Concomitant Treatment with Voriconazole and Flucloxacillin: A Combination to Avoid
Abstract
:1. Introduction
2. Results
2.1. Patients
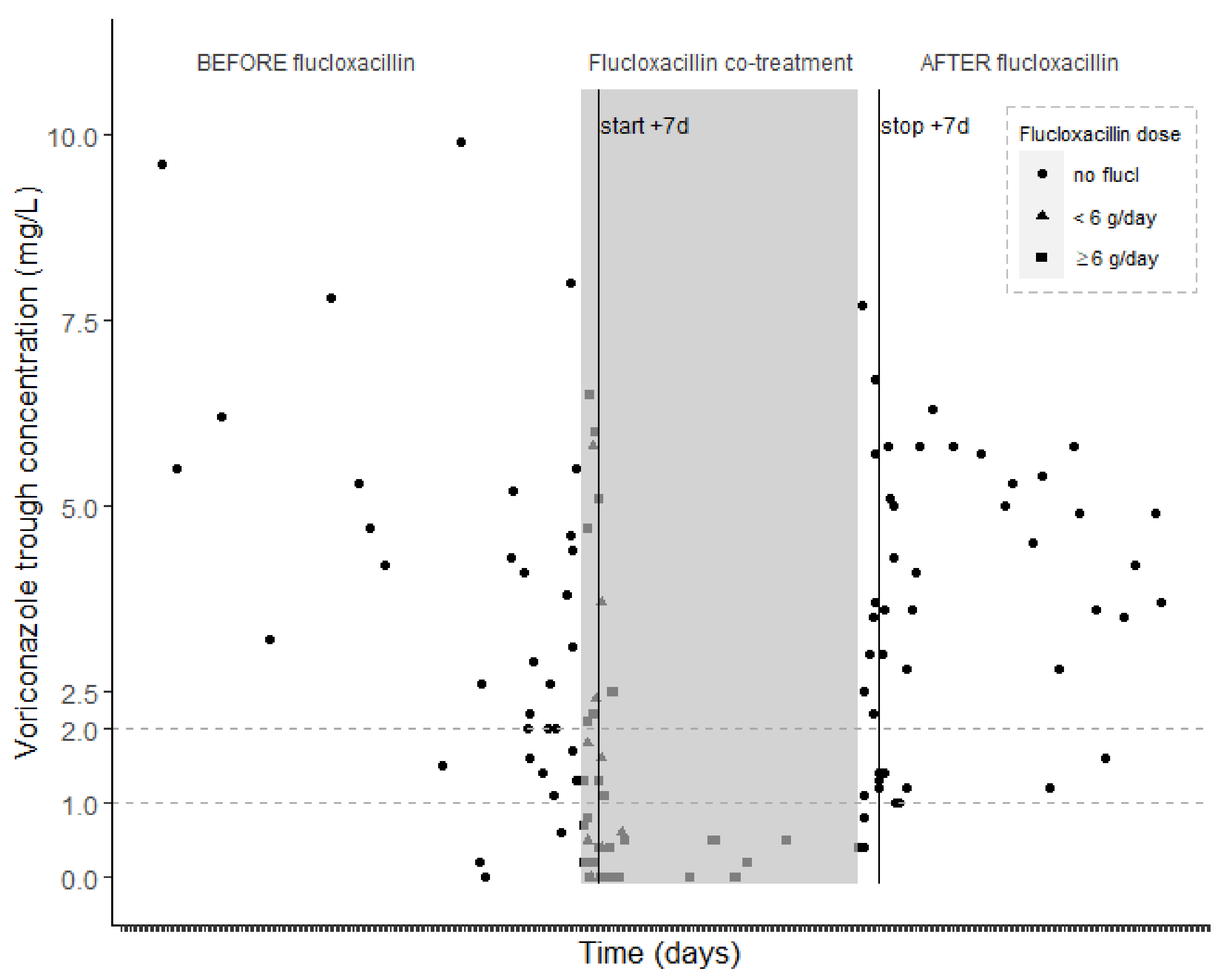
2.2. Voriconazole Concentrations
2.3. Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Study Design, Population and Setting
4.2. Data Collection
4.3. Voriconazole Measured Concentrations
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Florl, C.; Lewis, R.E.; Munoz, P.E.; Verweij, F.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24, e1–e38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellmann, R.; Smuszkiewicz, P. Pharmacokinetics of antifungal drugs: Practical implications for optimized treatment of patients. Infection 2017, 45, 737–779. [Google Scholar] [CrossRef] [PubMed]
- Yanni, S.B.; Annaert, P.P.; Augustijns, P.; Bridges, A.; Gao, Y.; Benjamin, D.K., Jr.; Thakker, D.R. Role of flavin-containing monooxygenase in oxidative metabolism of voriconazole by human liver microsomes. Drug Metab. Dispos. 2008, 36, 1119–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfizer. Summary of Product Characteristics (SmPC) Vfend; Pfizer: New York, NY, USA, 2021. [Google Scholar]
- Pascual, A.; Calandra, T.; Bolay, S.; Buclin, T.; Bille, J.; Marchetti, O. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Diss. 2008, 46, 201–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyakis, S.; van Hal, S.J.; Ray, J.; Marriott, D. Voriconazole concentrations and outcome of invasive fungal infections. Clin. Microbiol. Infect. 2010, 16, 927–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, R.; Brüggemann, R.; Padoin, C.; Maertens, J.; Marchetti, O.; Groll, A.; Johnson, E.; Arendrup, M. Triazole Antifungal Therapeutic Drug Monitoring; ECIL 6 Meeting. 2015. (France). Available online: https://www.ebmt.org/sites/default/files/migration_legacy_files/document/ECIL%206-Triazole-TDM-07-12-2015-Lewis-R-et-al.pdf (accessed on 10 September 2021).
