Studies of the Specific Activity of Aerosolized Isoniazid against Tuberculosis in a Mouse Model
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Isoniazid Aerosol Generation and Inhalation Equipment
2.2. Inhalation Dose
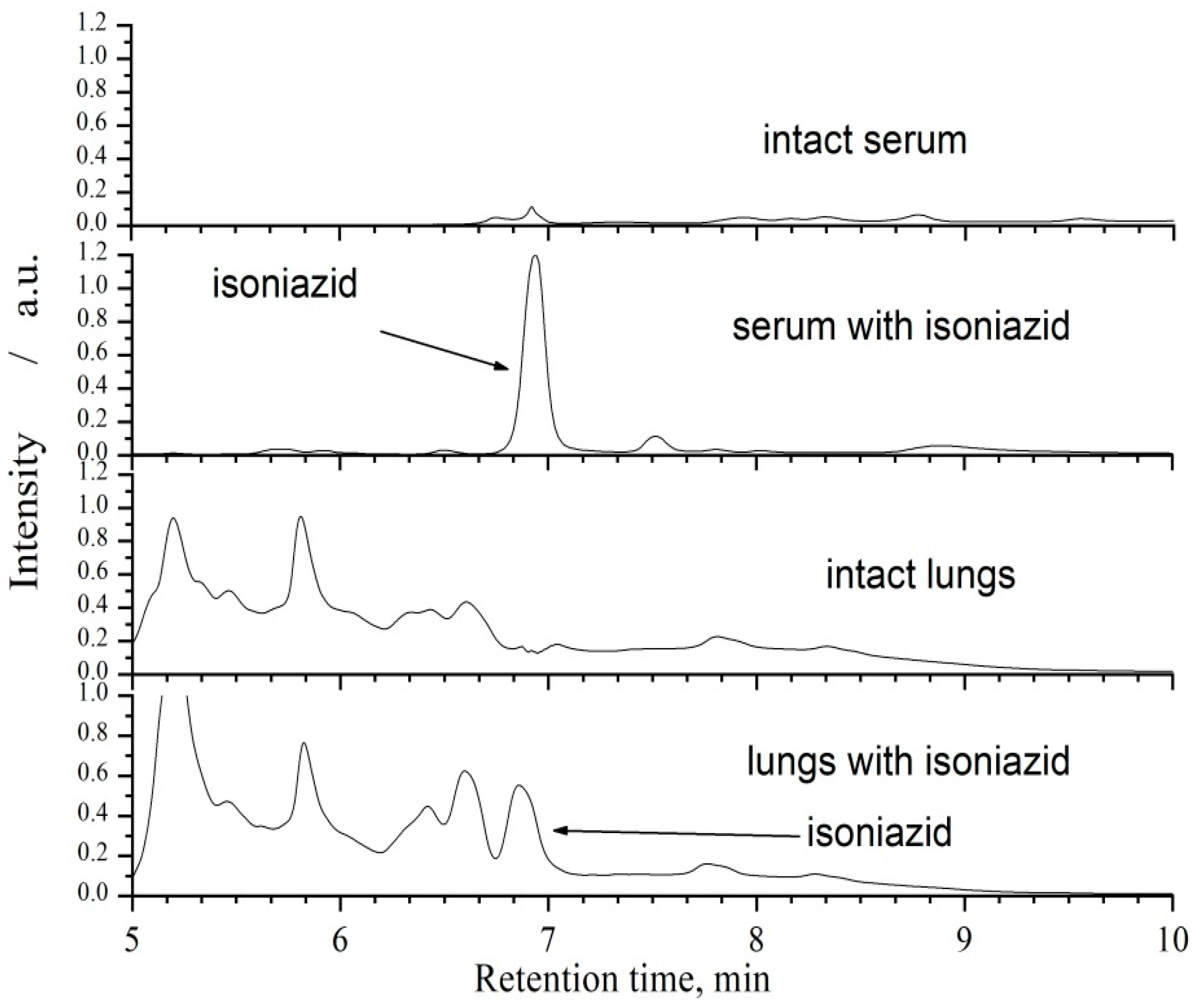
2.3. Sample Preparation and Chromatographic Analysis
2.4. Specific Activity in the Model of Acute Tuberculosis Infection
2.4.1. Tuberculosis Infection Model and Experimental Groups
2.4.2. Histologic Examination
2.4.3. Bacteriological Examination
2.4.4. Statistical Processing
3. Results and Discussion
3.1. Pharmacokinetics of Aerosolized Isoniazid
3.2. Specific Activity in the Model of Acute Tuberculosis Infection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Misra, A.; Hickey, A.J.; Rossi, C.; Borchard, G.; Terada, H.; Makino, K.; Fourie, P.B.; Colombo, P. Inhaled drug therapy for treatment of tuberculosis. Tuberculosis 2011, 91, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, M.; Hickey, A.J.; Ekins, S. Why wait? The case for treating tuberculosis with inhaled drugs. Pharm. Res. 2019, 36, 166. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Sharma, S.; Sharma, J. Potential strategies for the management of drug-resistant tuberculosis. J. Glob. Antimicrob. Resist. 2020, 22, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Dohál, M.; Dvořáková, V.; Šperková, M.; Pinková, M.; Spitaleri, A.; Norman, A.; Cabibbe, A.M.; Rasmussen, E.M.; Porvazník, I.; Škereňová, M.; et al. Whole genome sequencing of multidrug-resistant Mycobacterium tuberculosis isolates collected in the Czech Republic, 2005–2020. Sci. Rep. 2022, 12, 7149. [Google Scholar] [CrossRef] [PubMed]
- WHO. Manual for Selection of Molecular WHO-Recommended Rapid Diagnostic Tests for Detection of Tuberculosis and Drug-Resistant Tuberculosis; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Patil, K.; Bagade, S.; Bonde, S.; Sharma, S.; Saraogi, G. Recent therapeutic approaches for the management of tuberculosis: Challenges and opportunities. Biomed. Pharmacother. 2018, 99, 735–745. [Google Scholar] [CrossRef]
- Hinshaw, H.C.; Pyle, M.M.; Feldman, W.H. Streptomycin in tuberculosis. Am. J. Med. 1947, 2, 429–435. [Google Scholar] [CrossRef] [Green Version]
- Levaditi, C.; Vaisman, A.; Lévy, P. Effets curatifs de la streptomycine administrée en inlialations à des souris contaminées par le Mycobacteriuin tuberculosis. Comptes Rendus Séances L’académie Sci. 1948, 227, 987–989. [Google Scholar]
- Larroude, C. Treatment of tuberculosis of the larynx by streptomycin aerosol. Acta Oto-Laryngol. 1948, 36, 363–371. [Google Scholar] [CrossRef]
- Prigal, S.J.; Tchertkoff, V.; Brooks, A.M. Streptomycin blood levels following inhalation of steam generated aerosols. Dis. Chest 1950, 17, 304–311. [Google Scholar] [CrossRef]
- Miller, J.B.; Abramson, H.A.; Ratner, B. Aerosol streptomycin treatment of advanced pulmonary tuberculosis in children. Am. J. Dis. Child. 1950, 80, 207–237. [Google Scholar] [CrossRef]
