Antibiotics in Necrotizing Soft Tissue Infections
Abstract
1. Introduction
2. Microbiology of NSTIs
3. Microbial Documentation of NSTIs: A Challenge for Microbiologists
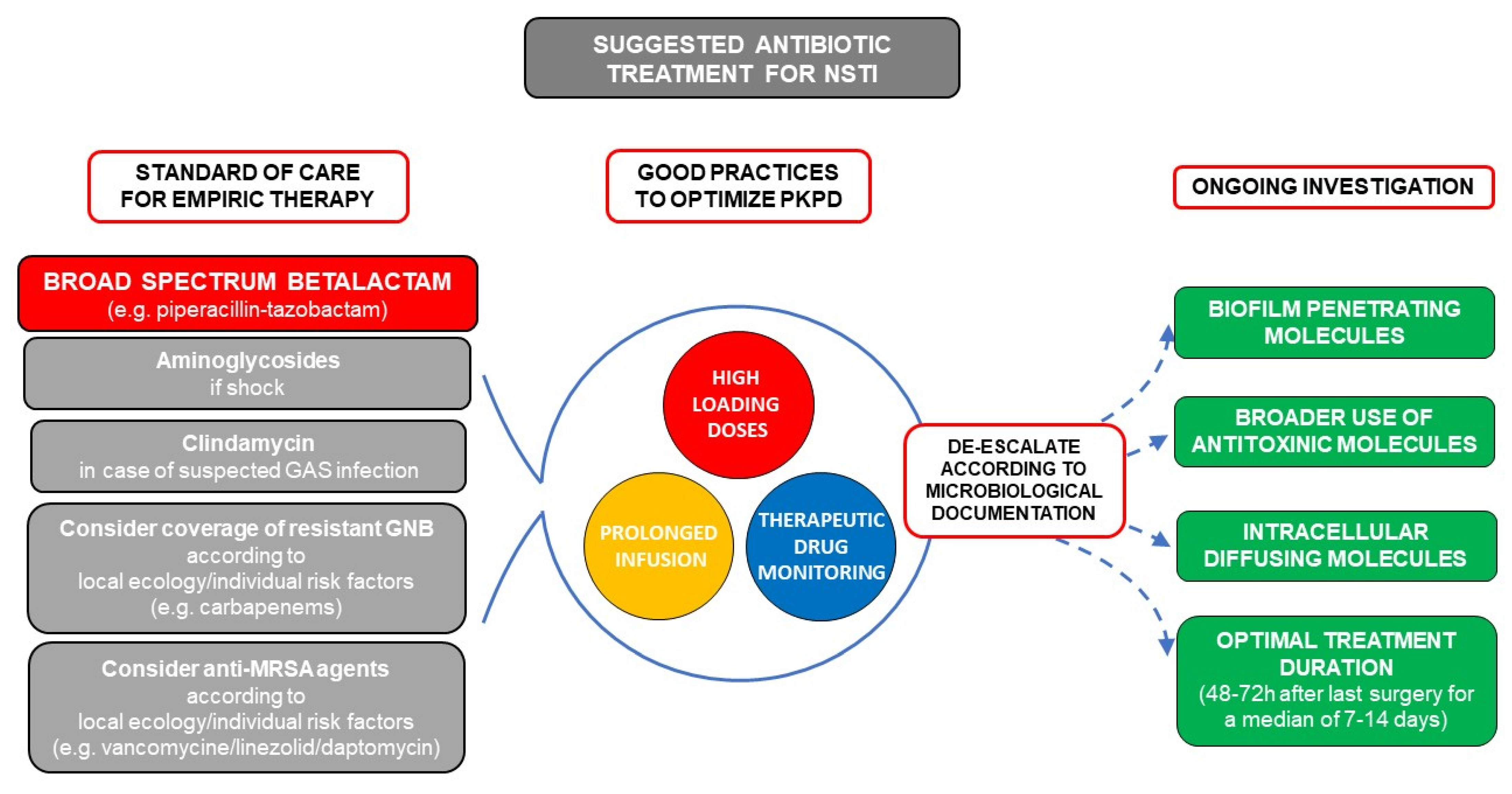
4. Antibiotic Treatment
4.1. Available Data Regarding Choice of Molecules Specifically in NSTIs
4.2. Specificities for GAS Infections: Anti-Toxinic Molecules
4.3. Perspectives on Other NSTI Specificities
4.4. Duration of Treatment and De-Escalation
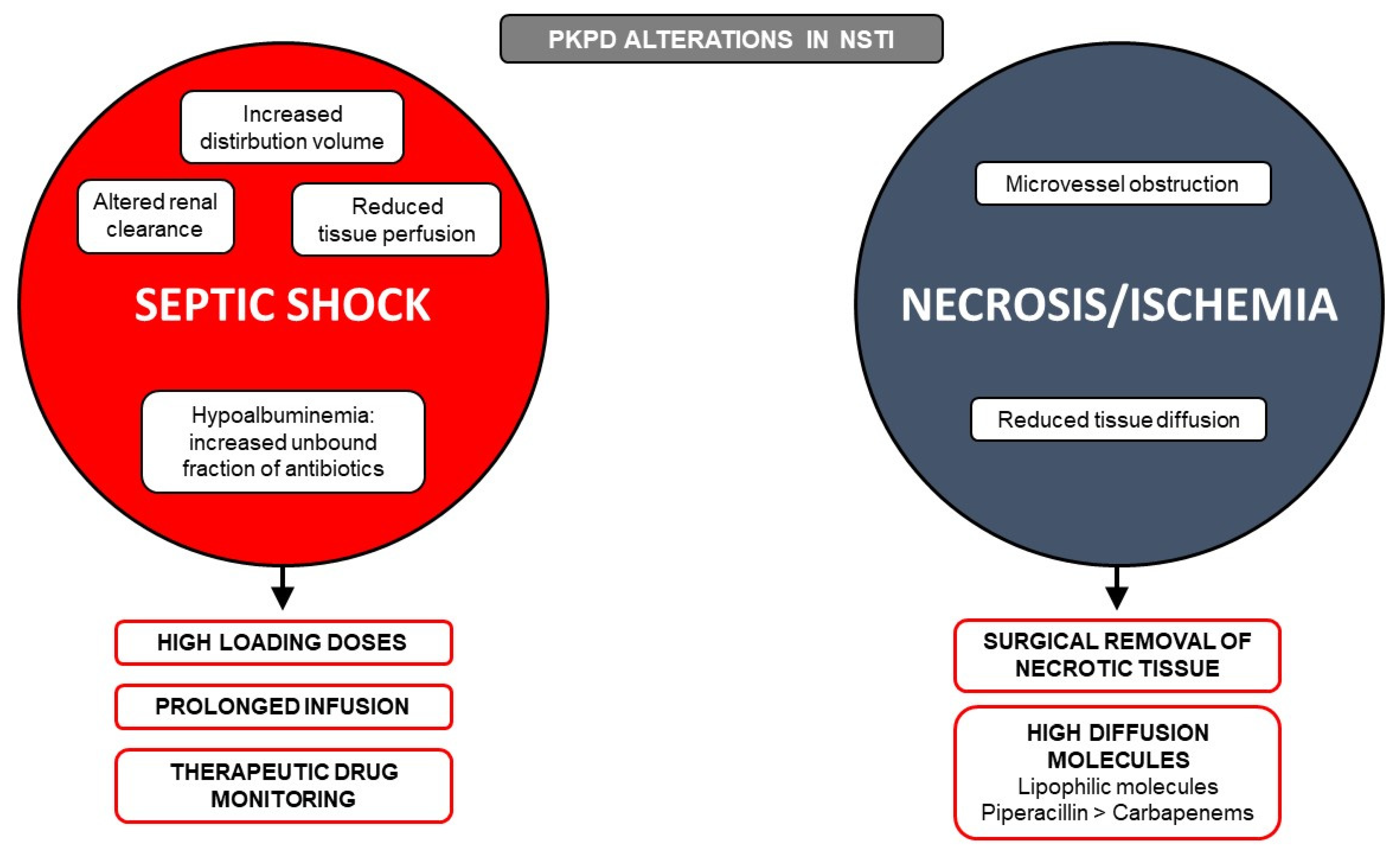
5. Pharmacokinetics (PK) and Pharmacodynamics (PD) Targets and Optimization
6. Tissue Diffusion of Antibiotics
6.1. Available Data from Uncomplicated SSTI
| Molecule | Guidelines | Pharmacokinetic Parameters | Tissue Penetration (Tissue/Blood Ratio) | Antimicrobial Spectrum/Anti-Toxinic Activity and other Specific Aspects | Dosing Regimen § | ||||
|---|---|---|---|---|---|---|---|---|---|
| Distribution (Vd) | Protein Binding | PK/PD Targets in Severe Infections | Soft Tissue of Healthy Subjects | Impact of Necrosis on Tissue Diffusion | Impact of Altered Tissue Perfusion on Tissue Diffusion [84,94,95,96,97,98] | ||||
| Piperacillin + tazobactam | USA 2014 (IDSA) World 2018 (WSES/SIS-E) Germany 2018 South Korea 2017 | Hydrophilic (0.24 L/kg) | Low (16%) | fT > 4−8 × MIC = 100% | Low (0.27) [81] * | Medium to low impact (tissue concentrations appear sufficient to achieve PK/PD targets) | No data | Methicillin-susceptible S. aureus, S. pyogenes, Enterobacteriaceae, nonfermenting bacilli, anaerobic bacteria | 4 g q6h IV Consider prolonged (4 h) or continuous infusion with loading dose |
| Cefotaxime | USA 2014 Norway 2013 South Korea 2017 | Hydrophilic (0.28 L/kg) | Low (30–51%) | fT > 4−8 × MIC = 100% | Medium (0.54) [99] π | Medium to low impact (tissue concentrations appear sufficient to achieve PK/PD targets) | No data (but high decrease of tissue concentration with others cephalosporins: cefepime [100] and ceftazidime [101]) | Methicillin-susceptible S. aureus, S. pyogenes, Enterobacteriaceae | 2 g q6–8h IV |
