Protective Effects of Grape Seed Oligomeric Proanthocyanidins in IPEC-J2–Escherichia coli/Salmonella Typhimurium Co-Culture
Abstract
:1. Introduction
2. Results
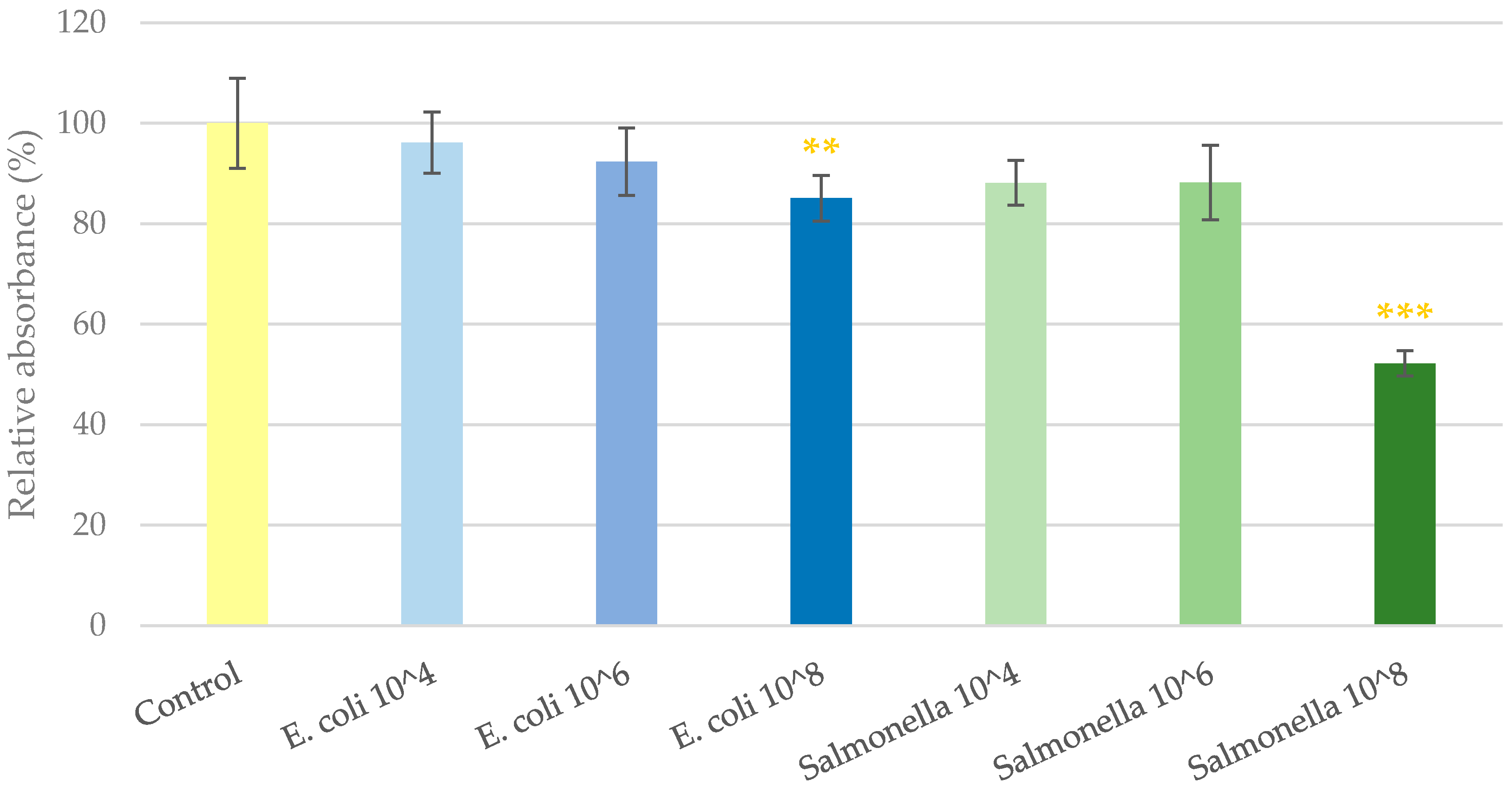
2.1. Cell Viability Determination
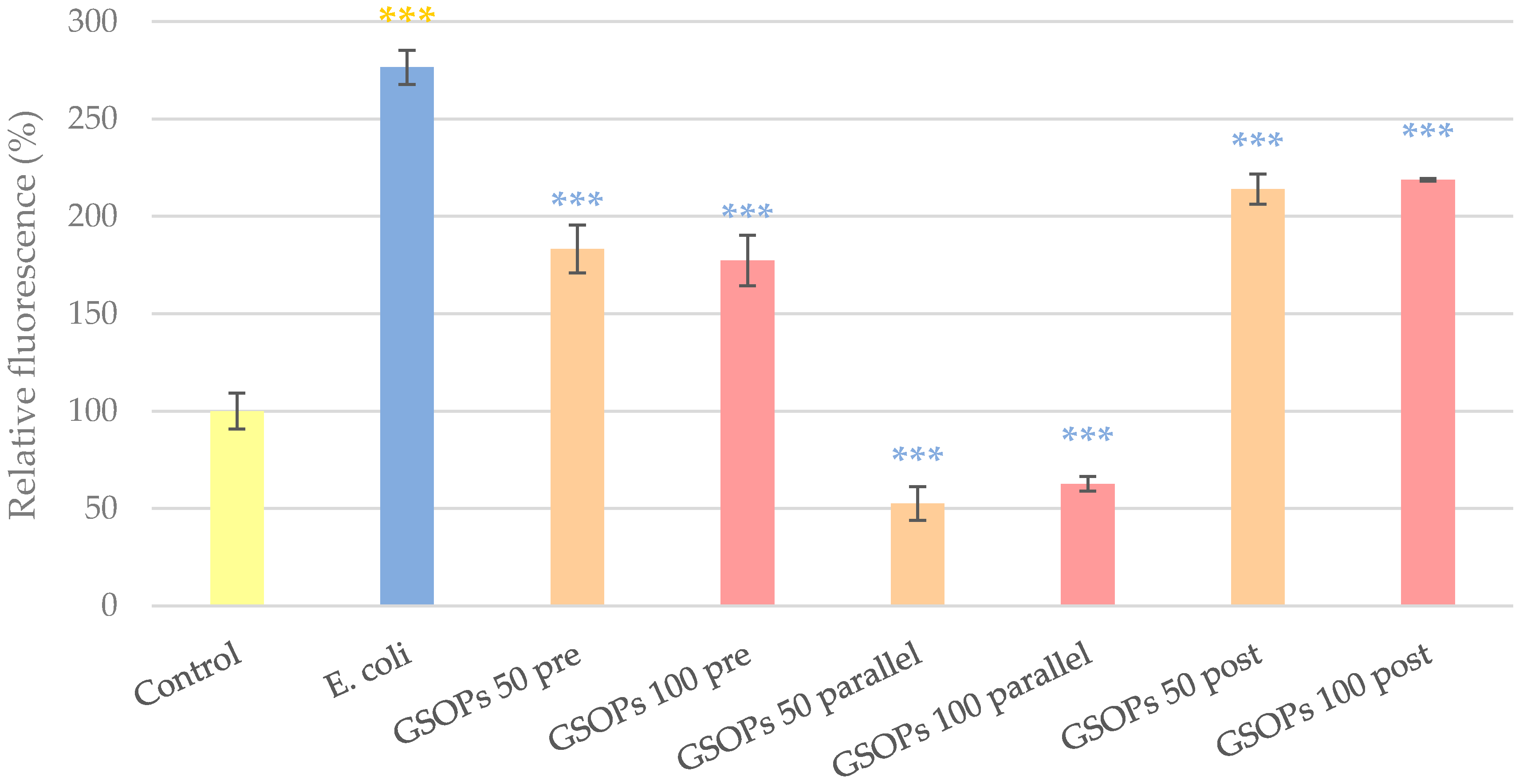
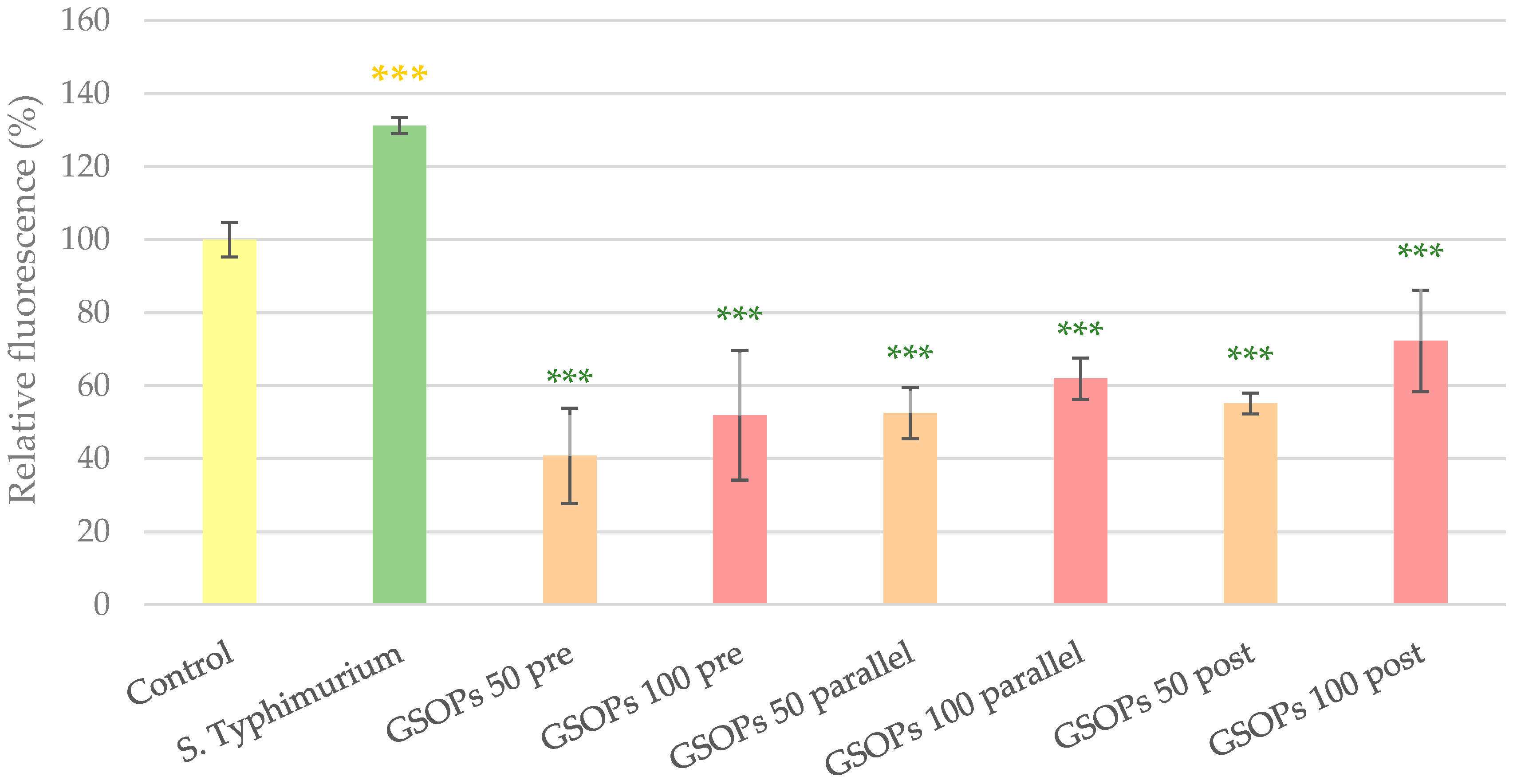
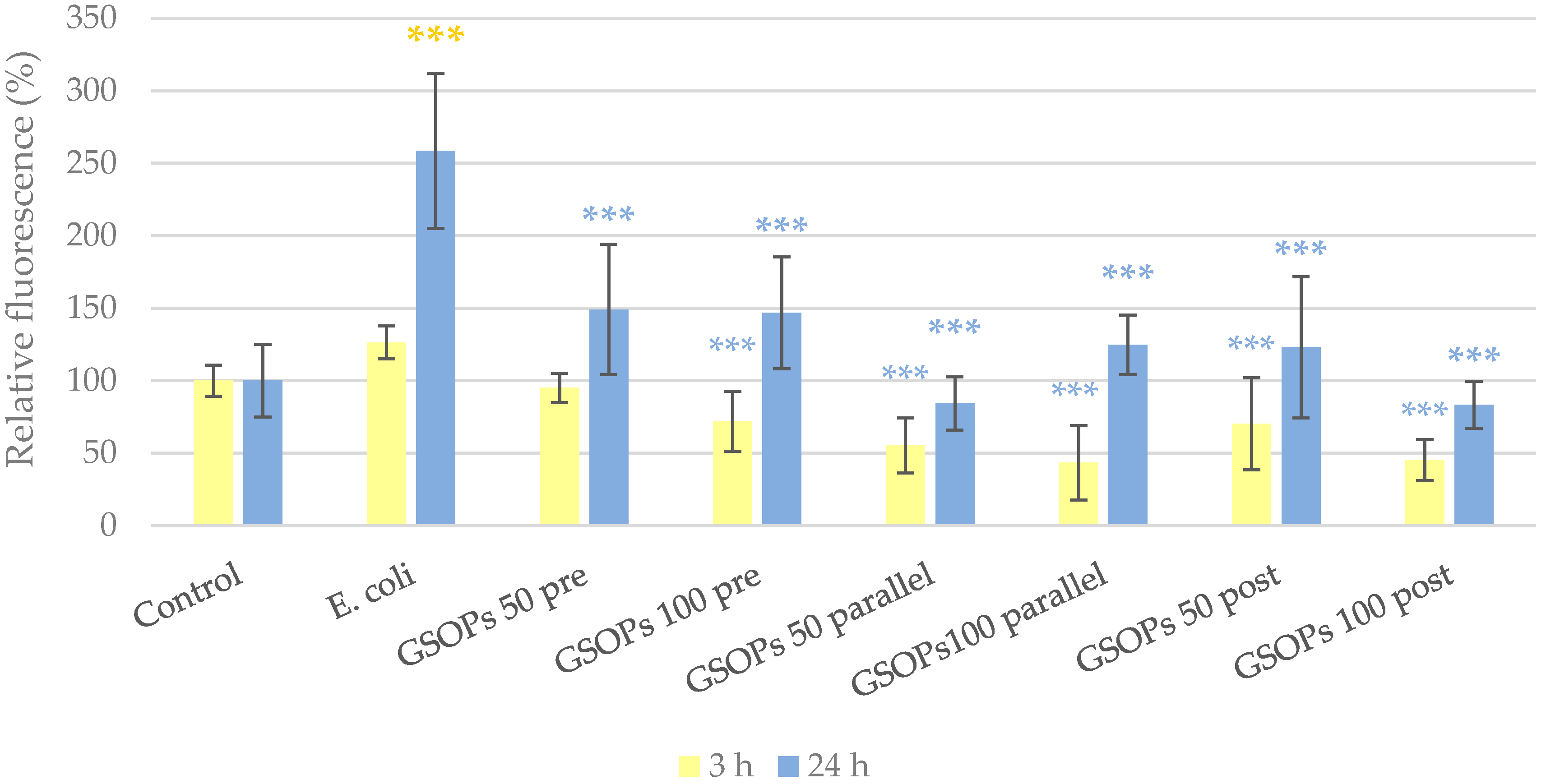
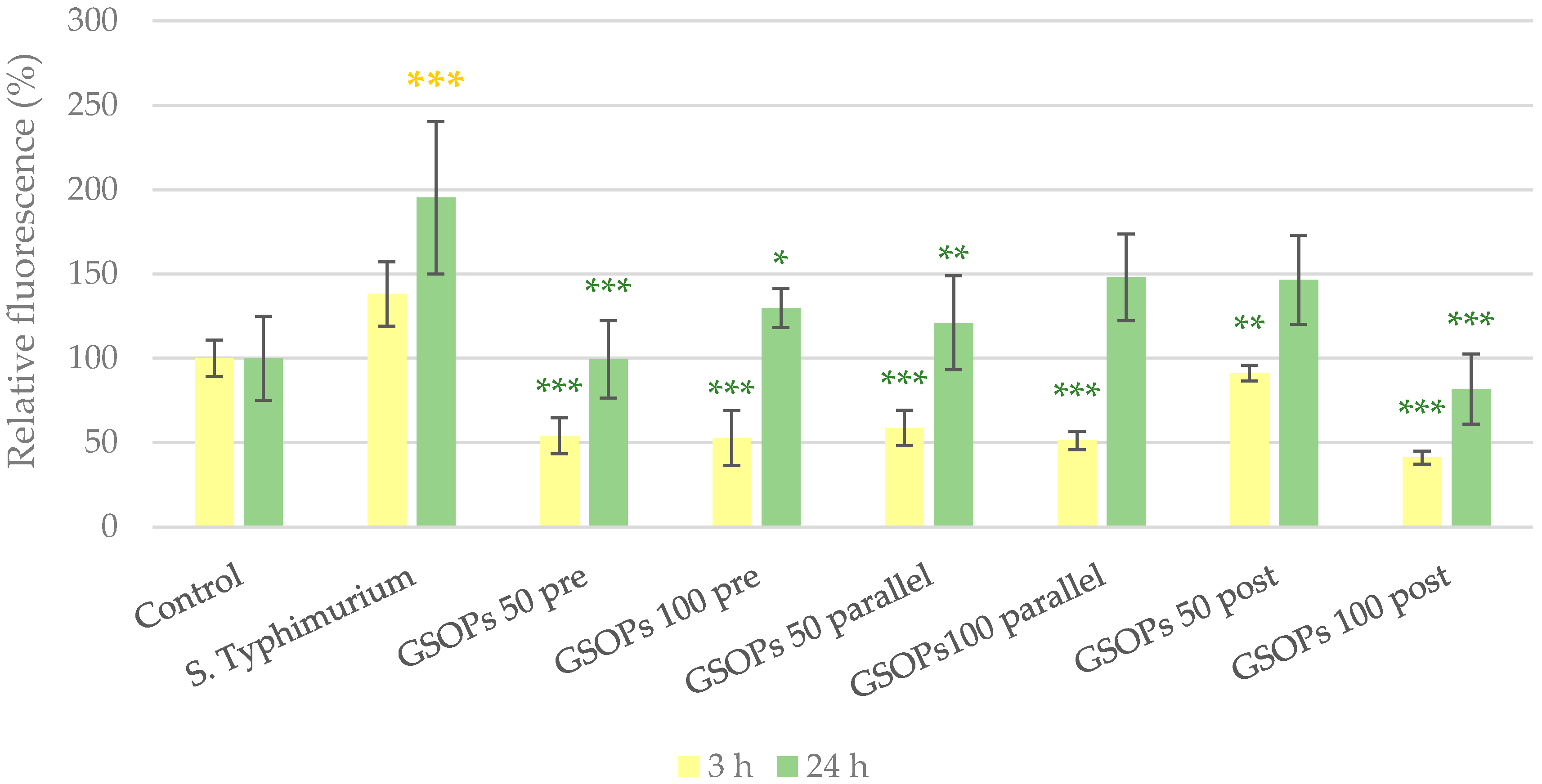
2.2. Intracellular Reactive Oxygen Species Level
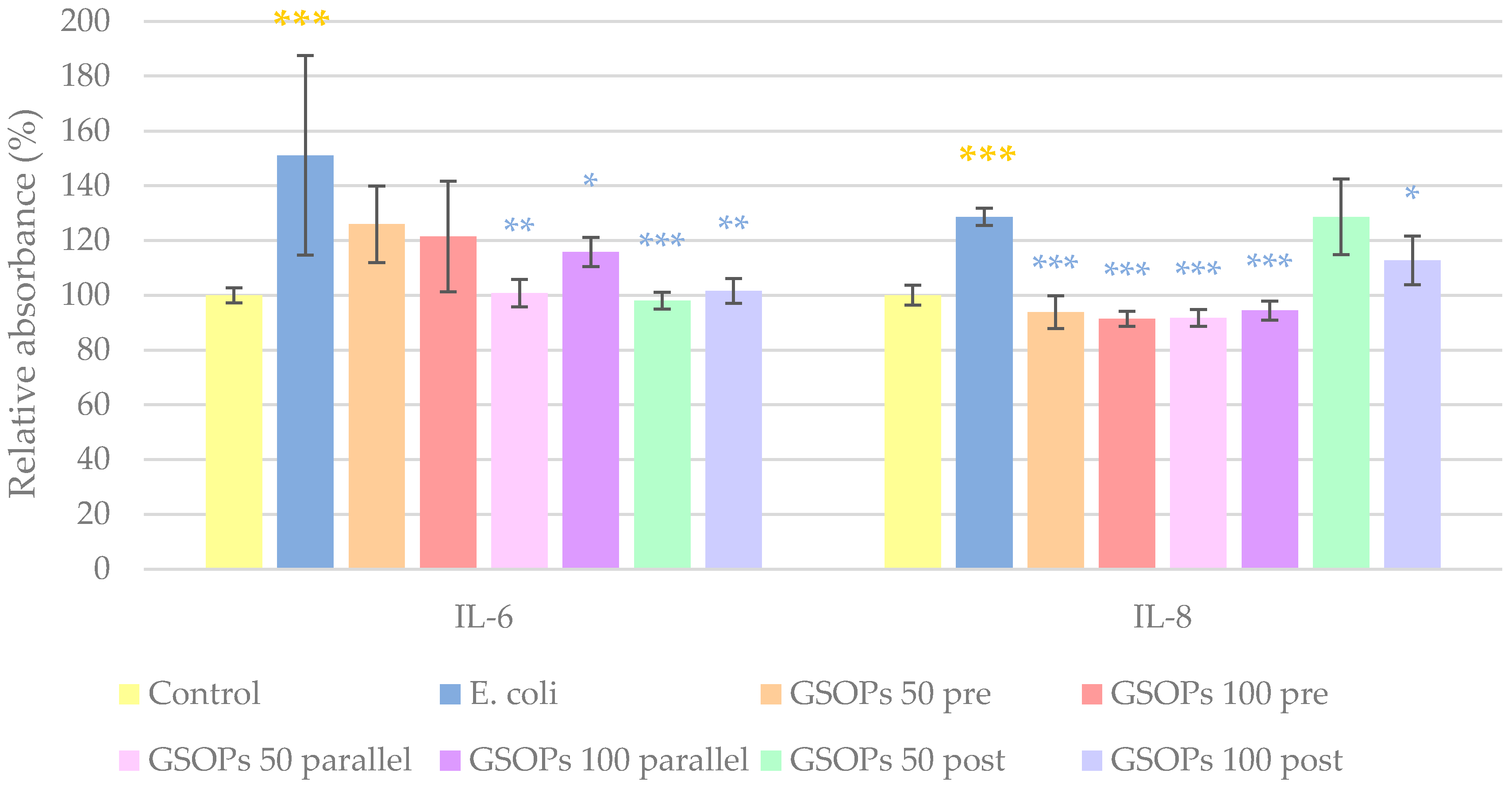
2.3. Interleukin Levels
2.4. Paracellular Permeability
2.5. Bacterial Adhesion
3. Discussion
4. Materials and Methods
4.1. Chemicals and Instruments
4.2. IPEC-J2 Cell Line
4.3. Bacterial Strains
4.4. Cell Viability Determination
4.5. Experimental Design
4.6. IC ROS Level Determination (DCFH-DA)
4.7. Interleukin Level Determination (ELISA)
4.8. Paracellular Permeability Determination (FD4)
4.9. Bacterial Adhesion Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Li, S.; Pi, D.; Zhu, H.; Hou, Y.; Shi, H.; Leng, W. Asparagine attenuates intestinal injury, improves energy status and inhibits AMP-activated protein kinase signaling pathways in weaned piglets challenged with Escherichia coli lipopolysaccharide. Br. J. Nutr. 2015, 114, 553–565. [Google Scholar] [CrossRef] [Green Version]
- Pi, D.; Liu, Y.; Shi, H.; Li, S.; Odle, J.; Lin, X.; Zhu, H.; Chen, F.; Hou, Y.; Leng, W. Dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge. J. Nutr. Biochem. 2014, 25, 456–462. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, J.; Sun, Z.; Li, J.; Sun, W.; Mao, J.; Wang, Y. Protective effects of taurine on growth performance and intestinal epithelial barrier function in weaned piglets challenged without or with lipopolysaccharide. Anim. Prod. Sci. 2017, 58, 2011–2020. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, J.; Wu, G.; Chen, D.; Yu, B.; Huang, Z.; Luo, Y.; Zheng, P.; Luo, J.; Mao, X.; et al. Amelioration of Enterotoxigenic Escherichia coli- Induced Intestinal Barrier Disruption by Low-Molecular-Weight Chitosan in Weaned Pigs is Related to Suppressed Intestinal Inflammation and Apoptosis. Int. J. Mol. Sci. 2019, 20, 3485. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, T.S.; Giromini, C.; Rebucci, R.; Baldi, A. Omega-3 Polyunsaturated Fatty Acids Counteract Inflammatory and Oxidative Damage of Non-Transformed Porcine Enterocytes. Animals 2020, 10, 956. