Antimicrobial Selection for the Treatment of Clinical Mastitis and the Efficacy of Penicillin Treatment Protocols in Large Estonian Dairy Herds
Abstract
:1. Introduction
2. Results
2.1. Incidence Rate of Antimicrobial-Treated CM and Number of Treatment Courses per Cow
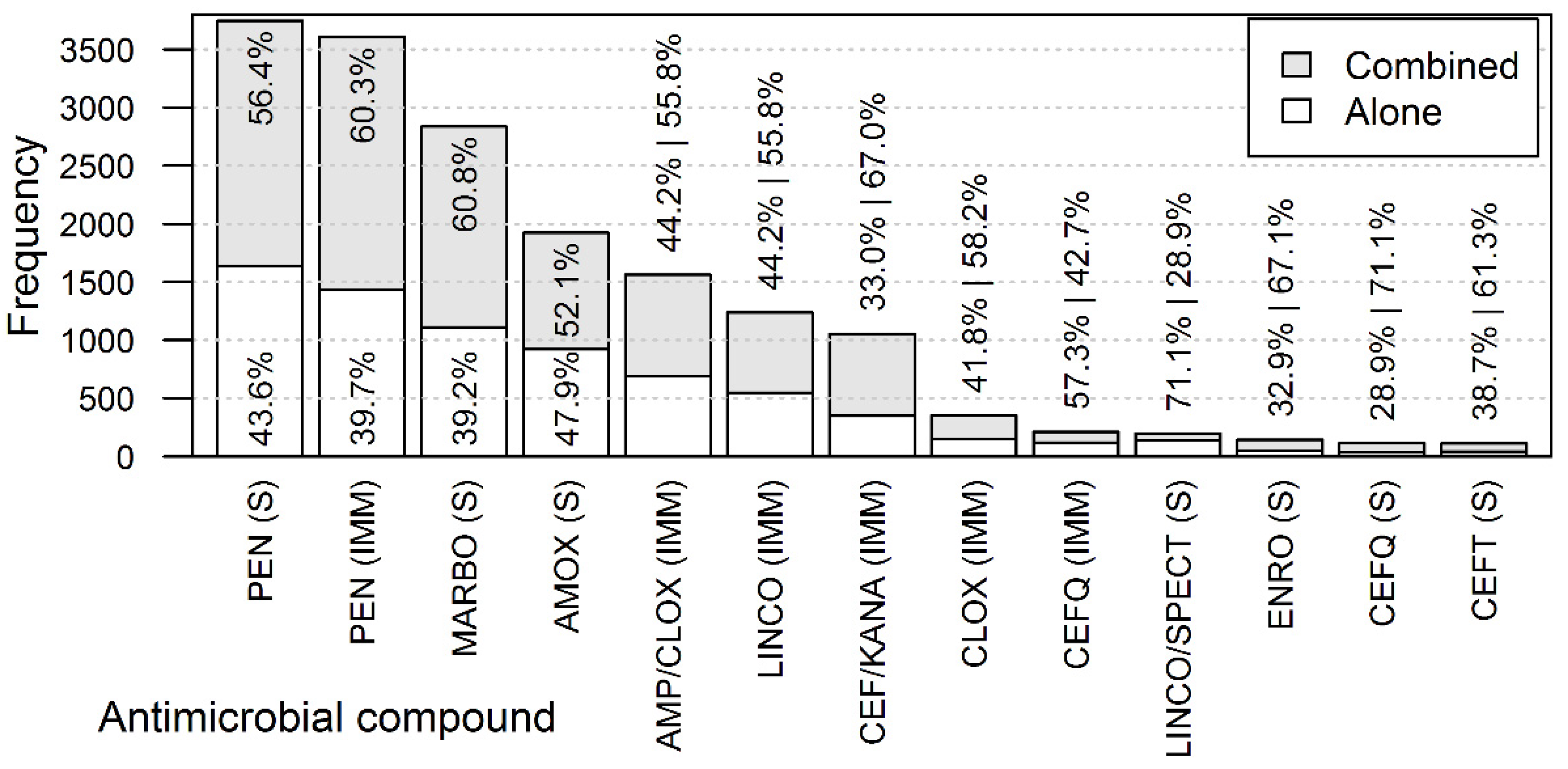
2.2. Antimicrobial Compounds Used in the CM Treatments
2.3. Combination of Antimicrobials within a Treatment Course
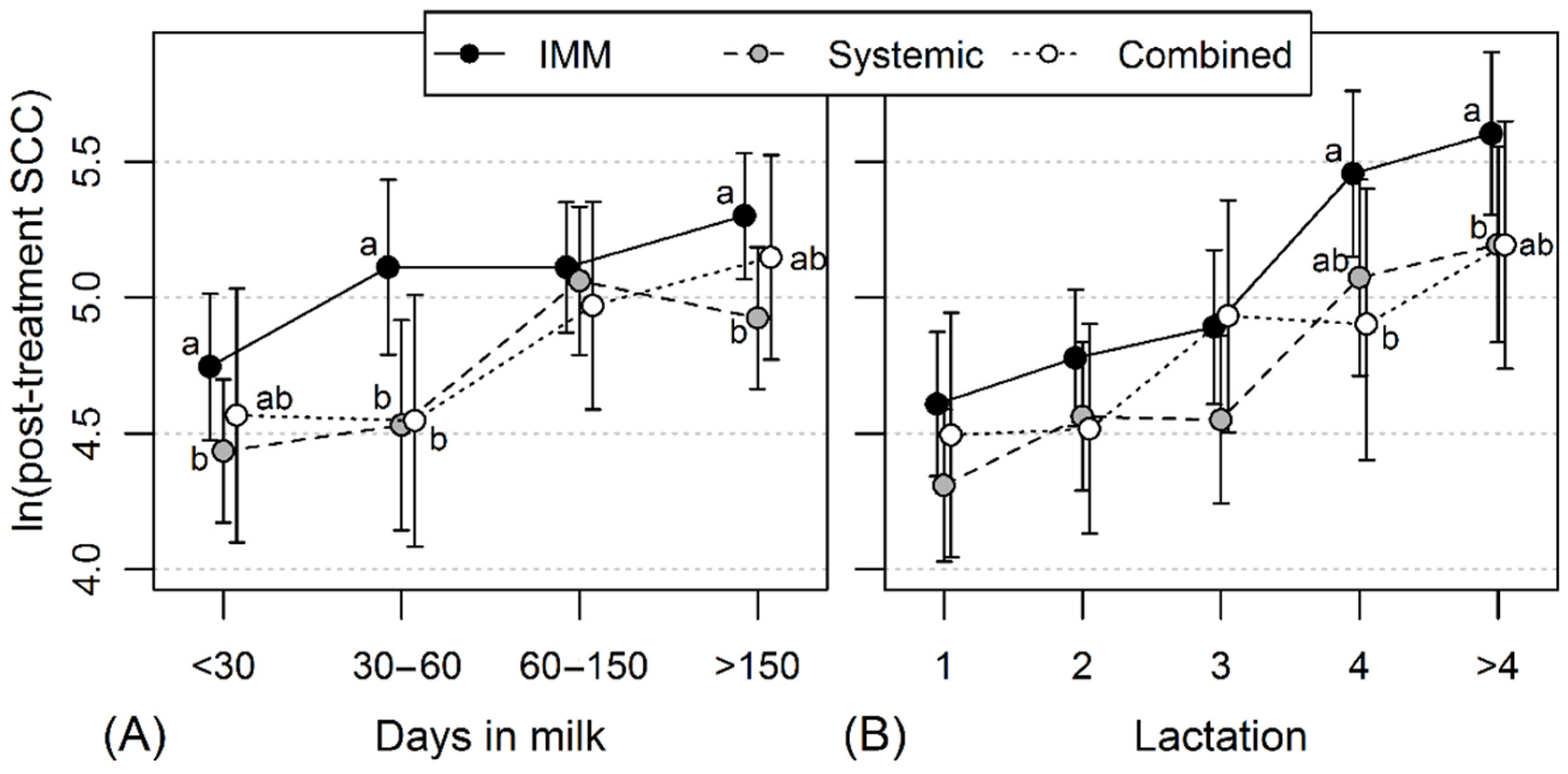
2.4. The Effect of Different Penicillin Treatment Schemes of CM on Post-Treatment Milk SCC
3. Discussion
3.1. Incidence Rate of Antimicrobial-Treated CM
3.2. Antimicrobial Usage in the Treatment of CM Cases
3.3. Association between Different CM Penicillin Treatment Protocols and the Level of Post-Treatment Milk SCC
3.4. Limitations of the Study
4. Materials and Methods
4.1. Collection of CM Treatment Data
4.2. Analysis of the Efficacy of Different Penicillin Treatment Protocols on Post-Treatment Milk SCC
4.3. Definitions of a CM Treatment and a Treatment Course
4.4. Data Editing
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruegg, P.L. What is success? A narrative review of research evaluating outcomes of antimicrobials used for treatment of clinical mastitis. Front. Vet. Med. 2021, 8, 639641. [Google Scholar] [CrossRef] [PubMed]
- Krogh, M.A.; Nielsen, C.L.; Sørensen, J.T. Antimicrobial use in organic and conventional dairy herds. Animal 2020, 14, 2187–2193. [Google Scholar] [CrossRef]
- European Medicines Agency. Categorisation of Antibiotics in the European Union, EMA/CVMP/CHMP/682198/2017. 2020. Available online: https://www.ema.europa.eu/en/documents/report/categorisation-antibiotics-european-union-answer-request-european-commission-updating-scientific_en.pdf (accessed on 24 November 2021).
