barupal, D.K.; Fiehn, O. Chemical Similarity Enrichment Analysis (ChemRICH) as Alternative to Biochemical Pathway Mapping for Metabolomic Datasets. Sci. Rep. 2017, 7, 14567. [Google Scholar] [CrossRef] [Green Version]
- Holman, J.D.; Tabb, D.L.; Mallick, P. Employing ProteoWizard to convert raw mass spectrometry data. Curr. Protoc. Bioinform. 2014, 46, 13–24. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Conway, J.R.; Lex, A.; Gehlenborg, N. UpSetR: An R package for the visualization of intersecting sets and their properties. Bioinformatics 2017, 33, 2938–2940. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Ludwig, M.; Nothias, L.-F.; Dührkop, K.; Koester, I.; Fleischauer, M.; Hoffmann, M.A.; Petras, D.; Vargas, F.; Morsy, M.; Aluwihare, L.; et al. Database-independent molecular formula annotation using Gibbs sampling through ZODIAC. Nat. Mach. Intell. 2020, 2, 629–641. [Google Scholar] [CrossRef]
- Lowe, H.J.; O Barnett, G. Understanding and using the medical subject headings (MeSH) vocabulary to perform literature searches. JAMA 1994, 271, 1103–1108. [Google Scholar] [CrossRef]
- Michał Krassowski, M.A.; Lagger, C. Krassowski/Complex-Upset: V1.3.3 (v1.3.3). 2021. Available online: https://zenodo.org/record/5762625#.Y2MyX3ZByUk (accessed on 2 March 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meza-Villezcas, A.; Carballo-Castañeda, R.A.; Moreno-Ulloa, A.; Huerta-Saquero, A. Metabolomic Profiling of the Responses of Planktonic and Biofilm Vibrio cholerae to Silver Nanoparticles. Antibiotics 2022, 11, 1534. https://doi.org/10.3390/antibiotics11111534
Meza-Villezcas A, Carballo-Castañeda RA, Moreno-Ulloa A, Huerta-Saquero A. Metabolomic Profiling of the Responses of Planktonic and Biofilm Vibrio cholerae to Silver Nanoparticles. Antibiotics. 2022; 11(11):1534. https://doi.org/10.3390/antibiotics11111534
Chicago/Turabian StyleMeza-Villezcas, Anaid, Rommel A. Carballo-Castañeda, Aldo Moreno-Ulloa, and Alejandro Huerta-Saquero. 2022. "Metabolomic Profiling of the Responses of Planktonic and Biofilm Vibrio cholerae to Silver Nanoparticles" Antibiotics 11, no. 11: 1534. https://doi.org/10.3390/antibiotics11111534
APA StyleMeza-Villezcas, A., Carballo-Castañeda, R. A., Moreno-Ulloa, A., & Huerta-Saquero, A. (2022). Metabolomic Profiling of the Responses of Planktonic and Biofilm Vibrio cholerae to Silver Nanoparticles. Antibiotics, 11(11), 1534. https://doi.org/10.3390/antibiotics11111534