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert. Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Kovács, D.; Karancsi, Z.; Farkas, O.; Jerzsele, Á. Antioxidant activity of flavonoids in LPS-treated IPEC-J2 porcine intestinal epithelial cells and their antibacterial effect against bacteria of swine origin. Antioxidants 2020, 9, 1267. [Google Scholar] [CrossRef] [PubMed]
- De Azevedo, E.C.; Martins, B.T.F.; Casas, M.R.T.; Possebon, F.S.; Junior, J.P.A.; Nero, L.A.; Yamatogi, R.S. Multidrug resistance and virulence profiles of Salmonella isolated from swine lymph nodes. Microb. Drug. Resist. 2020, 27, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Fallah, N.; Ghaemi, M.; Ghazvini, K.; Rad, M.; Jamshidi, A. Occurrence, pathotypes, and antimicrobial resistance profiles of diarrheagenic Escherichia coli strains in animal source food products from public markets in Mashhad, Iran. Food Control 2021, 121, 107640. [Google Scholar] [CrossRef]
- Porter, S.; Strain, S.A.J.; Bagdonaite, G.; McDowell, S.; Bronckaers, T.; Sherrey, M.; Devine, P.; Pascual-Linaza, A.V.; Spence, N.; Porter, R.; et al. Trends in Salmonella serovars and antimicrobial resistance in pigs and poultry in Northern Ireland between 1997 and 2016. Vet. Rec. 2020, 186, 156. [Google Scholar] [CrossRef] [PubMed]
- Garrido, V.; Sánchez, S.; Román, B.S.; Fraile, L.; Migura-García, L.; Grilló, M.-J. Salmonella infection in mesenteric lymph nodes of breeding sows. Foodborne Pathog. Dis. 2020, 17, 411–417. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017/2018. EFSA J. 2020, 18, 6007. [Google Scholar] [CrossRef] [Green Version]
- Cádiz-Gurrea, M.D.L.L.; Borrás-Linares, I.; Lozano-Sánchez, J.; Joven, J.; Fernández-Arroyo, S.; Segura-Carretero, A. Cocoa and grape seed byproducts as a source of antioxidant and anti-inflammatory proanthocyanidins. Int. J. Mol. Sci. 2017, 18, 376. [Google Scholar] [CrossRef]