| Meropenem | USA 2014 World 2018 Germany 2018 South Korea 2017 | Hydrophilic (0.25 L/kg) | Very low (2%) | fT > 4−8 × MIC = 100% | Low (0.35–0.48) [87] * | Medium to high decrease in drug concentration (tissue concentrations could not be sufficient to achieve PK/PD targets) | Low impact | Methicillin-susceptible S. aureus, S. pyogenes, Enterobacteriaceae, nonfermenting bacilli anaerobic bacteria activity on multi-drug resistant gram-negative bacilli | 1–2 g q8h IV Consider prolonged infusion 3 h |
| Gentamycin | Norway 2013 | Hydrophilic (0.26 L/kg) | Very low (0–3%) | Cmax/MIC > 8–10 | Medium (0.60) [81] * | High decrease in drug concentration | No data | S. aureus, S. pyogenes, Enterobacteriaceae, nonfermenting bacilli Rapid bactericidal action Should be added in cases of septic shock | 5–8 mg/kg over 30 min, q24h |
| Amikacin | France 2018 | Hydrophilic (0.26 L/kg) | Very low (< 10%) | Cmax/MIC > 8–10 | High (1.03) [86] π | High decrease in drug concentration | No data | S. aureus, S. pyogenes, Enterobacteriaceae, nonfermenting bacilli Rapid bactericidal action Should be added in cases of septic shock | 25–30 m/kg over 30 min, q24h |
| Metronidazole | USA 2014 Norway 2013 South Korea 2017 | Lipophilic (0.65 L/kg) | Very low (< 10%) | AUC24/MIC Cmax/MIC No target defined | High (0.67) [85] * | No or low impact | No impact [102] | Anaerobic bacteria | 500 mg q8h IV |
| Vancomycin | USA 2014 South Korea 2017 | Hydrophilic (0.70 L/kg) | Medium (55%) | AUC24/MIC > 400–600 | Low (0.30) [81] * | Medium to high decrease in drug concentration | High impact [103] | Methicillin-resistant S. aureus | Consider continuous infusion of 30 mg/kg/24 h with loading dose of 30 mg/kg and TDM |
| Daptomycin | World 2018 | Hydrophilic (0.10 L/kg) | High (92%) | AUC24/MIC > 666 [104] | High (0.74–0,93) [81] * | No data | No impact | Methicillin-resistant S. aureus | 8–12 mg/kg q24h |