- Ashbee, H.R.; Barnes, R.A.; Johnson, E.M.; Richardson, M.D.; Gorton, R.; Hope, W.W. Therapeutic drug monitoring (TDM) of antifungal agents: Guidelines from the British Society for Medical Mycology. J. Antimicrob. Chemother. 2014, 69, 1162–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patterson, T.F.; Thompson, G.R., III; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clini. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.; Kluwe, F.; Mikus, G.; Michelet, R.; Kloft, C. Novel insights into the complex pharmacokinetics of voriconazole: A review of its metabolism. Drug Metab. Rev. 2019, 51, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.; Larcombe, R.; Chaptini, C.; Gordon, D.L. Interaction between voriconazole and flucloxacillin during treatment of disseminated Scedosporium apiospermum infection. J. Antimicrob. Chemother. 2015, 70, 2171–2173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muilwijk, E.W.; Dekkers, B.G.J.; Henriet, S.S.V.; Verweij, P.E.; Witjes, B.; Lashof, A.; Groeneveld, G.H.; van der Hoeven, J.; Alffenaar, J.W.C.; Russel, F.G.M.; et al. Flucloxacillin Results in Suboptimal Plasma Voriconazole Concentrations. Antimicrob. Agents Chemother. 2017, 61, e00915-00917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huwyler, J.; Wright, M.B.; Gutmann, H.; Drewe, J. Induction of cytochrome P450 3A4 and P-glycoprotein by the isoxazolyl-penicillin antibiotic flucloxacillin. Curr. Drug Metab. 2006, 7, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Stage, T.B.; Graff, M.; Wong, S.; Rasmussen, L.L.; Nielsen, F.; Pottegård, A.; Brøsen, K.; Kroetz, D.L.; Khojasteh, S.C.; Damkier, P. Dicloxacillin induces CYP2C19, CYP2C9 and CYP3A4 in vivo and in vitro. Br. J. Clin. Pharmacol. 2018, 84, 510–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veenhof, H.; Schouw, H.M.; Besouw, M.T.P.; Touw, D.J.; Gracchi, V. Flucloxacillin decreases tacrolimus blood trough levels: A single-center retrospective cohort study. Eur. J. Clin. Pharmacol. 2020, 76, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.-Q.; Wang, Z.-J.; He, L.; Jiang, X.-H.; Wang, L. PXR polymorphisms and their impact on pharmacokinetics/pharmacodynamics of repaglinide in healthy Chinese volunteers. Eur. J. Clin. Pharmacol. 2013, 69, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Spriet, I.; Meersseman, W.; de Hoon, J.; von Winckelmann, S.; Wilmer, A.; Willems, L. Mini-series: II. clinical aspects. clinically relevant CYP450-mediated drug interactions in the ICU. Intensiv. Care Med. 2009, 35, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Hakkola, J.; Hukkanen, J.; Turpeinen, M.; Pelkonen, O. Inhibition and induction of CYP enzymes in humans: An update. Arch. Toxicol. 2020, 94, 3671–3722. [Google Scholar] [CrossRef] [PubMed]
- Van Daele, R.; Spriet, I.; Maertens, J. Posaconazole in prophylaxis and treatment of invasive fungal infections: A pharmacokinetic, pharmacodynamic and clinical evaluation. Expert Opin. Drug Metab. Toxicol. 2020, 16, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ramírez, J.; Gamazon, E.R.; Mirkov, S.; Chen, P.; Wu, K.; Sun, C.; Cox, N.J.; Cook, E., Jr.; Das, S.; et al. Genetic factors affecting gene transcription and catalytic activity of UDP-glucuronosyltransferases in human liver. Hum. Mol. Genet. 2014, 23, 5558–5569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Synold, T.W.; Dussault, I.; Forman, B.M. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat. Med. 2001, 7, 584–590. [Google Scholar] [CrossRef] [PubMed]