- Jonker, A.; Yu, P. The occurrence, biosynthesis, and molecular structure of proanthocyanidins and their effects on legume forage protein precipitation, digestion and absorption in the ruminant digestive tract. Int. J. Mol. Sci. 2017, 18, 1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbe, A.; Ramé, C.; Mellouk, N.; Estienne, A.; Bongrani, A.; Brossaud, A.; Riva, A.; Guérif, F.; Froment, P.; Dupont, J. Effects of grape seed extract and proanthocyanidin B2 on in vitro proliferation, viability, steroidogenesis, oxidative stress, and cell signaling in human granulosa cells. Int. J. Mol. Sci. 2019, 20, 4215. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Dwivedi, M.; Mahdi, A.A.; Gowda, G.A.N.; Khetrapal, C.L.; Bhandari, M. Inhibition of adherence of multi-drug resistant E. coli by proanthocyanidins. Urol. Res. 2012, 40, 143–150. [Google Scholar] [CrossRef]
- Harmidy, K.; Tufenkji, N.; Gruenheid, S. Perturbation of Host Cell Cytoskeleton by Cranberry Proanthocyanidins and Their Effect on Enteric Infections. PLoS ONE 2011, 6, e27267. [Google Scholar] [CrossRef] [PubMed]
- Nicolosi, D.; Tempera, G.; Genovese, C.; Furneri, P.M. Anti-Adhesion Activity of A2-type Proanthocyanidins (a Cranberry Major Component) on Uropathogenic, E. coli and P. mirabilis Strains. Antibiotics 2014, 3, 143–154. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, L.; Li, W.; Zhang, S.; Luo, L.; Wang, J.; Sun, B. In vitro evaluation of the anti-digestion and antioxidant effects of grape seed procyanidins according to their degrees of polymerization. J. Funct. Foods 2018, 49, 85–95. [Google Scholar] [CrossRef]
- González-Quilen, C.; Rodríguez-Gallego, E.; Beltrán-Debón, R.; Pinent, M.; Ardévol, A.; Blay, M.T.; Terra, X. Health-Promoting Properties of Proanthocyanidins for Intestinal Dysfunction. Nutrients 2020, 12, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schierack, P.; Nordhoff, M.; Pollmann, M.; Weyrauch, K.D.; Amasheh, S.; Lodemann, U.; Jores, J.; Tachu, B.; Kleta, S.; Blikslager, A.; et al. Characterization of a porcine intestinal epithelial cell line for in vitro studies of microbial pathogenesis in swine. Histochem. Cell. Biol. 2006, 125, 293–305. [Google Scholar] [CrossRef]
- Verhoeckx, K.; Cotter, P.; López-Expósito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer International Publishing: Cham, Switzerland, 2015; pp. 125–129. [Google Scholar] [CrossRef]
- Ayuso, M.; Van Cruchten, S.; Van Ginneken, C. A medium-throughput system for in vitro oxidative stress assessment in IPEC-J2 cells. Int. J. Mol. Sci. 2020, 21, 7263. [Google Scholar] [CrossRef]