- OIE Standards, Guidelines and Resolution on Antimicrobial Resistance and the Use of Antimicrobial Agents; World Organisation for Animal Health: Paris, France, 2015; Available online: https://web.oie.int/delegateweb/eng/ebook/AF-book-AMR-ANG_FULL.pdf?WAHISPHPSESSID=03152ead00d06990fa9066b7b71fcabc (accessed on 24 November 2021).
- Finnish Food Authority and University of Helsinki, Institute of Veterinary Medicine. Mikrobilääkkeiden Käyttösuositukset Eläinten Tärkeimpiin Tulehdus—Ja Tartuntatauteihin. 2016. Available online: https://www.ruokavirasto.fi/globalassets/tietoa-meista/asiointi/oppaat-ja-lomakkeet/viljelijat/elainten-pito/elainten-laakitseminen/mikrobilaakkeiden_kayttosuositukset_fi_2.pdf (accessed on 22 November 2021).
- Sølverød, L.; Whist, A.C.; Katholm, J.; Rattenborg, E.; Kulkas, L.; Sandgren, C.; Carlsson, J.; Landin, H. Nordic Guidelines for Mastitis Therapy; Swedish Dairy Association: Stockholm, Sweden, 2011; Available online: https://www.sva.se/media/qsljw2yb/nordic-guidelines-for-mastitis-therapy.pdf (accessed on 24 November 2021).
- Ruegg, P.L. Making antibiotic treatment decisions for clinical mastitis. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 413–425. [Google Scholar] [CrossRef]
- Vakkamäki, J.; Taponen, S.; Heikkilä, A.-M.; Pyörälä, S. Bacteriological etiology and treatment of mastitis in Finnish dairy herds. Acta Vet. Scand. 2017, 59, 33. [Google Scholar] [CrossRef] [Green Version]
- Cvetnić, L.; Samardžija, M.; Duvnjak, S.; Habrun, B.; Cvetnić, M.; Tkalec, V.J.; Đuričić, D.; Benić, M. Multi Locus Sequence Typing and spa Typing of Staphylococcus aureus Isolated from the Milk of Cows with Subclinical Mastitis in Croatia. Microorganisms 2021, 9, 725. [Google Scholar] [CrossRef]
- Carmo, L.P.; Nielsen, L.R.; Alban, L.; da Costa, P.M.; Schüpbach-Regula, G.; Magouras, I. Veterinary expert opinion on potential drivers and opportunities for changing antimicrobial usage practices in livestock in Denmark, Portugal, and Switzerland. Front. Vet. Sci. 2018, 5, 29. [Google Scholar] [CrossRef]
- Carmo, L.P.; Bouzalas, I.; Nielsen, L.R.; Alban, L.; da Costa, P.M.; Müntener, C.; Schüpbach, G.; Abreu, Y.; Magouras, I. Expert opinion on livestock antimicrobial usage indications and patterns in Denmark, Portugal and Switzerland. Vet. Rec. Open 2018, 5, e000288. [Google Scholar] [CrossRef] [Green Version]
- Grave, K.; Jensen, V.F.; Odensvik, K.; Wierup, M.; Bangen, M. Usage of veterinary therapeutic antimicrobials in Denmark, Norway and Sweden following termination of antimicrobial growth promoter use. Prev. Vet. Med. 2006, 75, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Estonian Agency of Medicines. Register of Medicinal Products. 2021. Available online: https://www.ravimiregister.ee/en/publichomepage.aspx?pv=PublicMedDetail&vid=1caeb73e-53ec-4c8f-b75d-dd18faf733dd (accessed on 24 November 2021).