- Efimov, I.I. Application of streptomycin aerosol in the treatment of pulmonary tuberculosis. Probl. Tuberk. 1958, 36, 106–107. [Google Scholar]
- Zarnizkaya, B.M. Aerosol therapy in the treatment of pulmonary tuberculosis. Probl. Tuberk. 1958, 36, 70–75. [Google Scholar]
- Lavor, Z.V. Treatment of the bronchial tuberculosis by drug aerosols together with the direct current therapy. Probl. Tuberk. 1976, 54, 38–41. [Google Scholar]
- Semenova, E.V. Basis for administration of streptomycin and isoniazide in the form of ultrasonic aerozols in the treatment of patients with intrathoracic tuberculosis. Antibiotiki 1977, 22, 469–470. [Google Scholar]
- Kaliberda, R.S.; Malevsky, K.V. Efficiency of aerosol chemical therapy in the pre- and postoperational treatment of patients with pulmonary tuberculosis. Probl. Tuberk. 1983, 61, 47–48. [Google Scholar]
- Aksenova, V.A. Ultrasonic inhalation of antibacterial drugs in combined treatment of children and adolescents with respiratory tuberculosis. Probl. Tuberk. 1985, 63, 27–29. [Google Scholar]
- Protsyuk, R.G. Effect of the drug aerosol inhalations to the external respiration of pulmonary tuberculosis patients. Clin. Med. 1985, 63, 50–55. [Google Scholar]
- Pilipchuk, N.S.; Protsiuk, R.G. Effect of inhalations of aerosols of antitubercular agents on pulmonary surfactants. Vrachebnoe Delo 1986, 7, 21–25. [Google Scholar]
- Abdurashitova, M.B. Aerogel of ftivazide in the pulmonary tuberculosis therapy. Probl. Tuberk. 1955, 2, 25–29. [Google Scholar]
- Kulik, N.M. Inhalation of cycloserine in the complex therapy of pulmonary tuberculosis. Probl. Tuberk. 1967, 5, 38–40. [Google Scholar]
- Gorbach, I.N.; Samtsov, V.S. Therapeutic potentialities of rifampicin-dimexid inhalations in phthisiopulmonology. Probl. Tuberk. 1991, 69, 34–35. [Google Scholar]
- Fridkin, M.M.; Krasnoschekova, A.M. Treatment of the pulmonary tuberculosis with the wet and dry aerosol inhalations of antibacterial drugs. Vrachebnoe Delo 1958, 10, 1049–1053. [Google Scholar]
- Gerasimov, A.I.; Ganushchak, M.M. Inhalation of medicinal agents in the complex treatment of patients with chronic destructive pulmonary tuberculosis. Vrachebnoe Delo 1972, 11, 111–113. [Google Scholar] [PubMed]
- Kulik, N.M. Inhalation therapy of phthisics with antituberculous drugs of the first and second line. Probl. Tuberk. 1974, 8, 34–37. [Google Scholar]
- Kulik, N.M. Aerosol therapy by a combination of trace elements and antituberculous agents in dealing with patients suffering from pulmonary tuberculosis. Probl. Tuberk. 1975, 53, 41–44. [Google Scholar]
- Kulik, N.M. Aerosols of antibacterial drugs and some pathoqenetic agents in treatment of patients with pulmonary tuberculosis. Probl. Tuberk. 1980, 58, 40–42. [Google Scholar]
- Abdurashitova, M.V.; Aun, V.Y. Effective treatment of patients with tuberculosis of the bronchi and lungs with the use ultrasonic ethambutol aerosols. Probl. Tuberk. 1979, 57, 27–30. [Google Scholar]