- Klingspor, S.; Bondzio, A.; Martens, H.; Aschenbach, J.R.; Bratz, K.; Tedin, K.; Einspanier, R.; Lodemann, U. Enterococcus faecium NCIMB 10415 modulates epithelial integrity, heat shock protein, and proinflammatory cytokine response in intestinal cells. Mediat. Inflamm. 2015, 2015, 304149. [Google Scholar] [CrossRef] [Green Version]
- Loss, H.; Aschenbach, J.R.; Tedin, K.; Ebner, F.; Lodemann, U. The inflammatory response to enterotoxigenic E. coli and probiotic E. faecium in a coculture model of porcine intestinal epithelial and dendritic cells. Mediat. Inflamm. 2018, 2018, 9368295. [Google Scholar] [CrossRef] [Green Version]
- Kern, M.; Günzel, D.; Aschenbach, J.R.; Tedin, K.; Bondzio, A.; Lodemann, U. Altered Cytokine Expression and Barrier Properties after In Vitro Infection of Porcine Epithelial Cells with Enterotoxigenic Escherichia coli and Probiotic Enterococcus faecium. Mediat. Inflamm. 2017, 2017, 2748192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zeng, Y.; Wang, S.; Liu, H.; Zhang, D.; Zhang, W.; Wang, Y.; Ji, H. Swine-Derived Probiotic Lactobacillus plantarum Inhibits Growth and Adhesion of Enterotoxigenic Escherichia coli and Mediates Host Defense. Front. Microbiol. 2018, 9, 1364. [Google Scholar] [CrossRef]
- Palma, E.; Tilocca, B.; Roncada, P. Antimicrobial Resistance in Veterinary Medicine: An Overview. Int. J. Mol. Sci. 2020, 21, 1914. [Google Scholar] [CrossRef] [Green Version]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Yang, L.; Xian, D.; Xiong, X.; Lai, R.; Song, J.; Zhong, J. Proanthocyanidins against Oxidative Stress: From Molecular Mechanisms to Clinical Applications. BioMed Res. Int. 2018, 2018, 8584136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Z.; Song, Z.; Zhao, Y.; Wang, X.; Liu, P. Grape seed proanthocyanidin extract protects human lens epithelial cells from oxidative stress via reducing NF-кB and MAPK protein expression. Mol. Vis. 2011, 17, 210–217. [Google Scholar] [PubMed]
- Wu, H.; Luo, T.; Li, Y.M.; Gao, Z.P.; Zhang, K.Q.; Song, J.Y.; Xiao, J.S.; Cao, Y.P. Granny Smith apple procyanidin extract upregulates tight junction protein expression and modulates oxidative stress and inflammation in lipopolysaccharide-induced Caco-2 cells. Food. Funct. 2018, 9, 3321–3329. [Google Scholar] [CrossRef]
