Synthetic Flavonoid BrCl-Flav—An Alternative Solution to Combat ESKAPE Pathogens
Abstract
1. Introduction
2. Results
2.1. BrCl-flav Exhibits Potent Antibacterial Activity against ESKAPE Pathogens
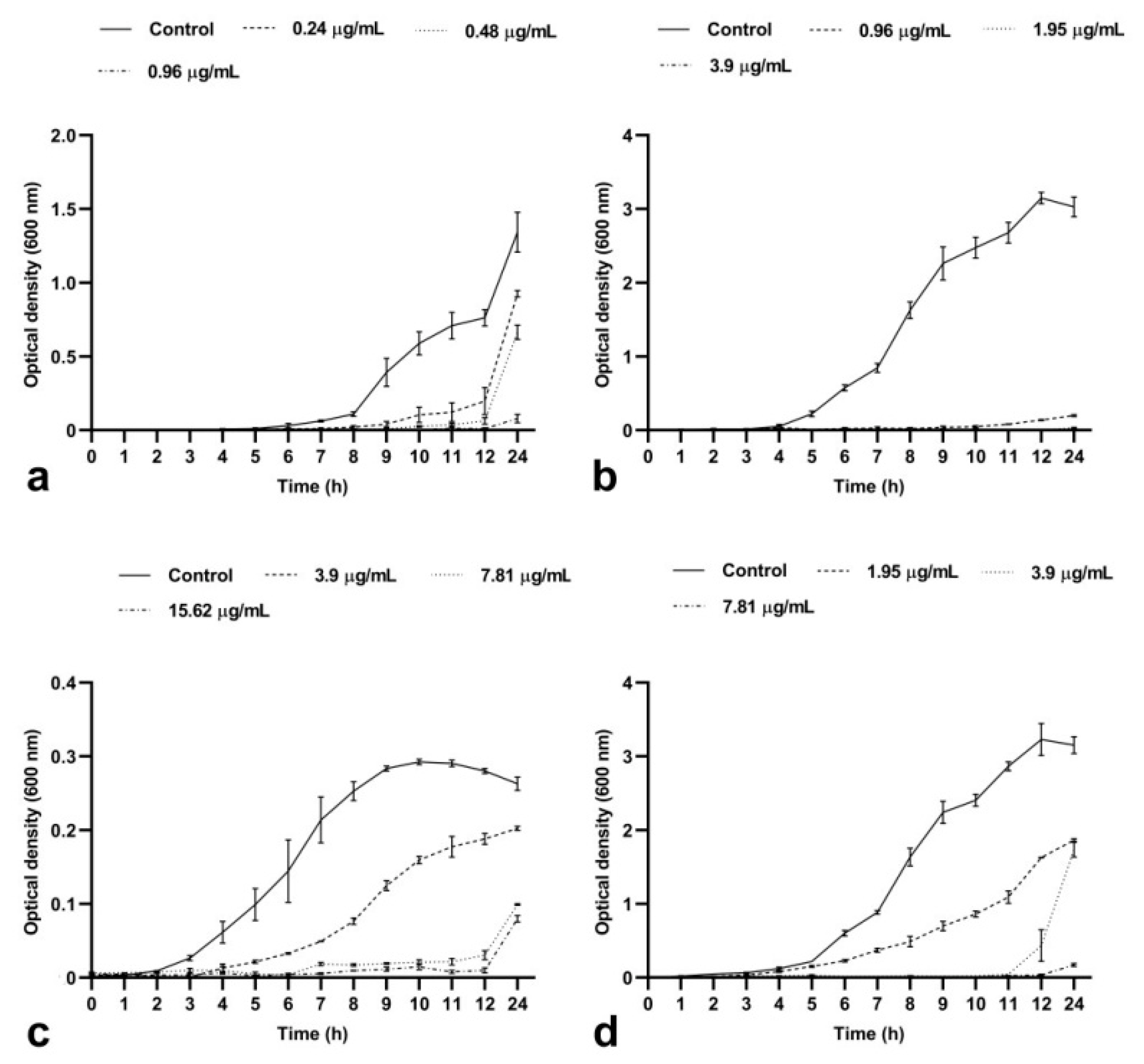
2.1.1. BrCl-flav Induced a Concentration- and Time-Dependent Bacteriostatic Effect
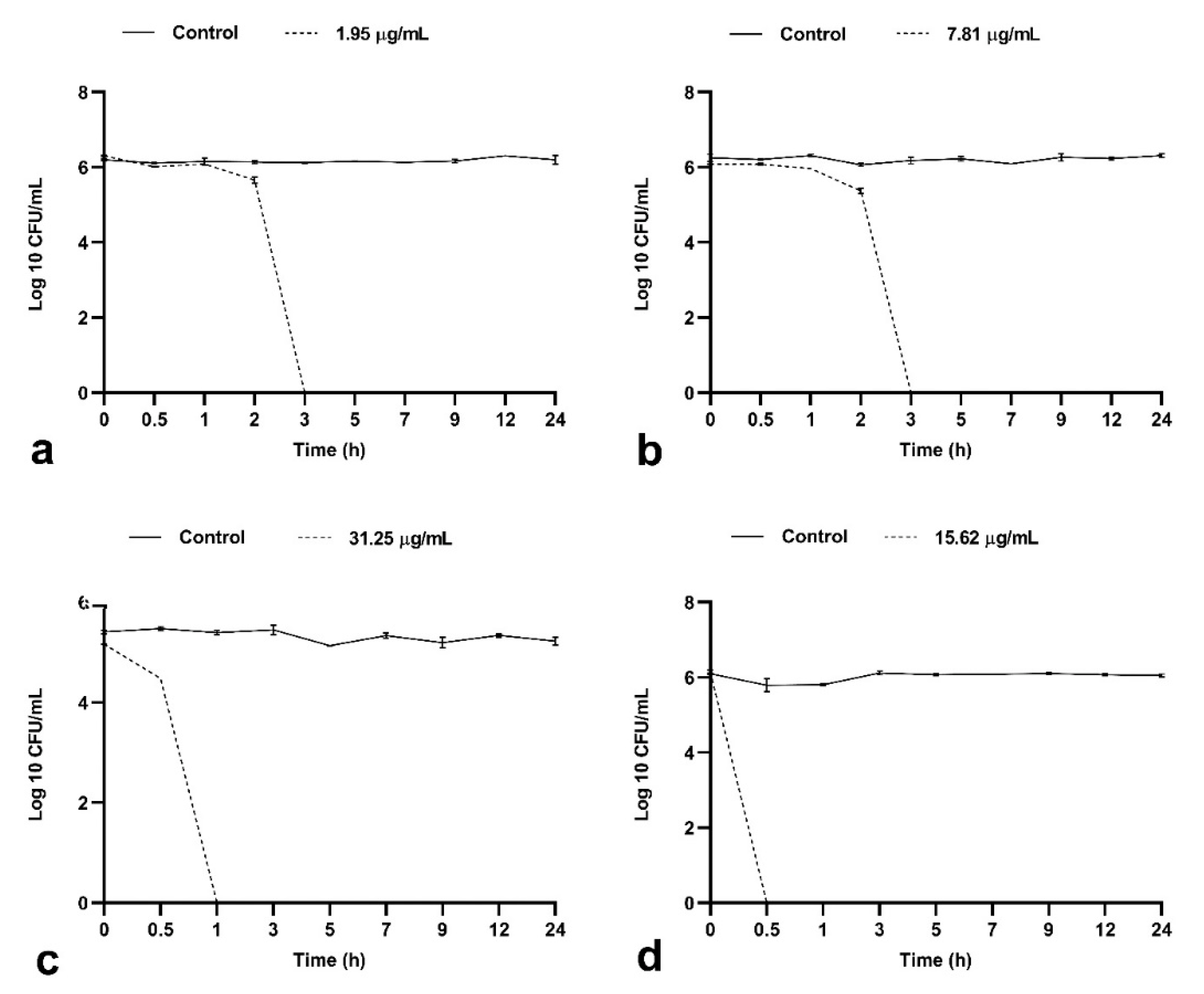
2.1.2. BrCl-flav Possesses Important Bactericidal Activity against Selected Clinical Isolates
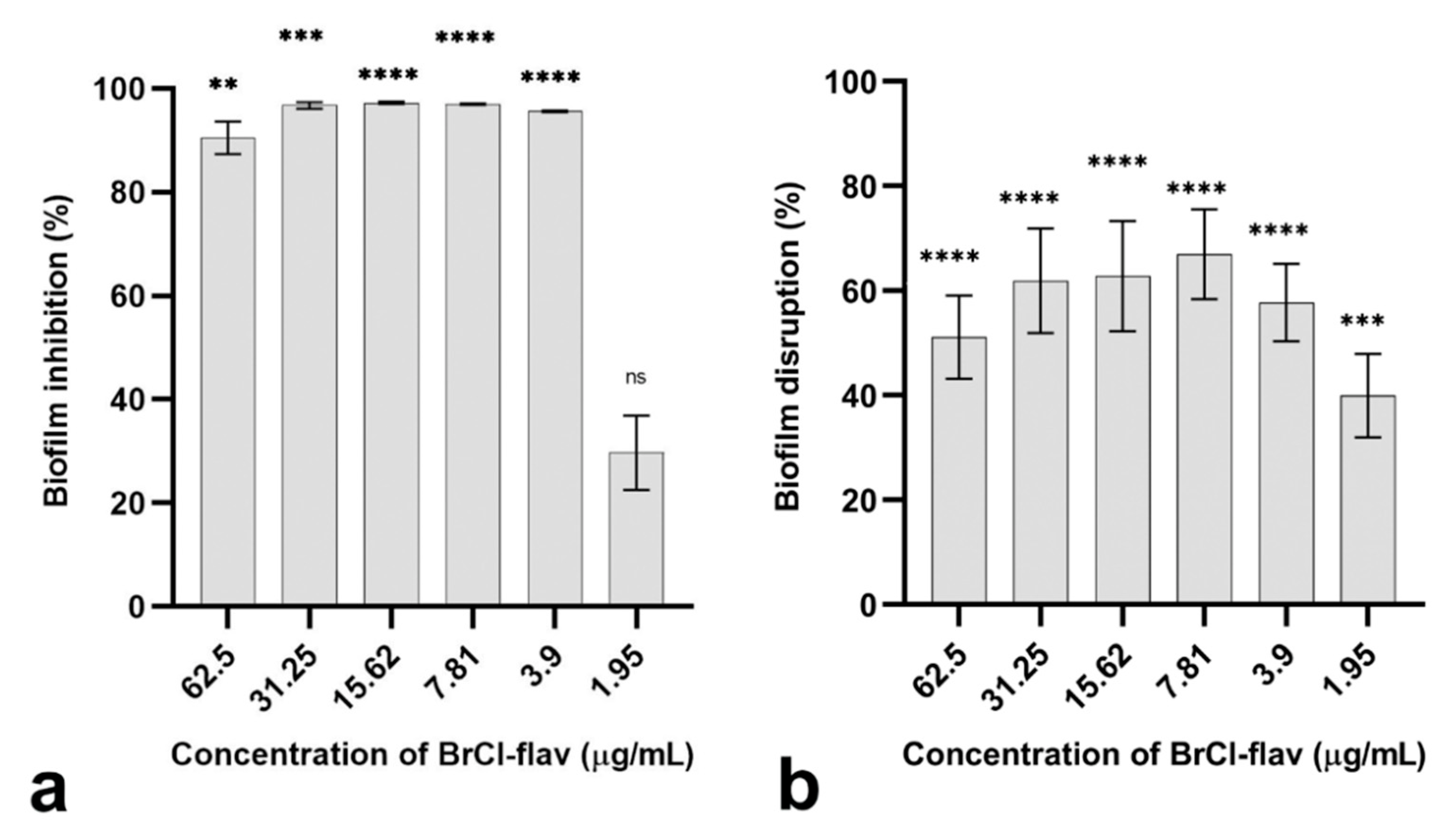
2.2. The Anti-Biofilm Activity of BrCl-flav
2.2.1. Bacterial Biofilm Formation Was Inhibited by BrCl-flav
2.2.2. BrCl-flav Showed Important Biofilm Disruption Potential
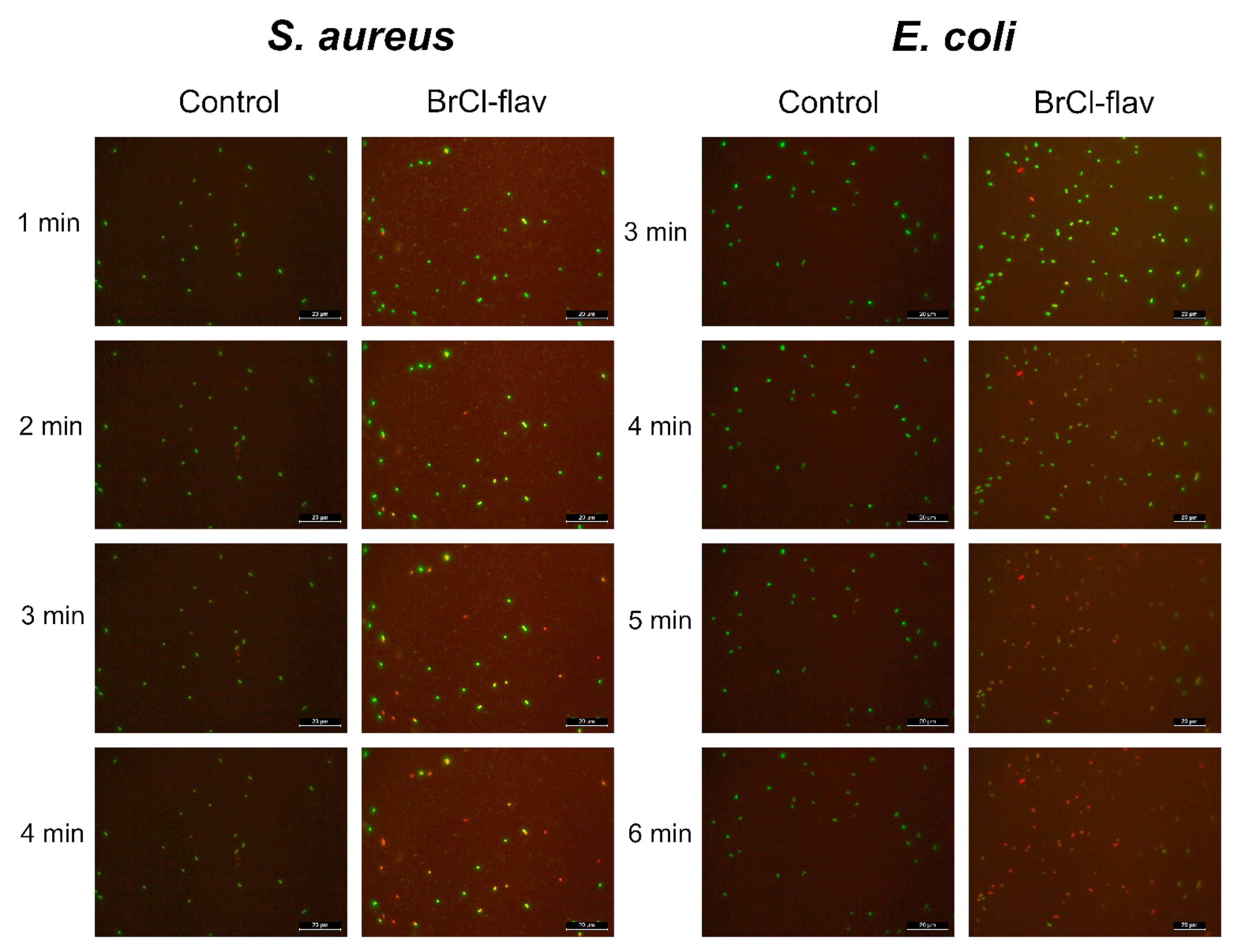
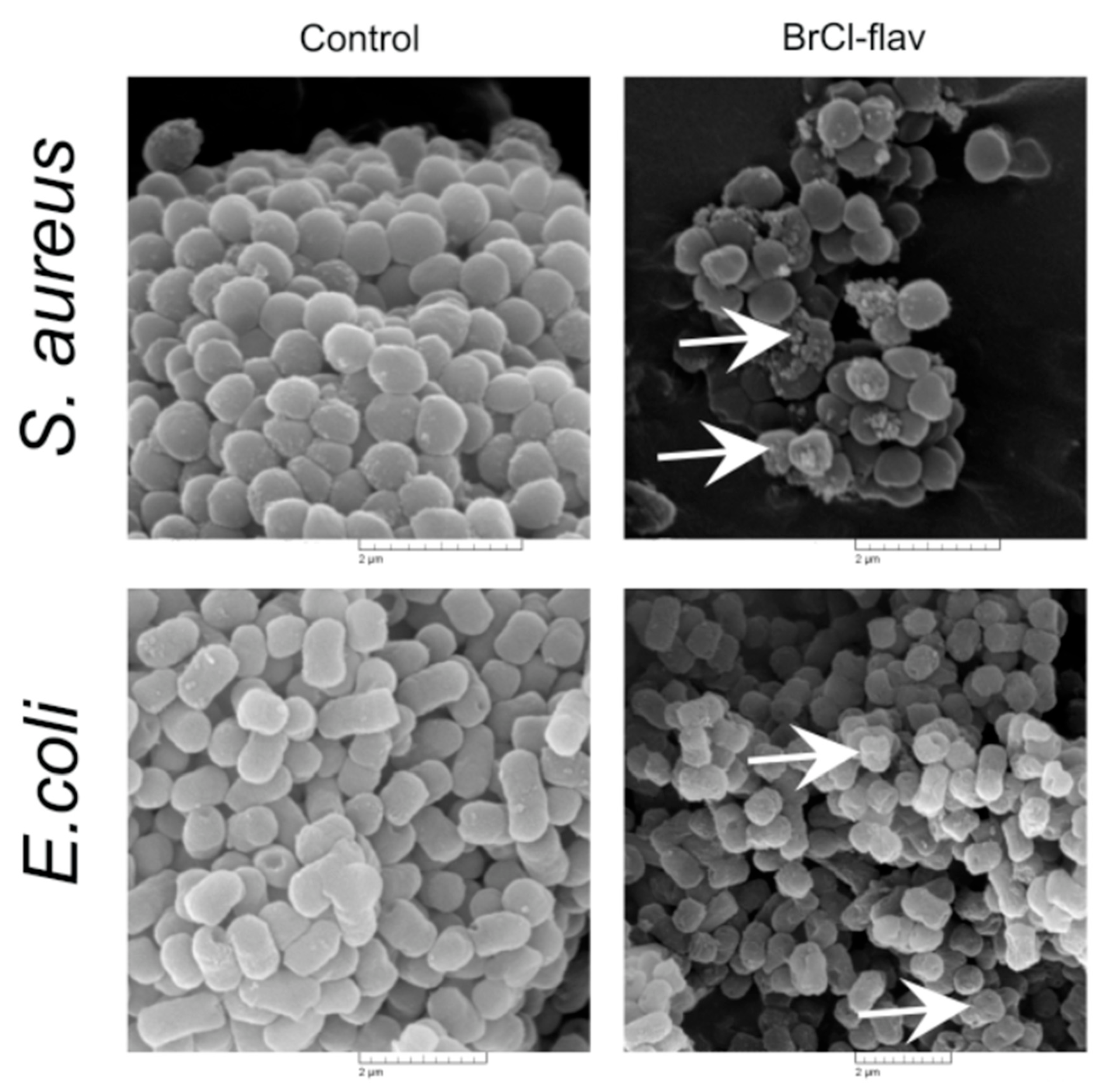
2.3. Mode of Action
2.3.1. BrCl-flav Impair the Cellular Membrane Integrity
2.3.2. The Effects of BrCl-flav on Bacterial Cell Morphology
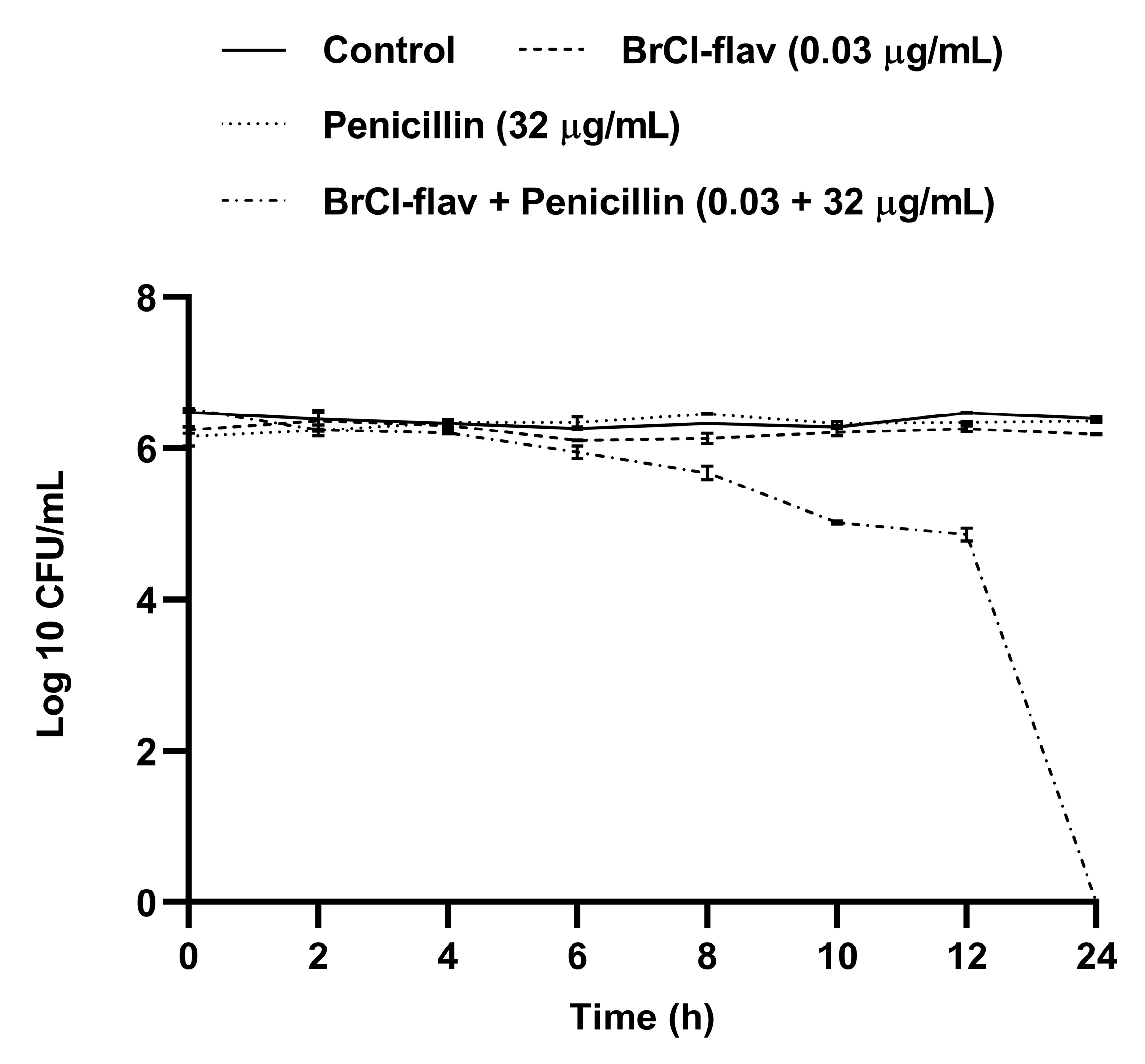
2.4. Effect of BrCl-flav in Combination with Antibiotics against a MRSA Strain
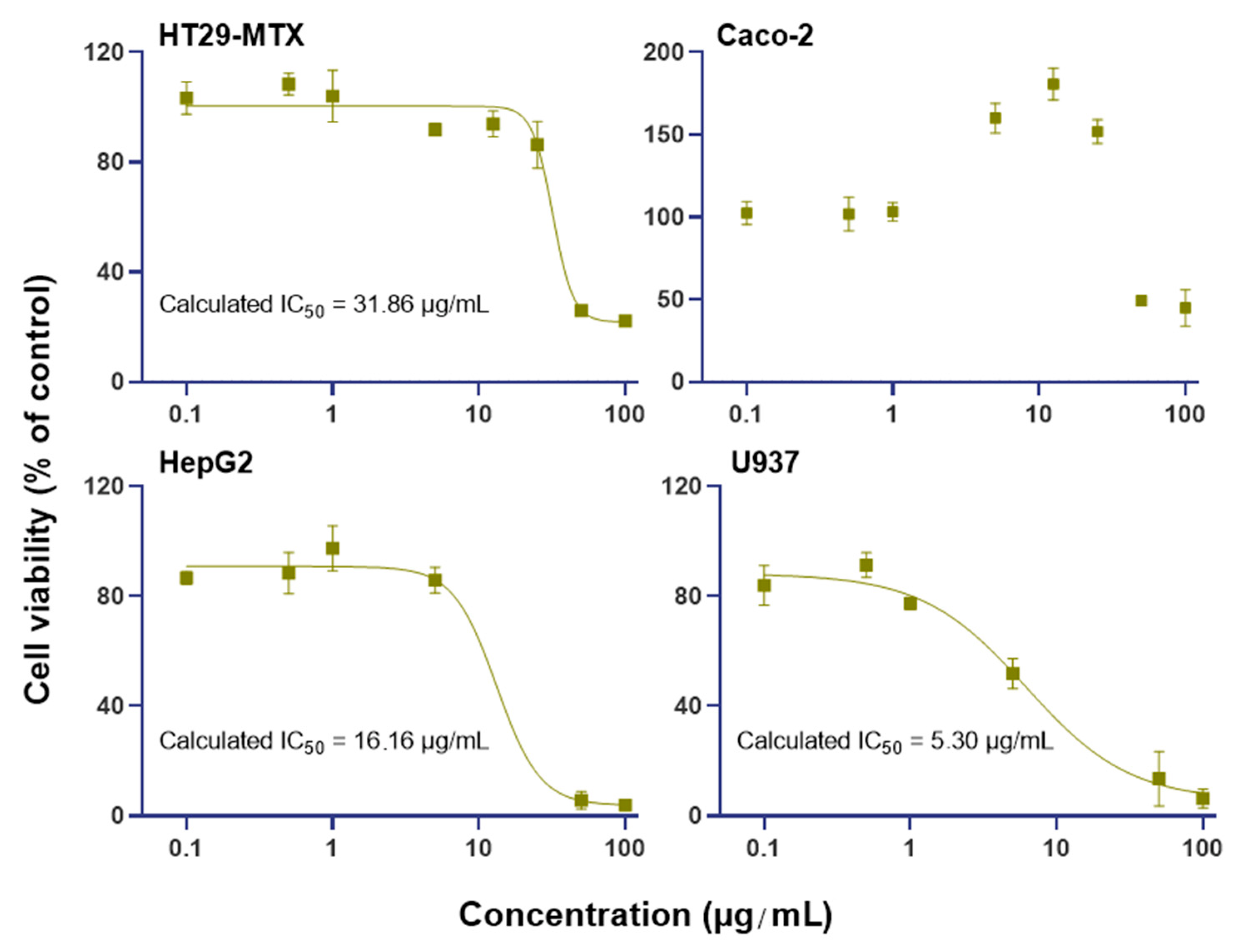
2.5. BrCl-flav Effect on Human Cell Viability
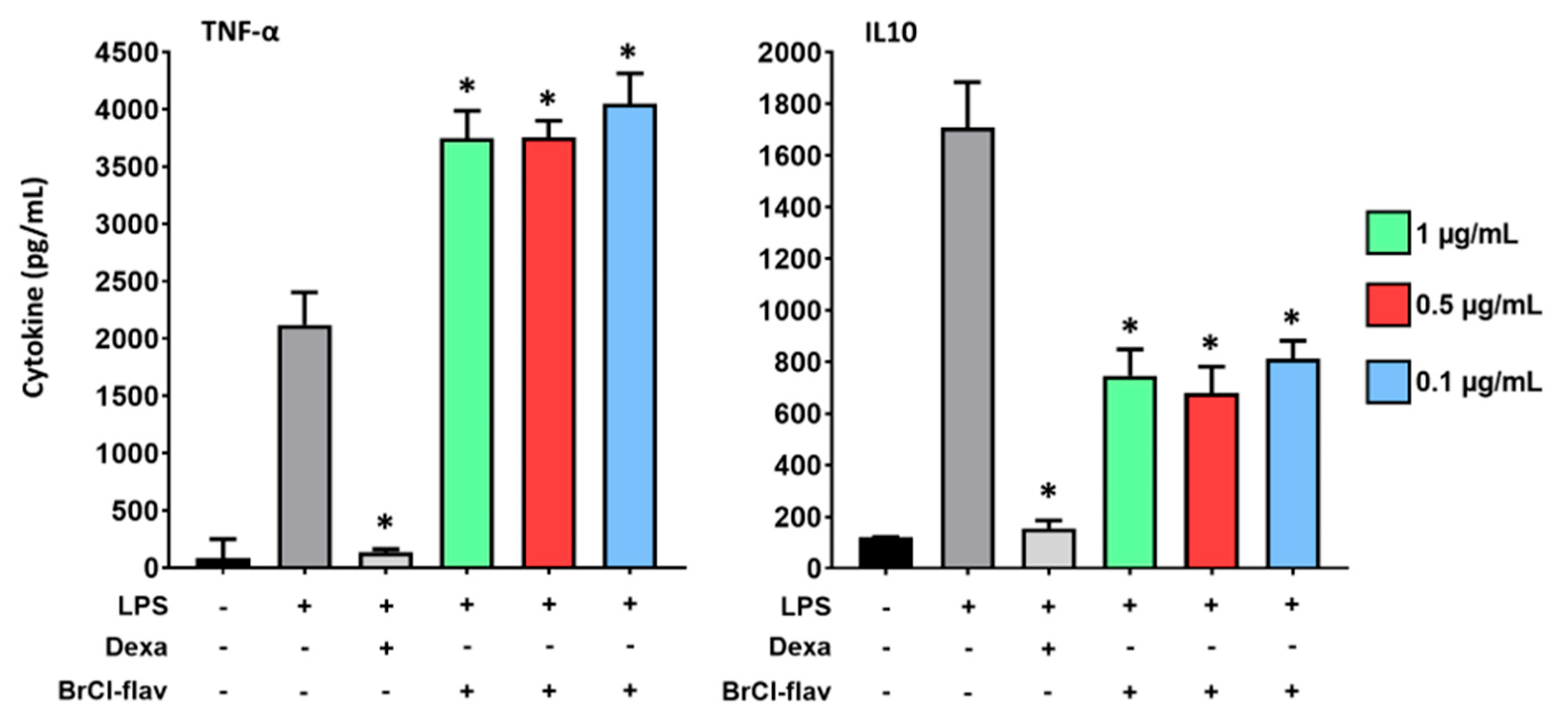
2.6. Inflammation Study
3. Discussion
4. Materials and Methods
4.1. Antibacterial Agents
4.2. Bacterial Strains
4.3. Antibacterial Susceptibility Testing
4.3.1. Disk Diffusion Method
4.3.2. Determination of Minimum Inhibitory Concentration and Minimum Bactericidal Concentration
4.4. Growth Inhibition Assay
4.5. Time-Kill Kinetic Assay
4.6. In Vitro Anti-Biofilm Activity Assay
4.7. Evaluation of Cell Membrane Integrity
4.8. Scanning Electron Microscopy
4.9. Checkerboard Assay
4.10. Cell Culture
4.11. U937 Differentiation into Macrophages
4.12. Cell Viability CCK-8 Assay
4.13. Inflammation Study
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Tawfiq, J.A.; Momattin, H.; Al-Ali, A.Y.; Eljaaly, K.; Tirupathi, R.; Haradwala, M.B.; Areti, S.; Alhumaid, S.; Rabaan, A.A.; Al Mutair, A.; et al. Antibiotics in the pipeline: A literature review (2017–2020). Infection 2022, 50, 553–564. [Google Scholar] [CrossRef] [PubMed]
- WHO Regional Office for Europe; European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe-2020 Data; WHO Regional Office for Europe: Copenhagen, Denmark, 2022. [Google Scholar]