- Krasnova, T.K.; Guryeva, I.G. Hydrocortisone ultrasound inhalations in combined therapy of patients with pulmonary tuberculosis. Probl. Tuberk. 1980, 58, 28–30. [Google Scholar]
- Shesterina, M.V.; Garvei, N.N.; Krasnova, T.K. Effect of ultrasound inhalations of tuberculostatic and pathogexetic drugs on mucociliary apparatus of bronchi in patients with pulmonary tuberculosis. Probl. Tuberk. 1981, 59, 50–53. [Google Scholar]
- Frolova, R.P. Threatment of patients with pulmonary and laryngeal tuberculosis. Probl. Tuberk. 1982, 60, 69–70. [Google Scholar]
- Protsyuk, R.G. Effect of inhalation of aerosols of tuberculostatic drugs on the time course of bacterial isolation in patients with pulmonary tuberculosis. Probl. Tuberk. 1983, 61, 38–42. [Google Scholar]
- Protsyuk, R.G. Aerosol therapy in combined preoperative treatment of patients with tuberculosis and nonspecific inflammatory diseases of the lungs. Probl. Tuberk. 1984, 62, 51–55. [Google Scholar]
- Agzamov, R.A.; Abdurashitova, M.V.; Kadyrova, R.A.; Aun, V.Y. Efficacy of aerosol therapy of bronchopulmonary tuberculosis. Probl. Tuberk. 1983, 61, 56–59. [Google Scholar]
- Hickey, A.J.; Durham, P.G.; Dharmadhikari, A.; Nardell, E.A. Inhaled drug treatment for tuberculosis: Past progress and future prospects. J. Control. Release 2016, 240, 127–134. [Google Scholar] [CrossRef]
- Kurunov, Y.N.; Ursov, I.G.; Krasnov, V.A.; Pelrenko, T.I.; Yakovchenko, N.N.; Svistelnik, A.V.; Filimonov, P.A. Effectiveness of liposomal antibacterial drugs in inhalation therapy of experimental tuberculosis. Probl. Tuberk. 1995, 73, 38–40. [Google Scholar]
- Kurunov, Y.N.; Krasnov, V.A.; Svistelnik, A.V.; Yakovchenko, N.N. Way of Treatment of Pulmonary Tuberculosis. RF Patent 2122855, 10 December 1998. [Google Scholar]
- Justo, O.R.; Moraes, A.M. Incorporation of antibiotics in liposomes designed for tuberculosis therapy by inhalation. Drug Deliv. 2003, 10, 201–207. [Google Scholar] [CrossRef]
- Pandey, R.; Sharma, S.; Khuller, G.K. Nebulization of liposome encapsulated antitubercular drugs in guinea pigs. Int. J. Antimicrob. Agents 2004, 24, 93–94. [Google Scholar] [CrossRef]
- Vyas, S.P.; Kannan, M.E.; Jain, S.; Mishra, V.; Singh, P. Design of liposomal aerosols for improved delivery of rifampicin to alveolar macrophages. Int. J. Pharm. 2004, 269, 37–49. [Google Scholar] [CrossRef]
- Zaru, M.; Mourtas, S.; Klepetsanis, P.; Fadda, A.M.; Antimisiaris, S.G. Liposomes for drug delivery to the lungs by nebulization. Eur. J. Pharm. Biopharm. 2007, 67, 655–666. [Google Scholar] [CrossRef]
- Olivier, K.N.; Griffith, D.E.; Eagle, G.; McGinnis, I.I.G.P.; Micioni, L.; Liu, K.; Daley, C.L.; Winthrop, K.L.; Ruoss, S.; Addrizzo-Harris, D.J.; et al. Randomized trial of liposomal amikacin for inhalation in nontuberculous mycobacterial lung disease. Am. J. Respir. Crit. Care Med. 2017, 195, 814–823. [Google Scholar] [CrossRef]