- Liu, H.-J.; Pan, X.X.; Liu, B.Q.; Gui, G.; Hu, L.; Jiang, C.-Y.; Han, Y.; Fan, Y.X.; Tang, Y.L.; Liu, W.T. Grape seed-derived procyanidins alleviate gout pain via NLRP3 inflammasome suppression. J. Neuroinflamm. 2017, 14, 74. [Google Scholar] [CrossRef]
- Pajuelo, D.; Quesada, H.; Díaz, S.; Fernández-Iglesias, A.; Arola-Arnal, A.; Bladé, C.; Salvadó, J.; Arola, L. Chronic dietary supplementation of proanthocyanidins corrects the mitochondrial dysfunction of brown adipose tissue caused by diet-induced obesity in Wistar rats. Br. J. Nutr. 2012, 107, 170–178. [Google Scholar] [CrossRef]
- Gil-Cardoso, K.; Comitato, R.; Ginés, I.; Ardévol, A.; Pinent, M.; Virgili, F.; Terra, X.; Blay, M. Protective Effect of Proanthocyanidins in a Rat Model of Mild Intestinal Inflammation and Impaired Intestinal Permeability Induced by LPS. Mol. Nutr. Food Res. 2019, 63, 1800720. [Google Scholar] [CrossRef]
- Hao, R.; Li, Q.; Zhao, J.; Li, H.; Wang, W.; Gao, J. Effects of grape seed procyanidins on growth performance, immune function and antioxidant capacity in weaned piglets. Livest. Sci. 2015, 178, 237–242. [Google Scholar] [CrossRef]
- Chedea, V.S.; Palade, L.M.; Marin, D.E.; Pelmus, R.S.; Habeanu, M.; Rotar, M.C.; Gras, M.A.; Pistol, G.C.; Taranu, I. Intestinal Absorption and Antioxidant Activity of Grape Pomace Polyphenols. Nutrients 2018, 10, 588. [Google Scholar] [CrossRef] [Green Version]
- Taranu, I.; Marin, D.E.; Palade, M.; Pistol, G.C.; Chedea, V.S.; Gras, M.A.; Rotar, C. Assessment of the efficacy of a grape seed waste in counteracting the changes induced by aflatoxin B1 contaminated diet on performance, plasma, liver and intestinal tissues of pigs after weaning. Toxicon 2019, 162, 24–31. [Google Scholar] [CrossRef]
- Günay, E.; Celik, S.; Sarinc-Ulasli, S.; Özyürek, A.; Hazman, Ö.; Günay, S.; Özdemir, M.; Ünlü, M. Comparison of the Anti-inflammatory Effects of Proanthocyanidin, Quercetin, and Damnacanthal on Benzo(a)pyrene Exposed A549 Alveolar Cell Line. Inflammation 2016, 39, 744–751. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, Y.I.; Kim, Y.; Choi, M.; Min, S.; Joo, Y.H.; Yim, S.V.; Chung, N. Grape seed proanthocyanidin inhibits inflammatory responses in hepatic stellate cells by modulating the MAPK, Akt and NF-κB signaling pathways. Int. J. Mol. Med. 2017, 40, 226–234. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Chen, J.; Liao, H.; Li, C.; Chen, M. Anti-inflammatory effect of lipophilic grape seed proanthocyanidin in RAW 264.7 cells and a zebrafish model. J. Funct. Foods 2020, 75, 104217. [Google Scholar] [CrossRef]