- Dadgostar, P. Antimicrobial Resistance: Implications and costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Jit, M.; Ng, D.H.L.; Luangasanatip, N.; Sandmann, N.; Atkins, F.; Robotham, K.E.; Pouwels, J.V.; Pouwels, K.B. Quantifying the economic cost of antibiotic resistance and the impact of related interventions: Rapid methodological review, conceptual framework and recommendations for future studies. BMC Med. 2020, 18, 38. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti-Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Raj KC, H.; Gilmore, D.F.; Alam, M.A. Development of 4-[4-(Anilinomethyl)-3-phenyl-pyrazol-1-yl] Benzoic acid derivatives as potent anti-staphylococci and anti-enterococci agents. Antibiotics 2022, 11, 939. [Google Scholar] [CrossRef] [PubMed]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, J.; Das, S.; Fatima, Z.; Hameed, S. Multidrug resistance: An emerging crisis. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 541340. [Google Scholar] [CrossRef]
- Santajit, S.; Indrawattana, N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. BioMed Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef]
- Ma, Y.X.; Wang, C.Y.; Li, Y.Y.; Li, J.; Wan, Q.Q.; Chen, J.H.; Tay, F.R.; Niu, L.N. Considerations and caveats in combating ESKAPE pathogens against nosocomial infections. Adv. Sci. 2020, 7, 1901872. [Google Scholar] [CrossRef]
- Lin, Q.; Deslouches, B.; Montelaro, R.C.; Di, Y.P. Prevention of ESKAPE pathogen biofilm formation by antimicrobial peptides WLBU2 and LL37. Int. J. Antimicrob. Agents 2018, 52, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Lazar, V.; Holban, A.M.; Curutiu, C.; Chifiriuc, M.C. Modulation of quorum sensing and biofilms in less investigated Gram-negative ESKAPE pathogens. Front. Microbiol. 2021, 12, 676510. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Basukala, P.; Basukala, O.; Parajuli, K.; Pokhrel, B.M.; Rijal, B.P. Detection of biofilm production and antibiotic resistance pattern in clinical isolates from indwelling medical devices. Curr. Microbiol. 2015, 70, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef]
- Annunziato, G. Strategies to overcome antimicrobial resistance (AMR) making use of non-essential target inhibitors: A review. Int. J. Mol. Sci. 2019, 20, 5844. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Sarbu, L.G.; Bahrin, L.G.; Babii, C.; Stefan, M.; Birsa, M.L. Synthetic flavonoids with antimicrobial activity: A review. J. Appl. Microbiol. 2019, 127, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Bahrin, L.G.; Hopf, H.; Jones, P.G.; Sarbu, L.G.; Babii, C.; Mihai, A.C.; Stefan, M.; Birsa, L.M. Antibacterial structure-activity relationship studies of several tricyclic sulfur-containing flavonoids. Beilstein J. Org. Chem. 2016, 12, 1065–1071. [Google Scholar] [CrossRef]