- Kalmus, P.; Aasmäe, B. Juhend Antibiootikumide Kasutamiseks Põllumajandusloomadel. Maaeluministeerium. Estonian University of Life Sciences. 2020. Available online: https://www.agri.ee/sites/default/files/content/valjaanded/juhend-2020-antibiootikumiravi-loomad.pdf (accessed on 23 November 2021).
- Sérieys, F.; Raguet, Y.; Goby, L.; Schmidt, H.; Friton, G. Comparative efficacy of local and systemic antimicrobial treatment in lactating cows with clinical mastitis. J. Dairy Sci. 2005, 88, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Lago, A.; Godden, S.M.; Bey, R.; Ruegg, P.L.; Leslie, K. The selective treatment of clinical mastitis based on on-farm culture results: II. Effects on lactation performance, including clinical mastitis recurrence, somatic cell count, milk production, and cow survival. J. Dairy Sci. 2011, 94, 4457–4467. [Google Scholar] [CrossRef] [PubMed]
- Erskine, R.J.; Bartlett, P.C.; VanLente, J.L.; Phipps, C.R. Efficacy of systemic ceftiofur as a therapy for severe clinical mastitis in dairy cattle. J. Dairy Sci. 2002, 85, 2571–2575. [Google Scholar] [CrossRef]
- Suojala, L.; Simojoki, H.; Mustonen, K.; Kaartinen, L.; Pyörälä, S. Efficacy of enrofloxacin in the treatment of naturally occurring acute clinical Escherichia coli mastitis. J. Dairy Sci. 2010, 93, 1960–1969. [Google Scholar] [CrossRef] [Green Version]
- Kalmus, P.; Simojoki, H.; Orro, T.; Taponen, S.; Mustonen, K.; Holopainen, J.; Pyörälä, S. Efficacy of 5-day parenteral versus intramammary benzylpenicillin for treatment of clinical mastitis caused by gram-positive bacteria susceptible to penicillin in vitro. J. Dairy Sci. 2014, 97, 2155–2164. [Google Scholar] [CrossRef] [Green Version]
- Taponen, S.; Jantunen, A.; Pyörälä, E.; Pyörälä, S. Efficacy of targeted 5-day combined parenteral and intramammary treatment of clinical mastitis caused by penicillin-susceptible or penicillin-resistant Staphylococcus aureus. Acta Vet. Scand. 2003, 44, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Pyörälä, S.H.; Pyörälä, E.O. Efficacy of parenteral administration of three antimicrobial agents in treatment of clinical mastitis in lactating cows: 487 cases (1989–1995). J. Am. Vet. Med. Assoc. 1998, 212, 407–412. [Google Scholar]
- St Rose, S.G.; Swinkels, J.M.; Kremer, W.D.J.; Kruitwagen, C.L.J.J.; Zadoks, R.N. Effect of penethamate hydriodide treatment on bacteriological cure, somatic cell count and milk production of cows and quarters with chronic subclinical Streptococcus uberis or Streptococcus dysgalactiae infection. J. Dairy Res. 2003, 70, 387–394. [Google Scholar] [CrossRef]
- Ruegg, P. New perspectives in udder health management. Vet. Clin. N. Am. Food Anim. Pract. 2012, 28, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Levison, L.J.; Miller-Cushon, E.K.; Tucker, A.L.; Bergeron, R.; Leslie, K.E.; Barkema, H.W.; DeVries, T.J. Incidence rate of pathogen-specific clinical mastitis on conventional and organic Canadian dairy farms. J. Dairy Sci. 2016, 99, 1314–1350. [Google Scholar] [CrossRef]
- Gao, J.; Barkema, H.W.; Zhang, L.; Liu, G.; Deng, Z.; Cai, L.; Shan, R.; Zhang, S.; Zou, J.; Kastelic, J.P.; et al. Incidence of clinical mastitis and distribution of pathogens on large Chinese dairy farms. J. Dairy Sci. 2017, 100, 4797–4806. [Google Scholar] [CrossRef]