- Di Martino, P.; Agniel, R.; David, K.; Templer, C.; Gaillard, J.L.; Denys, P.; Botto, H. Reduction of Escherichia coli adherence to uroepithelial bladder cells after consumption of cranberry juice: A double-blind randomized placebo-controlled cross-over trial. World J. Urol. 2006, 24, 21–27. [Google Scholar] [CrossRef]
- Asadi, A.; Razavi, S.; Talebi, M.; Gholami, M. A review on anti-adhesion therapies of bacterial diseases. Infection 2019, 47, 13–23. [Google Scholar] [CrossRef] [PubMed]
- González de Llano, D.; Liu, H.; Khoo, C.; Moreno-Arribas, M.V.; Bartolomé, B. Some New Findings Regarding the Antiadhesive Activity of Cranberry Phenolic Compounds and Their Microbial-Derived Metabolites against Uropathogenic Bacteria. J. Agric. Food Chem. 2019, 67, 2166–2174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, K.; Chou, M.Y.; Howell, A.; Wobbe, C.; Grady, R.; Stapleton, A.E. Cranberry Products Inhibit Adherence of P-Fimbriated Escherichia Coli to Primary Cultured Bladder and Vaginal Epithelial Cells. J. Urol. 2007, 177, 2357–2360. [Google Scholar] [CrossRef] [Green Version]
- Margetis, D.; Roux, D.; Gaudry, S.; Messika, J.; Bouvet, O.; Branger, C.; Ponnuswamy, P.; Oufella, H.A.; Dreyfuss, D.; Denamur, E.; et al. Effects of Proanthocyanidins on Adhesion, Growth, and Virulence of Highly Virulent Extraintestinal Pathogenic Escherichia coli Argue for Its Use to Treat Oropharyngeal Colonization and Prevent Ventilator-Associated Pneumonia. Crit. Care Med. 2015, 43, e170–e178. [Google Scholar] [CrossRef]
- Feldman, M.; Tanabe, S.; Howell, A.; Grenier, D. Cranberry proanthocyanidins inhibit the adherence properties of Candida albicans and cytokine secretion by oral epithelial cells. BMC Complement. Altern. Med. 2012, 12, 6. [Google Scholar] [CrossRef] [Green Version]
- Alshaibani, D.; Zhang, R.; Wu, V.C. Antibacterial characteristics and activity of Vaccinium macrocarpon proanthocyanidins against diarrheagenic Escherichia coli. J. Funct. Foods 2017, 39, 133–138. [Google Scholar] [CrossRef]