- Bahrin, L.G.; Apostu, M.O.; Birsa, L.M.; Stefan, M. The antibacterial properties of sulfur containing flavonoids. Bioorg. Med. Chem. Lett. 2014, 24, 2315–2318. [Google Scholar] [CrossRef]
- Babii, C.; Savu, M.; Motrescu, I.; Birsa, L.M.; Sarbu, L.G.; Stefan, M. The antibacterial synthetic flavonoid BrCl-Flav exhibits important anti-Candida activity by damaging cell membrane integrity. Pharmaceuticals 2021, 14, 1130. [Google Scholar] [CrossRef] [PubMed]
- Babii, C.; Mihalache, G.; Bahrin, L.G.; Neagu, A.N.; Gostin, I.; Mihai, C.T.; Sarbu, L.G.; Birsa, L.M.; Stefan, M. A novel synthetic flavonoid with potent antibacterial properties: In vitro activity and proposed mode of action. PLoS ONE 2018, 13, e0194898. [Google Scholar] [CrossRef] [PubMed]
- Lebaron, P.; Catala, P.; Parthuisot, N. Effectiveness of SYTOX green stain for bacterial viability assessment. Appl. Environ. Microbiol. 1998, 64, 2697–2700. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- Babii, C.; Bahrin, L.G.; Neagu, A.N.; Gostin, I.; Mihasan, M.; Birsa, L.M.; Stefan, M. Antibacterial activity and proposed action mechanism of a new class of synthetic tricyclic flavonoids. J. Appl. Microbiol. 2016, 120, 630–637. [Google Scholar] [CrossRef]
- Shimamura, T.; Zhao, W.; Hu, Z.-Q. Mechanism of action and potential for use of tea catechin as an antiinfective agent. Anti-Infect. Agents Med. Chem. 2007, 6, 57–62. [Google Scholar] [CrossRef]
- Gutkind, G.; Norbedo, C.; Mollerach, M.; Ferraro, G.; Coussio, J.D.; de Torres, R. Antibacterial activity of Achyrocline flaccida. J. Ethnopharmacol. 1984, 10, 319–321. [Google Scholar] [CrossRef]
- Uchil, A.; Murali, T.S.; Nayak, R. Escaping ESKAPE: A chalcone perspective. Results Chem. 2021, 3, 100229. [Google Scholar] [CrossRef]
- Chan, B.C.; Yu, H.; Wong, C.W.; Lui, S.L.; Jolivalt, C.; Ganem-Elbaz, C.; Paris, J.M.; Morleo, B.; Litaudon, M.; Lau, C.B.; et al. Quick identification of kuraridin, a noncytotoxic anti-MRSA (methicillin-resistant Staphylococcus aureus) agent from Sophora flavescens using high-speed counter-current chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 880, 157–162. [Google Scholar] [CrossRef]
- Rukayadi, Y.; Han, S.; Yong, D.; Hwang, J.K. In vitro antibacterial activity of panduratin A against enterococci clinical isolates. Biol. Pharm. Bull. 2010, 33, 1489–1493. [Google Scholar] [CrossRef]