- Kaur, M.; Garg, T.; Narang, R.K. A review of emerging trends in the treatment of tuberculosis. Artif. Cells Nanomed. Biotechnol. 2016, 44, 78–484. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Hasegawa, T.; Nakajima, T.; Inagawa, H.; Kohchi, C.; Soma, G.; Makino, K.; Terada, H. Delivery of rifampicin–PLGA microspheres into alveolar macrophages is promising for treatment of tuberculosis. J. Control Release 2010, 142, 339–346. [Google Scholar] [CrossRef] [PubMed]
- AI-Hallak, K.; Sarfraz, M.K.; Azarmi, S.; Roa, W.H.; Finlay, W.H.; Löbenberg, R. Pulmonary delivery of inhalable nanoparticles: Dry-powder inhalers. Ther. Deliv. 2011, 2, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.-D.; Fattal, E.; Tsapis, N. Pulmonary drug delivery systems for tuberculosis treatment. Int. J. Pharm. 2015, 478, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Sanzhakov, M.A.; Ipatova, O.M.; Torkhovskaya, T.I.; Prozorovskyi, V.N.; Tikhonova, E.G.; Druzhilovskaya, O.S.; Medvedeva, N.V. Nanoparticles as drug delivery system for antituberculous drugs. Ann. Russ. Acad. Med. Sci. 2013, 68, 37–44. [Google Scholar] [CrossRef]
- Jawahar, N.; Reddy, G. Nanoparticles: A novel pulmonary drug delivery system for tuberculosis. J. Pharm. Sci. Res. 2012, 4, 1901–1906. [Google Scholar]
- Sung, J.C.; Pulliam, B.L.; Edwards, D.A. Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 2007, 25, 563–570. [Google Scholar] [CrossRef]
- Zainala, N.A.; Shukor, S.R.; Wabb, H.A.; Razakb, K. Study on the effect of synthesis parameters of silica nanoparticles entrapped with rifampicin. Chem. Eng. 2013, 32, 2245–2250. [Google Scholar]
- Carneiro, S.P.; Carvalho, K.V.; Soares, R.; Carneiro, C.M.; de Andrade, M.H.G.; Duarte, R.S.; Santos, O. Functionalized rifampicin-loaded nanostructured lipid carriers enhance macrophages uptake and antimycobacterial activity. Colloids Surf. B Biointerfaces 2019, 175, 306–313. [Google Scholar] [CrossRef]
- Djerafi, R.; Swanepoel, A.; Crampon, C.; Kalombo, L.; Labuschagne, P.; Badens, E.; Masmoudi, Y. Supercritical antisolvent co-precipitation of rifampicin and ethyl cellulose. Eur. J. Pharm. Sci. 2017, 102, 161–171. [Google Scholar] [CrossRef]
- Kujur, S.; Singh, A.; Singh, C. Inhalation potential of rifampicin-loaded novel metal–organic frameworks for improved lung delivery: Physicochemical characterization, in vitro aerosolization and antimycobacterial studies. J. Aerosol Med. Pulm. Drug Deliv. 2022, 35, 259–268. [Google Scholar] [CrossRef]
- Verma, R.; Mukker, J.; Kumar, K.; Misra, A. Intracellular time course, pharmacokinetics, and biodistribution of isoniazid and rifabutin following pulmonary delivery of inhalable microparticles to mice. Antimicrob. Agents Chemother. 2008, 52, 3195–3201. [Google Scholar] [CrossRef] [Green Version]
- Pilcer, G.; Amighi, K. Formulation strategy and use of excipients in pulmonary drug delivery. Int. J. Pharm. 2010, 392, 1–19. [Google Scholar] [CrossRef]
- Das, S.; Tucker, I.; Stewart, P. Inhaled dry powder formulations for treating tuberculosis. Curr. Drug Deliv. 2015, 12, 26–39. [Google Scholar] [CrossRef]