- Levy, J.; Boyer, R.R.; Neilson, A.P.; O’Keefe, S.F.; Chu, H.S.S.; Williams, R.C.; Dorenkott, M.R.; Goodrich, K.M. Evaluation of peanut skin and grape seed extracts to inhibit growth of foodborne pathogens. Food. Sci. Nutr. 2017, 5, 1130–1138. [Google Scholar] [CrossRef]
- Tang, C.; Xie, B.; Sun, Z. Antibacterial activity and mechanism of B-type oligomeric procyanidins from lotus seedpod on enterotoxigenic Escherichia coli. J. Funct. Foods 2017, 38, 454–463. [Google Scholar] [CrossRef]
- Fu, L.; Lu, W.Q.; Zhou, X.M. Phenolic Compounds and In Vitro Antibacterial and Antioxidant Activities of Three Tropic Fruits: Persimmon, Guava, and Sweetsop. BioMed Res. Int. 2016, 2016, 4287461. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.A.; Park, H.C.; Park, S.W.; Park, S.C.; Seo, M.G.; Her, M.; Kang, J.W. Synergism of the Combination of Traditional Antibiotics and Novel Phenolic Compounds against Escherichia coli. Pathogens 2020, 9, 811. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Loss, H.; Aschenbach, J.R.; Ebner, F.; Tedin, K.; Lodemann, U. Inflammatory Responses of Porcine MoDC and Intestinal Epithelial Cells in a Direct-Contact Co-culture System Following a Bacterial Challenge. Inflammation 2020, 43, 552–567. [Google Scholar] [CrossRef] [PubMed]
- Karancsi, Z.; Móritz, A.V.; Lewin, N.; Veres, A.M.; Jerzsele, Á.; Farkas, O. Beneficial Effect of a Fermented Wheat Germ Extract in Intestinal Epithelial Cells in case of Lipopolysaccharide-Evoked Inflammation. Oxidative Med. Cell. Longev. 2020, 2020, 1482482. [Google Scholar] [CrossRef] [PubMed]

| Escherichia coli | Salmonella Typhimurium | |||
|---|---|---|---|---|
| Treatment | Reduction | p Value | Reduction | p Value |
| GSOPs 50 pre | −62.35% | p < 0.001 | −51.14% | p < 0.05 |
| GSOPs 100 pre | −75.12% | p < 0.001 | −57.55% | p < 0.05 |
| GSOPs 50 parallel | −43.62% | p < 0.05 | −24.03% | p = 0.16 |
| GSOPs 100 parallel | −44.25% | p < 0.01 | −30.66% | p = 0.11 |
| GSOPs 50 post | −23.27% | p = 0.46 | −5.38% | p = 0.34 |
| GSOPs 100 post | −56.35% | p < 0.001 | −17.39% | p = 0.21 |
| GSOPs | Bacterium | |
|---|---|---|
| Control | − | − |
| E. coli | − | E. coli 106 CFU/mL |
| S. Typhimurium | − | S. Typhimurium 106 CFU/mL |