- Kant, R.; Kumar, D.; Agarwal, D.; Gupta, R.D.; Tilak, R.; Awasthi, S.K.; Agarwal, A. Synthesis of newer 1,2,3-triazole linked chalcone and flavone hybrid compounds and evaluation of their antimicrobial and cytotoxic activities. Eur. J. Med. Chem. 2016, 113, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Singh, G.; Kaur, H.; Saxena, A.K.; Ishar, M.P. Synthesis of β-ionone derived chalcones as potent antimicrobial agents. Bioorg. Med. Chem. Lett. 2012, 22, 6343–6346. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-F.; Zheng, C.-J.; Sun, L.-P.; Liu, X.-K.; Piao, H.-R. Synthesis of new chalcone derivatives bearing 2,4-thiazolidinedione and benzoic acid moieties as potential anti-bacterial agents. Eur. J. Med. Chem. 2011, 46, 3469–3473. [Google Scholar] [CrossRef] [PubMed]
- Henry, E.J.; Bird, S.J.; Gowland, P.; Collins, M.; Cassella, J.P. Ferrocenyl chalcone derivatives as possible antimicrobial agents. J. Antibiot. 2020, 73, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Ušjak, D.; Ivković, B.; Božić, D.D.; Bošković, L.; Milenković, M. Antimicrobial activity of novel chalcones and modulation of virulence factors in hospital strains of Acinetobacter baumannii and Pseudomonas aeruginosa. Microb. Pathog. 2019, 131, 186–196. [Google Scholar] [CrossRef]
- Kuete, V.; Ngameni, B.; Tangmouo, J.G.; Bolla, J.M.; Alibert-Franco, S.; Ngadjui, B.T.; Pagès, J.M. Efflux pumps are involved in the defense of Gram-negative bacteria against the natural products isobavachalcone and diospyrone. Antimicrob. Agents Chemother. 2010, 54, 1749–1752. [Google Scholar] [CrossRef]
- Shah, D.R.; Lakum, H.P.; Chikhalia, K.H. Synthesis and in vitro antimicrobial evaluation of piperazine substituted quinazoline-based thiourea/thiazolidinone/chalcone hybrids. Bioorg. Khim. 2015, 41, 235–248. [Google Scholar] [CrossRef]
- Ayman, M.; El-Messery, S.M.; Habib, E.E.; Al-Rashood, S.T.; Almehizia, A.A.; Alkahtani, H.M.; Hassan, G.S. Targeting microbial resistance: Synthesis, antibacterial evaluation, DNA binding and modeling study of new chalcone-based dithiocarbamate derivatives. Bioorg. Chem. 2019, 85, 282–292. [Google Scholar] [CrossRef]