- Brunaugh, A.D.; Jan, S.U.; Ferrati, S.; Smyth, H.D.C. Excipient-free pulmonary delivery and macrophage targeting of clofazimine via air jet micronization. Mol. Pharm. 2017, 14, 4019–4031. [Google Scholar] [CrossRef]
- Chan, J.G.Y.; Chan, H.-K.; Prestidge, C.A.; Denman, J.A.; Young, P.M.; Traini, D. A novel dry powder inhalable formulation incorporating three first-line anti-tubercular antibiotics. Eur. J. Pharm. Biopharm. 2013, 83, 285–292. [Google Scholar] [CrossRef]
- Roy, C.J.; Sivasubramani, S.K.; Dutta, N.K.; Mehra, S.; Golden, N.A.; Killeen, S.; Talton, J.D.; Hammoud, B.E.; Didier, P.J.; Kaushal, D. Aerosolized gentamicin reduces the burden of tuberculosis in a murine model. Antimicrob. Agents Chemother. 2012, 56, 883–886. [Google Scholar] [CrossRef] [Green Version]
- Durham, P.G.; Zhang, Y.; German, N.; Mortensen, N.; Dhillon, J.; Mitchison, D.A.; Fourie, P.B.; Hickey, A.J. Spray dried aerosol particles of pyrazinoic acid salts for tuberculosis therapy. Mol. Pharm. 2015, 12, 2574–2581. [Google Scholar] [CrossRef]
- Valiulin, S.V.; Onischuk, A.A.; Baklanov, A.M.; Dubtsov, S.N.; An’kov, S.V.; Tolstikova, T.G. Plokhotnichenko ME, Dultseva GG, Mazunina PS. Excipient-free isoniazid aerosol administration in mice: Evaporation-nucleation particle generation, pulmonary delivery and body distribution. Int. J. Pharm. 2019, 563, 101–109. [Google Scholar] [CrossRef]
- Dubtsov, S.; Ovchinnikova, T.; Valiulin, S.; Chen, X.; Manninen, H.E.; Aalto, P.P.; Petaj, T. Laboratory verification of Aerosol Diffusion Spectrometer and the application to ambient measurements of new particle formation. J. Aerosol Sci. 2017, 105, 10–23. [Google Scholar] [CrossRef]
- Onischuk, A.A.; Valiulin, S.V.; Baklanov, A.M.; Moiseenko, P.P.; Mitrochenko, V.G. Determination of the aerosol particle size distribution by means of the diffusion battery: Analytical inversion. Aerosol Sci. Technol. 2018, 52, 841–853. [Google Scholar] [CrossRef]
- Onischuk, A.A.; Baklanov, A.M.; Valiulin, S.V.; Moiseenko, P.P.; Mitrochenko, V.G. Aerosol diffusion battery: The retrieval of particle size distribution with the help of analytical formulas. Aerosol Sci. Technol. 2018, 52, 165–181. [Google Scholar] [CrossRef]
- Onischuk, A.A.; Valiulin, S.V.; Baklanov, A.M.; Moiseenko, P.P.; Mitrochenko, V.G.; Dultseva, G.G. Aerosol diffusion battery: Analytical inversion from noisy penetration. Measurement 2020, 164, 108049. [Google Scholar] [CrossRef]
- Valiulin, S.V.; Onischuk, A.A.; Baklanov, A.M.; Dubtsov, S.N.; Dultseva, G.G.; An’kov, S.V.; Tolstikova, T.G.; Rusinov, V.L.; Charushin, V.N. An integrated aerosol setup for therapeutics and toxicological testing: Generation techniques and measurement instrumentation. Meas. J. Int. Meas. Confed. 2021, 181, 109659. [Google Scholar] [CrossRef]
- Onischuk, A.A.; Baklanov, A.M.; Dubtsov, S.N.; An’kov, S.V.; Shkil, N.N.; Nefedova, E.V.; Plokhotnichenko, M.E.; Tolstikova, T.G.; Dolgov, A.M.; Dultseva, G.G. Aerosol inhalation delivery of cefazolin in mice: Pharmacokinetic measurements and antibacterial effect. Int. J. Pharm. 2021, 607, 121013. [Google Scholar]