| Linezolid | USA 2014 World 2018 South Korea 2017 | Lipophilic (0.65 L/kg) | Low (31%) | AUC24/MIC > 80–120 fT > 1 × MIC = 85% [105] | High (0.75–1.32) [81] * | No data | No impact | Methicillin-resistant S. aureus In vitro evidence of anti-toxinic action | 600 mg q12h IV (higher doses might be needed in obese patients [80]) |
| Clindamycin | World 2018 Germany 2018 | Lipophilic (1.1 L/kg) | High (90%) | AUC24/MIC No target defined | High (1.06) [106] x | No data | No impact | S. aureus, S. pyogenes Anaerobic bacteria (but with high proportion of resistant strains), High evidence of in vivo and in vitro anti-toxinic action | 600–900 mg q8h IV |
6.2. Available Data on Antibiotic Diffusion in Necrotic Tissue
6.3. Available Data on Antibiotic Diffusion in the Presence of Altered Perfusion
7. Suggested Empiric Treatment for Suspected NSTI Based on Basic Microbiology
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stevens, D.L.; Bryant, A.E. Necrotizing Soft-Tissue Infections. N. Engl. J. Med. 2017, 377, 2253–2265. [Google Scholar] [CrossRef]
- Peetermans, M.; de Prost, N.; Eckmann, C.; Norrby-Teglund, A.; Skrede, S.; De Waele, J.J. Necrotizing Skin and Soft-Tissue Infections in the Intensive Care Unit. Clin. Microbiol. Infect. 2019, 26, 8–17. [Google Scholar] [CrossRef]
- Urbina, T.; Madsen, M.B.; de Prost, N. Understanding Necrotizing Soft Tissue Infections in the Intensive Care Unit. Intensive Care Med. 2020, 46, 1739–1742. [Google Scholar] [CrossRef] [PubMed]
- Madsen, M.B.; Skrede, S.; Perner, A.; Arnell, P.; Nekludov, M.; Bruun, T.; Karlsson, Y.; Hansen, M.B.; Polzik, P.; Hedetoft, M.; et al. Patient’s Characteristics and Outcomes in Necrotising Soft-Tissue Infections: Results from a Scandinavian, Multicentre, Prospective Cohort Study. Intensive Care Med. 2019, 45, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Urbina, T.; Hua, C.; Sbidian, E.; Bosc, R.; Tomberli, F.; Lepeule, R.; Decousser, J.