- An, J.; Zuo, G.Y.; Hao, X.Y.; Wang, G.C.; Li, Z.S. Antibacterial and synergy of a flavanonol rhamnoside with antibiotics against clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA). Phytomedicine 2011, 18, 990–993. [Google Scholar] [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure-activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- Mohamed, M.F.; Brezden, A.; Mohammad, H.; Chmielewski, J.; Seleem, M.N. Targeting biofilms and persisters of ESKAPE pathogens with P14KanS, a kanamycin peptide conjugate. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 848–859. [Google Scholar] [CrossRef] [PubMed]
- Raorane, C.J.; Lee, J.H.; Kim, Y.G.; Rajasekharan, S.K.; García-Contreras, R.; Lee, J. Antibiofilm and antivirulence efficacies of flavonoids and curcumin against Acinetobacter baumannii. Front. Microbiol. 2019, 10, 990. [Google Scholar] [CrossRef] [PubMed]
- Maxam, A.M.; Gilbert, W. Sequencing end-labeled DNA with base-specific chemical cleavages. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1980; Volume 65, pp. 499–560. [Google Scholar]
- Pbinzbach, H.; Futterer, E. The 1, 2- and 1, 3-Dithiolium ions. In Advances in Heterocyclic Chemistry; Academic Press: Cambridge, MA, USA, 1967; Volume 7, pp. 39–151. [Google Scholar]
- Birsa, M.L. Reaction of 4-(2′-Hydroxyaryl)-1,3-dithiolium salts with sodium sulfide. A selective synthesis of 2′-hydroxyacetophenones. Synth. Commun. 2003, 33, 3071–3076. [Google Scholar] [CrossRef]
- Buchmann, D.; Schultze, N.; Borchardt, J.; Böttcher, I.; Schaufler, K.; Guenther, S. Synergistic antimicrobial activities of epigallocatechin gallate, myricetin, daidzein, gallic acid, epicatechin, 3-hydroxy-6-methoxyflavone and genistein combined with antibiotics against ESKAPE pathogens. J. Appl. Microbiol. 2022, 132, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.U.; Khurram, M.; Khattak, B.; Khan, J. Antibiotic additive and synergistic action of rutin, morin and quercetin against methicillin resistant Staphylococcus aureus. BMC Complement. Altern. Med. 2015, 15, 59. [Google Scholar] [CrossRef]
- Tran, T.D.; Do, T.H.; Tran, N.C.; Ngo, T.D.; Huynh, T.N.P.; Tran, C.D.; Thai, K.M. Synthesis and anti methicillin resistant Staphylococcus aureus activity of substituted chalcones alone and in combination with non-beta-lactam antibiotics. Bioorg. Med. Chem. Lett. 2012, 22, 4555–4560. [Google Scholar] [CrossRef]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic compounds diminish antibiotic resistance of Staphylococcus aureus clinical strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef]
- Chung, P.Y.; Navaratnam, P.; Chung, L.Y. Synergistic antimicrobial activity between pentacyclic triterpenoids and antibiotics against Staphylococcus aureus strains. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 25. [Google Scholar] [CrossRef]