- Arms, A.D.; Travis, C.C. Reference Physiological Parameters in Pharmacokinetic Modeling; EPA Report no. EPA/600/6-88/004; Available from NTIS Springfield, VA PB88-196019; U.S. Environmental Protection Agency, Office of Health and Environmental Assessment: Washington, DC, USA, 1988.
- Currie, W.D.; van Schaik, S.; Vargas, I.; Enhorning, G. Breathing and pulmonary surfactant function in mice 24 h after ozone exposure. Eur. Respir. J. 1998, 12, 288–293. [Google Scholar] [CrossRef] [Green Version]
- Schaper, M.; Brost, M.A. Respiratory effects of trimellitic anhydride aerosols in mice. Arch. Toxicol. 1991, 65, 671–677. [Google Scholar] [CrossRef]
- Hamelmann, E.; Schwarze, J.; Takeda, K.; Oshiba, A.; Larsen, G.L.; Irvin, C.G.; Gelfand, E.W. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am. J. Respir. Crit. Care Med. 1997, 156, 766–775. [Google Scholar] [CrossRef] [Green Version]
- Vijayaraghavan, R. Modifications of breathing pattern induced by inhaled sulphur mustard in mice. Arch. Toxicol. 1997, 71, 157–164. [Google Scholar] [CrossRef]
- Stephenson, E.N.; Moeller, R.B.; York, C.G.; Young, H.W. Nose-only versus whole-body aerosol exposure for induction of upper respiratory infections of laboratory mice. Am. Ind. Hyg. Assoc. J. 1988, 49, 128–135. [Google Scholar] [CrossRef]
- Yeh, H.C.; Snipes, M.; Eidson, A.F.; Hobbs, C.H.; Henry, M.C. Comparative evaluation of nose-only for rats-aerosol characteristics and lung deposition versus whole-body inhalation exposures. Inhal. Toxicol. 1990, 2, 205–221. [Google Scholar] [CrossRef]
- Chen, C.; Ortega, F.; Alameda, L.; Ferrer, S.; Simonsson, U.S.H. Population pharmacokinetics, optimised design and sample size determination for rifampicin, isoniazid, ethambutol and pyrazinamide in the mouse. Eur. J. Pharm. Sci. 2016, 93, 319–333. [Google Scholar] [CrossRef]
- Ibrahim, M.; Garcia-Contreras, L. Preclinical pharmacokinetics of antitubercular drugs. In Drug Delivery Systems for Tuberculosis Prevention and Treatment; Hickey, A.J., Ed.; John Wiley & Sons: Chichester, UK, 2016; pp. 131–155. [Google Scholar]
- Kumar, N.; Vishwas, K.G.; Kumar, M.; Reddy, J.; Parab, M.; Manikanth, C.L.; Pavithra, B.S.; Shandil, R.K. Pharmacokinetics and dose response of anti-TB drugs in rat infection model of tuberculosis. Tuberculosis 2014, 94, 282–286. [Google Scholar] [CrossRef] [Green Version]
- Jayaram, R.; Shandil, R.K.; Gaonkar, S.; Kaur, P.; Suresh, B.L.; Mahesh, B.N.; Jayashree, R.; Nandi, V.; Bharath, S.; Kantharaj, E.; et al. Isoniazid pharmacokinetics-pharmacodynamics in an aerosol infection model of tuberculosis. Antimicrob. Agents Chemother. 2004, 48, 2951–2957. [Google Scholar] [CrossRef] [Green Version]