| Pre-treatment 50 E. coli | 50 μg/mL 1 h prior to infection | E. coli 106 CFU/mL |
| Pre-treatment 50 S. Typhimurium | 50 μg/mL 1 h prior to infection | S. Typhimurium 106 CFU/mL |
| Pre-treatment 100 E. coli | 100 μg/mL 1 h prior to infection | E. coli 106 CFU/mL |
| Pre-treatment 100 S. Typhimurium | 100 μg/mL 1 h prior to infection | S. Typhimurium 106 CFU/mL |
| Parallel treatment 50 E. coli | 50 μg/mL together with infection | E. coli 106 CFU/mL |
| Parallel treatment 50 S. Typhimurium | 50 μg/mL together with infection | S. Typhimurium 106 CFU/mL |
| Parallel treatment 100 E. coli | 100 μg/mL together with infection | E. coli 106 CFU/mL |
| Parallel treatment 100 S. Typhimurium | 100 μg/mL together with infection | S. Typhimurium 106 CFU/mL |
| Post-treatment 50 E. coli | 50 μg/mL 1 h after infection | E. coli 106 CFU/mL |
| Post-treatment 50 S. Typhimurium | 50 μg/mL 1 h after infection | S. Typhimurium 106 CFU/mL |
| Post-treatment 100 E. coli | 100 μg/mL 1 h after infection | E. coli 106 CFU/mL |
| Post-treatment 100 S. Typhimurium | 100 μg/mL 1 h after infection | S. Typhimurium 106 CFU/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, D.; Palkovicsné Pézsa, N.; Jerzsele, Á.; Süth, M.; Farkas, O. Protective Effects of Grape Seed Oligomeric Proanthocyanidins in IPEC-J2–Escherichia coli/Salmonella Typhimurium Co-Culture. Antibiotics 2022, 11, 110. https://doi.org/10.3390/antibiotics11010110
Kovács D, Palkovicsné Pézsa N, Jerzsele Á, Süth M, Farkas O. Protective Effects of Grape Seed Oligomeric Proanthocyanidins in IPEC-J2–Escherichia coli/Salmonella Typhimurium Co-Culture. Antibiotics. 2022; 11(1):110. https://doi.org/10.3390/antibiotics11010110
Chicago/Turabian StyleKovács, Dóra, Nikolett Palkovicsné Pézsa, Ákos Jerzsele, Miklós Süth, and Orsolya Farkas. 2022. "Protective Effects of Grape Seed Oligomeric Proanthocyanidins in IPEC-J2–Escherichia coli/Salmonella Typhimurium Co-Culture" Antibiotics 11, no. 1: 110. https://doi.org/10.3390/antibiotics11010110
APA StyleKovács, D., Palkovicsné Pézsa, N., Jerzsele, Á., Süth, M., & Farkas, O. (2022). Protective Effects of Grape Seed Oligomeric Proanthocyanidins in IPEC-J2–Escherichia coli/Salmonella Typhimurium Co-Culture. Antibiotics, 11(1), 110. https://doi.org/10.3390/antibiotics11010110