- Eumkeb, G.; Sakdarat, S.; Siriwong, S. Reversing β-lactam antibiotic resistance of Staphylococcus aureus with galangin from Alpinia officinarum Hance and synergism with ceftazidime. Phytomedicine 2010, 18, 40–45. [Google Scholar] [CrossRef]
- Wada, H.; Saito, K.; Kanda, T.; Kobayashi, I.; Fujii, H.; Fujigaki, S.; Maekawa, N.; Takatsu, H.; Fujiwara, H.; Sekikawa, K.; et al. Tumor necrosis factor-α (TNF-α) plays a protective role in acute viral myocarditis in mice. Circulation 2001, 103, 743–749. [Google Scholar] [CrossRef]
- Iyer, S.S.; Cheng, G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012, 32, 23–63. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.L.; Goldstein, M.M.; Chan, J.; Triebold, K.J.; Pfeffer, K.; Lowenstein, C.J.; Schreiber, R.; Mak, T.W.; Bloom, B.R. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 1995, 2, 561–572. [Google Scholar] [CrossRef]
- Gan, X.-H.; Bonavida, B. Preferential induction of TNF-α and IL-1β and inhibition of IL-10 secretion by human peripheral blood monocytes by synthetic aza–alkyl lysophospholipids. Cell. Immunol. 1999, 193, 125–133. [Google Scholar] [CrossRef]
- Bahrin, L.G.; Sarbu, L.G.; Hopf, H.; Jones, P.G.; Babii, C.; Stefan, M.; Birsa, M.L. The influence of halogen substituents on the biological properties of sulfur-containing flavonoids. Bioorg. Med. Chem. 2016, 24, 3166–3173. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. Supplement M100, 31st ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- Aqil, F.; Ahmad, I.; Owais, M. Evaluation of anti-methicillin-resistant Staphylococcus aureus (MRSA) activity and synergy of some bioactive plant extracts. Biotechnol. J. 2006, 1, 1093–1102. [Google Scholar] [CrossRef]
- Onsare, J.G.; Arora, D.S. Antibiofilm potential of flavonoids extracted from Moringa oleifera seed coat against Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans. J. Appl. Microbiol. 2015, 118, 313–325. [Google Scholar] [CrossRef]
- Djordjevic, D.; Wiedmann, M.; McLandsborough, L.A. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 2002, 68, 2950–2958. [Google Scholar] [CrossRef]
- Stepanovic, S.; Vukovic, D.; Dakic, I.; Savic, B.; Svabic-Vlahovic, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Qu, S.; Dai, C.; Shen, Z.; Tang, Q.; Wang, H.; Zhai, B.; Zhao, L.; Hao, Z. Mechanism of synergy between tetracycline and quercetin against antibiotic resistant Escherichia coli. Front. Microbiol. 2019, 10, 2536. [Google Scholar] [CrossRef]