- De Groote, M.A.; Gilliland, J.C.; Wells, C.L.; Brooks, E.J.; Woolhiser, L.K.; Gruppo, V.; Peloquin, C.A.; Orme, I.M.; Lenaerts, A.J. Comparative studies evaluating mouse models used for efficacy testing of experimental drugs against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2011, 55, 1237–1247. [Google Scholar] [CrossRef]



| Group No. | Group Title | Experimental Action |
|---|---|---|
| 1 | Control (−) | Not infected. Inhalation with pure air for 20 min in the whole-body chamber, once a day. |
| 2 | Control (+) | Infected. Inhalation with pure air for 20 min in the whole-body chamber, once a day. |
| 3 | Per-oral | Infected. Treated with isoniazid, administered through gastric tube in the form of aqueous suspension once a day in the dose of 10 mg/kg. |
| 4 | Aerosol (1) | Infected. Treated with isoniazid through inhalation for 20 min in the whole-body chamber once a day. Dose 5.0 ± 0.5 mg/kg. |
| 5 | Aerosol (2) | Infected. Treated with isoniazid through inhalation for 20 min in the whole-body chamber once a day. Dose 8.0 ± 0.8 mg/kg. |
| Group | 28th Day | 56th Day | ||
|---|---|---|---|---|
| Number of Granulomas | Number of MBT | Number of Granulomas | Number of MBT | |
| Control (+) | 0.30 ± 0.89 | 1.30 ± 12.05 | 0.23 ± 0.54 | 0.65 ± 2.89 |
| Per-oral | 0.03 ± 0.19 * | 0 * | 0.01 ± 0.09 * | 0 * |
| Aerosol (1) | 0.01 ± 0.08 * | 0 * | 0.02 ± 0.17 * | 0 * |
| Aerosol (2) | 0.01 ± 0.11 * | 0 * | 0.01 ± 0.09 * | 0 * |
| Group | AFM | CFU | ||
|---|---|---|---|---|
| 28th Day | 56th Day | 28th Day | 56th Day | |
| Control (+) | 105.5 ± 57.7 | 67.6 ± 46.5 | 77.2 ± 67.3 | 22.6 ± 18.6 |
| Per-oral | 0.17 ± 0.23 * | 0 * | 0.5 ± 0.76 | 0 |
| Aerosol (1) | 0 * | 0 * | 0 | 0 |
| Aerosol (2) | 0 * | 0 * | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valiulin, S.V.; Onischuk, A.A.; Baklanov, A.M.; Dubtsov, S.N.; Dultseva, G.G.; An’kov, S.V.; Tolstikova, T.G.; Belogorodtsev, S.N.; Schwartz, Y.S. Studies of the Specific Activity of Aerosolized Isoniazid against Tuberculosis in a Mouse Model. Antibiotics 2022, 11, 1527. https://doi.org/10.3390/antibiotics11111527
Valiulin SV, Onischuk AA, Baklanov AM, Dubtsov SN, Dultseva GG, An’kov SV, Tolstikova TG, Belogorodtsev SN, Schwartz YS. Studies of the Specific Activity of Aerosolized Isoniazid against Tuberculosis in a Mouse Model. Antibiotics. 2022; 11(11):1527. https://doi.org/10.3390/antibiotics11111527
Chicago/Turabian StyleValiulin, Sergey V., Andrey A. Onischuk, Anatoly M. Baklanov, Sergey N. Dubtsov, Galina G. Dultseva, Sergey V. An’kov, Tatiana G. Tolstikova, Sergey N. Belogorodtsev, and Yakov Sh. Schwartz. 2022. "Studies of the Specific Activity of Aerosolized Isoniazid against Tuberculosis in a Mouse Model" Antibiotics 11, no. 11: 1527. https://doi.org/10.3390/antibiotics11111527
APA StyleValiulin, S. V., Onischuk, A. A., Baklanov, A. M., Dubtsov, S. N., Dultseva, G. G., An’kov, S. V., Tolstikova, T. G., Belogorodtsev, S. N., & Schwartz, Y. S. (2022). Studies of the Specific Activity of Aerosolized Isoniazid against Tuberculosis in a Mouse Model. Antibiotics, 11(11), 1527. https://doi.org/10.3390/antibiotics11111527