- O’Reilly, K.M.; Green, M.J.; Peeler, E.J.; Fitzpatrick, J.L.; Green, L.E. Investigation of risk factors for clinical mastitis in British dairy herds with bulk milk somatic cell counts less than 150.000 cells/mL. Vet. Rec. 2006, 158, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Olde Riekerink, R.G.M.; Barkema, H.W.; Kelton, D.F.; Scholl, D.T. Incidence rate of clinical mastitis on Canadian dairy farms. J. Dairy Sci. 2008, 91, 1366–1377. [Google Scholar] [CrossRef]
- van den Borne, B.H.P.; van Schaik, G.; Lam, T.J.G.M.; Nielen, M. Variation in herd level mastitis indicators between primi- and multiparae in Dutch dairy herds. Prev. Vet. Med. 2010, 96, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Suojala, L.; Kaartinen, L.; Pyörälä, S. Treatment for bovine Escherichia coli mastitis—An evidence-based approach. J. Vet. Pharmacol. Ther. 2013, 36, 521–531. [Google Scholar] [CrossRef]
- Scott, H.M.; Acuff, G.; Bergeron, G.; Bourassa, M.W.; Gill, J.; Graham, D.W.; Kahn, L.H.; Morley, P.S.; Salois, M.J.; Simjee, S.; et al. Critically important antimicrobials: Criteria and approaches for measuring and reducing their use in food animal agriculture. Ann. N. Y. Acad. Sci. 2019, 144, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Riigi Teataja. Animal Disease Control Act. 2010. Available online: https://www.riigiteataja.ee/en/eli/518062015013/consolide (accessed on 12 November 2021).
- Owens, W.E.; Watts, J.L.; Boddie, R.L.; Nickerson, S.C. Antibiotic treatment of mastitis: Comparison of intramammary and intramammary plus intramuscular therapies. J. Dairy Sci. 1988, 71, 3143–3147. [Google Scholar] [CrossRef]
- Erskine, R.J.; Wagner, S.; DeGraves, F.J. Mastitis therapy and pharmacology. Vet. Clin. N. Am. Food Anim. Pract. 2003, 19, 109–138. [Google Scholar] [CrossRef]
- Peiter, M.; Phillips, H.N.; Endres, M.I. Association between early postpartum rumination time and peak milk yield in dairy cows. J. Dairy Sci. 2021, 104, 5898–5908. [Google Scholar] [CrossRef]
- Esposito, G.; Irons, P.C.; Webb, E.C.; Chapwanya, A. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim. Reprod. Sci. 2014, 144, 60–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDougall, S.; Clausen, L.; Hintukainen, J.; Hunnam, J. Randomized, controlled, superiority study of extended duration of therapy with an intramammary antibiotic for treatment of clinical mastitis. J. Dairy Sci. 2019, 102, 4376–4386. [Google Scholar] [CrossRef]
- Wiggans, G.R.; Shook, G.E. A lactation measure of somatic cell count. J. Dairy Sci. 1987, 70, 2666–2672. [Google Scholar] [CrossRef]
- Barkema, H.W.; Schukken, Y.H.; Zadoks, R.N. Invited review: The role of cow, pathogen, and treatment regimen in the therapeutic success of bovine staphylococcus aureus mastitis. J. Dairy Sci. 2006, 89, 1877–1895. [Google Scholar] [CrossRef] [Green Version]
- Putz, E.J.; Palmer, M.V.; Ma, H.; Casas, E.; Reinhardt, T.A.; Lippolis, J.D. Case report: Characterization of a persistent, treatment-resistant, novel Staphylococcus aureus infection causing chronic mastitis in a Holstein dairy cow. BMC Vet. Res. 2020, 16, 336. [Google Scholar] [CrossRef] [PubMed]