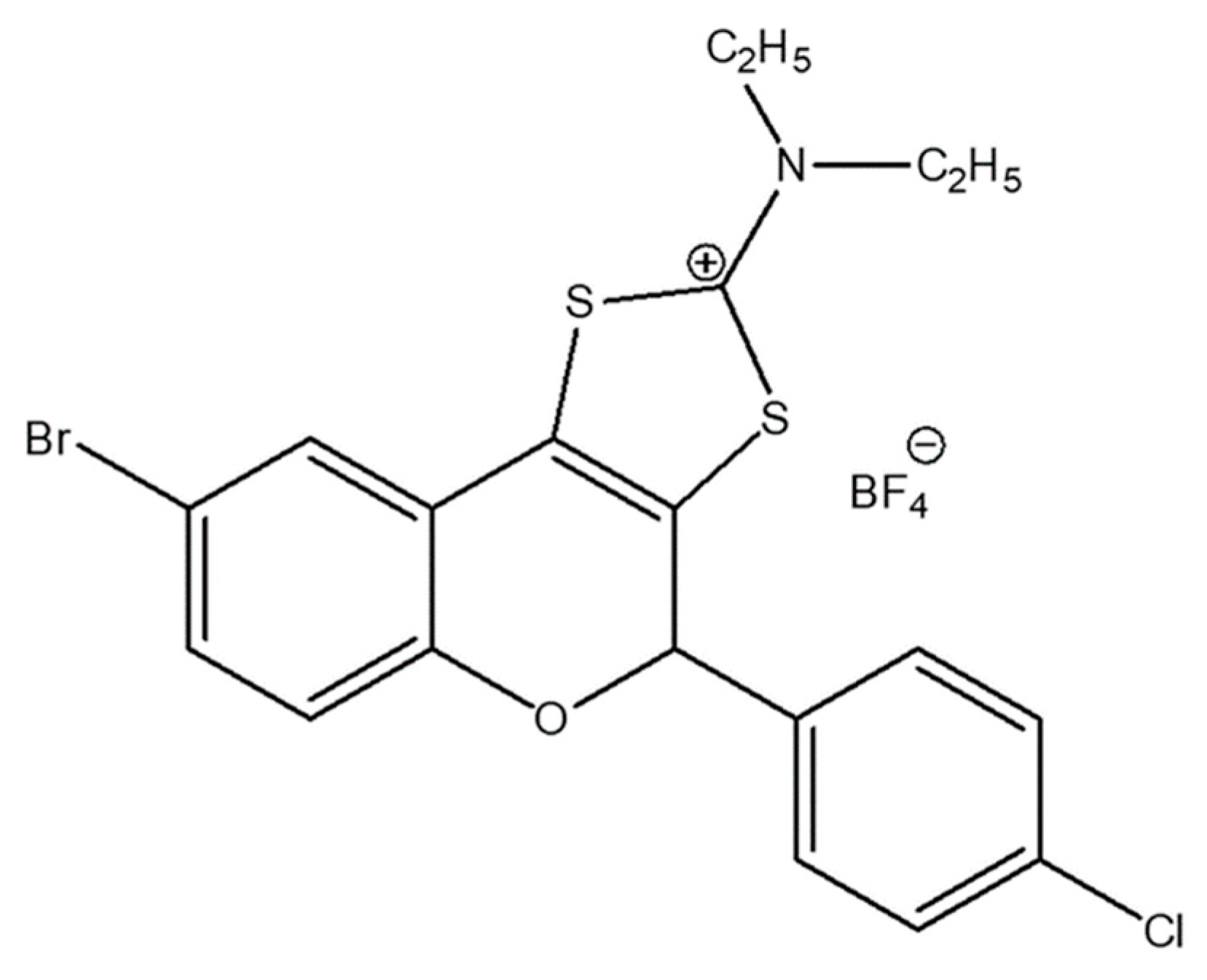

| Bacterial Strains | BrCl-flav | DMSO | Control | ||
|---|---|---|---|---|---|
| MIC | MBC | MIC | MIC | MBC | |
| Staphylococcus aureus medbio1-2012 | 0.48 | 1.95 | >125 | 7.8 c | 31.25 c |
| S. aureus prxbio1 | 1.95 | 7.81 | >125 | 3.9 c | 125 c |
| S. aureus prxbio2 | 7.81 | 15.6 | 250 | 7.8 c | 125 c |
| S. aureus prxbio3 | 0.48 | 0.97 | 250 | 1.9 c | 62.5 c |
| S. aureus prxbio4 | 0.24 | 0.97 | 250 | 3.9 c | 15.62 c |
| S. aureus prxbio5 | 0.24 | 0.48 | 250 | 7.8 c | 62.5 c |
| S. aureus prxbio6 | 7.81 | 250 | 250 | 7.8 c | 125 c |
| S. aureus prxbio7 | 0.48 | 0.97 | 250 | 7.8 c | 62.5 c |
| Streptococcus spp. prxbio9 | 3.9 | 7.81 | 250 | <0.9 c | >125 c |
| S. pneumoniae prxbio10 | 0.48 | 0.97 | 250 | 3.9 g | 31.25 g |
| Enterococcus faecium medbio2-2012 | 7.81 | 31.3 | >125 | <0.9 c | 15.62 c |
| E. faecalis prxbio8 | 31.3 | 62.5 | 125 | 7.8 c | > 125 c |
| Acinetobacter baumannii medbio3-2013 | 3.9 | 15.6 | >125 | <0.9 g | <0.9 g |
| Escherichia coli medbio4-2013 | 3.9 | 15.6 | >125 | <0.9 g | <0.9 g |
| Enterobacter cloacae medbio5-2013 | 125 | 250 | 125 | <0.9 g | <0.9 g |
| Klebsiella pneumoniae medbio6-2013 | 125 | >250 | 125 | >125 g | >125 g |
| K. pneumoniae prxbio11 | 125 | >250 | 125 | <0.9 g | <0.9 g |
| K. pneumoniae prxbio12 | 125 | >250 | 125 | <0.9 g | <0.9 g |
| Pseudomonas aeruginosa medbio7-2013 | 31.3 | - | >125 | <0.9 g | <0.9 g |
| Salmonella enterica medbio8-2013 | 125 | - | >125 | <0.9 g | <0.9 g |
| Haemophillus spp. prxbio13 | 0.48 | 1.95 | 250 | 0.9 g | 125 g |
| S. aureus ATCC 43300 | 3.9 | 31.3 | 125 | 7.8 c | 62.5 c |
| K. pneumoniae ATCC BAA-1705 | 125 | 250 | 125 | 3.9 g | 15.62 g |
| Bacterial Strain | MIC (µg/mL) | FICI | Effect | |||
|---|---|---|---|---|---|---|
| Alone | In Combination | |||||
| BrCl-flav | Penicillin | BrCl-flav | Penicillin | |||
| S. aureus medbio1-2012 | 0.48 | 128 | 0.96 | 8 | 2.06 | IND |
| 0.48 | 16 | 1.125 | IND | |||
| 0.24 | 32 | 0.75 | ADD | |||
| 0.12 | 32 | 0.5 | SYN | |||
| 0.06 | 32 | 0.375 | SYN | |||
| 0.03 | 32 | 0.312 | SYN | |||
| 0.015 | 32 | 0.281 | SYN | |||
| 0.007 | 32 | 0.264 | SYN | |||
| BrCl-flav | Ciprofloxacin | BrCl-flav | Ciprofloxacin | FICI | Effect | |
| 0.48 | 156.25 | 0.48 | 9.76 | 1.06 | IND | |
| 0.48 | 4.88 | 1.03 | IND | |||
| 0.48 | 2.44 | 1.015 | IND | |||
| 0.48 | 1.22 | 1.007 | IND | |||