| Total | During Flucloxacillin Co-Treatment | Without Flucloxacillin Co-Treatment | p-Value | |
|---|---|---|---|---|
| Continuous voriconazole trough concentrations of SAMPLE SET A a | ||||
| Number of Cmin | 128 | 45 | 83 | NA |
| Trough concentration (mg/L), median (IQR) | 2.2 (0.8–4.5) | 0.5 (0–1.8) | 3.5 (1.7–5.1) | 0.002 |
| Previous daily dose (mg/kg), median (IQR) | 7.4 (5.3–10.2) | 7.9 (6.8–11.0) | 7.3 (5.3–9.6) | 0.54 |
| Mode of administration, IV, n (%) | 40 (31) | 20 (44) | 20 (24) | 0.19 |
| Day of flucloxacillin therapy, median (IQR) | NA | 8 (4–13) | NA | NA |
| Categorical voriconazole trough concentrations of SAMPLE SET B b | ||||
| Number of Cmin | 145 | 51 | 94 | NA |
| Subtherapeutic Cmin (<1 mg/L), n (%) | 42 (29) | 35 (69) | 7 (7) | <0.0001 |
| Subtherapeutic Cmin (<2 mg/L), n (%) | 65 (45) | 40 (78) | 25 (27) | 0.0001 |
| Previous daily dose (mg/kg), median (IQR) | 7.3 (5.3–9.6) | 7.9 (6.8–10.7) | 7.3 (5.3–8.5) | 0.79 |
| Mode of administration, IV, n (%) | 49 (34) | 22 (43) | 27 (29) | 0.01 |
| Number of patients with at least one subtherapeutic (<1 mg/L) Cmin, ratio * | 24/33 | 24/31 | 5/22 | NA |
| Number of patients with at least one subtherapeutic (<2 mg/L) Cmin, ratio * | 27/33 | 26/31 | 15/22 | NA |
| Day of flucloxacillin therapy, median (IQR) | NA | 8 (5–12) | NA | NA |
| Therapeutic Cmin under Flucloxacillin Therapy | Cmin > 1 mg/L | Cmin > 2 mg/L |
|---|---|---|
| Number of therapeutic Cmin under flucloxacillin, n (%) | 16 (31) | 11 (22) |
| Trough concentration (mg/L), median (IQR) | 2.3 (1.8-4.8) | 3.7 (2.3–5.5) |
| Previous daily dose (mg/kg), median (IQR) | 8.0 (7.2–11.4) | 7.5 (7.0–9.7) |
| Day of flucloxacillin therapy, median (IQR) | 6 (4–8) | 6 (4–7) |
| Dose was previously increased under flucloxacillin association, (yes), n (%) | 2 (13) | 0 (0) |
| Number of patients with only therapeutic Cmin under flucloxacillin, ratio * | 7/31 | 5/31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Daele, R.; Wauters, J.; De Cock, P.; Buyle, F.; Leys, J.; Van Brantegem, P.; Gijsen, M.; Annaert, P.; Debaveye, Y.; Lagrou, K.; et al. Concomitant Treatment with Voriconazole and Flucloxacillin: A Combination to Avoid. Antibiotics 2021, 10, 1112. https://doi.org/10.3390/antibiotics10091112
Van Daele R, Wauters J, De Cock P, Buyle F, Leys J, Van Brantegem P, Gijsen M, Annaert P, Debaveye Y, Lagrou K, et al. Concomitant Treatment with Voriconazole and Flucloxacillin: A Combination to Avoid. Antibiotics. 2021; 10(9):1112. https://doi.org/10.3390/antibiotics10091112
Chicago/Turabian StyleVan Daele, Ruth, Joost Wauters, Pieter De Cock, Franky Buyle, John Leys, Pieter Van Brantegem, Matthias Gijsen, Pieter Annaert, Yves Debaveye, Katrien Lagrou, and et al. 2021. "Concomitant Treatment with Voriconazole and Flucloxacillin: A Combination to Avoid" Antibiotics 10, no. 9: 1112. https://doi.org/10.3390/antibiotics10091112
APA StyleVan Daele, R., Wauters, J., De Cock, P., Buyle, F., Leys, J., Van Brantegem, P., Gijsen, M., Annaert, P., Debaveye, Y., Lagrou, K., Peetermans, W. E., Brüggemann, R. J., & Spriet, I. (2021). Concomitant Treatment with Voriconazole and Flucloxacillin: A Combination to Avoid. Antibiotics, 10(9), 1112. https://doi.org/10.3390/antibiotics10091112