| Active Compound(s) in the Antimicrobial Product | Number of Treatments (%; 95% Confidence Intervals) | ||
|---|---|---|---|
| Systemic | IMM 1 | Total | |
| Penicillins | |||
| Procaine benzylpenicillin | 2498 (27.4; 26.5–28.3) | 3605 (44.8; 43.7–45.9) | 6103 (35.5; 34.8–36.2) |
| Procaine benzylpenicillin/dihydrostreptomycin | 1249 (13.7; 13.0–14.4) | * | 1249 (7.3; 6.9–7.7) |
| Amoxicillin | 1926 (21.1; 20.3–22.0) | * | 1926 (11.2; 10.7–11.7) |
| Ampicillin/cloxacillin | * | 1562 (19.4; 18.5–20.3) | 1562 (9.1; 8.7–9.5) |
| Cloxacillin | * | 354 (4.4; 4.0–4.9) | 354 (2.1; 1.9–2.3) |
| Cephalosporins | |||
| Cefalexin/kanamycin | * | 1054 (13.1; 12.3–13.8) | 1054 (6.1; 5.8–6.5) |
| Cefacetrile/rifaximin | * | 30 (0.4; 0.3–0.5) | 30 (0.2; 0.1–0.2) |
| Ceftiofur | 111 (1.2; 1.0–1.5) | * | 111 (0.6; 0.5–0.8) |
| Cefquinome | 114 (1.2; 1.0–1.5) | 211 (2.6; 2.3–3.0) | 325 (1.9; 1.7–2.1) |
| Fluoroquinolones | |||
| Marbofloxacin | 2839 (31.1; 30.2–32.1) | * | 2839 (16.5; 16.0–17.1) |
| Enrofloxacin | 143 (1.6; 1.3–1.8) | * | 143 (0.8; 0.7–1.0) |
| Lincosamides | |||
| Lincomycin | * | 347 (4.3; 3.9–4.8) | 347 (2.0; 1.8–2.2) |
| Lincomycin/neomycin | * | 889 (11.0; 10.4–11.7) | 889 (5.2; 4.8–5.5) |
| Lincomycin/spectinomycin | 197 (2.1; 1.9–2.5) | * | 197 (1.1; 1.0–1.3) |
| Other antimicrobials | |||
| Tetracycline | 42 (0.5; 0.3–0.6) | * | 42 (0.2; 0.2–0.3) |
| Sulfadiazine/trimethoprim | 9 (0.1; 0.0–0.2) | * | 9 (0.1; 0.02–0.1) |
| Total | 9128 (100.0) | 8052 (100.0) | 17,180 (100.0) |
| Characteristic | Mean (Standard Error) | Median | Range (Min; Max) |
|---|---|---|---|
| Herd size (n of cows) | 660 (472.3) | 566 | 100; 2398 |
| 305-day milk yield (kg) | 10,702 (175.1) | 10,698 | 7915; 13,226 |
| Herd SCC (× 1000/mL) | 212 (5.4) | 191 | 128; 537 |
| IMI rate 1 | 25.0 (1.6) | 23.2 | 14.5; 63.5 |
| New IMI rate 2 | 6.8 (0.3) | 6.8 | 5.2; 8.2 |
| Chronic IMI rate 3 | 18.2 (1.9) | 16.4 | 8.6; 46.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timonen, A.; Sammul, M.; Taponen, S.; Kaart, T.; Mõtus, K.; Kalmus, P. Antimicrobial Selection for the Treatment of Clinical Mastitis and the Efficacy of Penicillin Treatment Protocols in Large Estonian Dairy Herds. Antibiotics 2022, 11, 44. https://doi.org/10.3390/antibiotics11010044
Timonen A, Sammul M, Taponen S, Kaart T, Mõtus K, Kalmus P. Antimicrobial Selection for the Treatment of Clinical Mastitis and the Efficacy of Penicillin Treatment Protocols in Large Estonian Dairy Herds. Antibiotics. 2022; 11(1):44. https://doi.org/10.3390/antibiotics11010044
Chicago/Turabian StyleTimonen, Anri, Marju Sammul, Suvi Taponen, Tanel Kaart, Kerli Mõtus, and Piret Kalmus. 2022. "Antimicrobial Selection for the Treatment of Clinical Mastitis and the Efficacy of Penicillin Treatment Protocols in Large Estonian Dairy Herds" Antibiotics 11, no. 1: 44. https://doi.org/10.3390/antibiotics11010044
APA StyleTimonen, A., Sammul, M., Taponen, S., Kaart, T., Mõtus, K., & Kalmus, P. (2022). Antimicrobial Selection for the Treatment of Clinical Mastitis and the Efficacy of Penicillin Treatment Protocols in Large Estonian Dairy Herds. Antibiotics, 11(1), 44. https://doi.org/10.3390/antibiotics11010044