| 0.24 | 39.06 | 0.74 | ADD | |||
| 0.24 | 19.53 | 0.62 | ADD | |||
| 0.12 | 78.12 | 0.74 | ADD | |||
| 0.06 | 78.12 | 0.615 | ADD | |||
| 0.06 | 156.25 | 1.125 | IND | |||
| 0.03 | 156.25 | 1.06 | IND | |||
| 0.015 | 156.25 | 1.031 | IND | |||
| 0.007 | 156.25 | 1.015 | IND | |||
| 0.003 | 156.25 | 1.007 | IND | |||
| 0.001 | 156.25 | 1.003 | IND | |||
| 0.0009 | 156.25 | 1.001 | IND | |||
| 0.0004 | 156.25 | 1.0009 | IND | |||
| 0.0002 | 156.25 | 1.0004 | IND | |||
| BrCl-flav | Tetracycline | BrCl-flav | Tetracycline | FICI | Effect | |
| 0.48 | 19.531 | 0.48 | 4.882 | 1.025 | IND | |
| 0.48 | 2.441 | 1.012 | IND | |||
| 0.48 | 1.222 | 1.06 | IND | |||
| 0.48 | 0.61 | 1.031 | IND | |||
| 0.48 | 0.305 | 1.015 | IND | |||
| 0.48 | 0.152 | 1.007 | IND | |||
| 0.24 | 9.76 | 0.999 | ADD | |||
| 0.12 | 19.531 | 1.25 | IND | |||
| 0.06 | 19.531 | 1.125 | IND | |||
| 0.03 | 19.531 | 1.06 | IND | |||
| 0.015 | 19.531 | 1.031 | IND | |||
| 0.07 | 19.531 | 1.015 | IND | |||
| 0.003 | 19.531 | 1.007 | IND | |||
| 0.001 | 19.531 | 1.003 | IND | |||
| 0.0009 | 19.531 | 1.001 | IND | |||
| 0.0004 | 19.531 | 1.0009 | IND | |||
| 0.0002 | 19.531 | 1.0004 | IND | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldovan, C.-V.; Savu, M.; Dussert, E.; Aboubacar, H.; Sarbu, L.G.; Matiut, S.; Cudennec, B.; Krier, F.; Ravallec, R.; Birsa, L.M.; et al. Synthetic Flavonoid BrCl-Flav—An Alternative Solution to Combat ESKAPE Pathogens. Antibiotics 2022, 11, 1389. https://doi.org/10.3390/antibiotics11101389
Moldovan C-V, Savu M, Dussert E, Aboubacar H, Sarbu LG, Matiut S, Cudennec B, Krier F, Ravallec R, Birsa LM, et al. Synthetic Flavonoid BrCl-Flav—An Alternative Solution to Combat ESKAPE Pathogens. Antibiotics. 2022; 11(10):1389. https://doi.org/10.3390/antibiotics11101389
Chicago/Turabian StyleMoldovan, Cristina-Veronica, Mihaela Savu, Elodie Dussert, Haïrati Aboubacar, Laura Gabriela Sarbu, Simona Matiut, Benoit Cudennec, François Krier, Rozenn Ravallec, Lucian Mihail Birsa, and et al. 2022. "Synthetic Flavonoid BrCl-Flav—An Alternative Solution to Combat ESKAPE Pathogens" Antibiotics 11, no. 10: 1389. https://doi.org/10.3390/antibiotics11101389
APA StyleMoldovan, C.-V., Savu, M., Dussert, E., Aboubacar, H., Sarbu, L. G., Matiut, S., Cudennec, B., Krier, F., Ravallec, R., Birsa, L. M., & Stefan, M. (2022). Synthetic Flavonoid BrCl-Flav—An Alternative Solution to Combat ESKAPE Pathogens. Antibiotics, 11(10), 1389. https://doi.org/10.3390/antibiotics11101389