-W.; Mekontso Dessap, A.; Chosidow, O.; de Prost, N.; et al. Impact of a Multidisciplinary Care Bundle for Necrotizing Skin and Soft Tissue Infections: A Retrospective Cohort Study. Ann. Intensive Care 2019, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Urbina, T.; Canoui-Poitrine, F.; Hua, C.; Layese, R.; Alves, A.; Ouedraogo, R.; Bosc, R.; Sbidian, E.; Chosidow, O.; Dessap, A.M.; et al. Long-Term Quality of Life in Necrotizing Soft-Tissue Infection Survivors: A Monocentric Prospective Cohort Study. Ann. Intensive Care 2021, 11, 102. [Google Scholar] [CrossRef]
- Hua, C.; Sbidian, E.; Hemery, F.; Decousser, J.W.; Bosc, R.; Amathieu, R.; Rahmouni, A.; Wolkenstein, P.; Valeyrie-Allanore, L.; Brun-Buisson, C.; et al. Prognostic Factors in Necrotizing Soft-Tissue Infections (NSTI): A Cohort Study. J. Am. Acad. Dermatol. 2015, 73, 1006–1012.e8. [Google Scholar] [CrossRef]
- Nawijn, F.; Smeeing, D.P.J.; Houwert, R.M.; Leenen, L.P.H.; Hietbrink, F. Time Is of the Essence When Treating Necrotizing Soft Tissue Infections: A Systematic Review and Meta-Analysis. World J. Emerg. Surg. 2020, 15, 4. [Google Scholar] [CrossRef]
- Audureau, E.; Hua, C.; de Prost, N.; Hemery, F.; Decousser, J.W.; Bosc, R.; Lepeule, R.; Chosidow, O.; Sbidian, E. Henri Mondor Hospital Necrotizing Fasciitis group Mortality of Necrotizing Fasciitis: Relative Influence of Individual and Hospital-Level Factors, a Nationwide Multilevel Study, France, 2007–2012. Br. J. Dermatol. 2017, 177, 1575–1582. [Google Scholar] [CrossRef]
- Hua, C.; Bosc, R.; Sbidian, E.; De Prost, N.; Hughes, C.; Jabre, P.; Chosidow, O.; Le Cleach, L. Interventions for Necrotizing Soft Tissue Infections in Adults. Cochrane Database Syst. Rev. 2018, 5, CD011680. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.C.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C.; et al. Practice Guidelines for the Diagnosis and Management of Skin and Soft Tissue Infections: 2014 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, e10–e52. [Google Scholar] [CrossRef]
- Sartelli, M.; Guirao, X.; Hardcastle, T.C.; Kluger, Y.; Boermeester, M.A.; Raşa, K.; Ansaloni, L.; Coccolini, F.; Montravers, P.; Abu-Zidan, F.M.; et al. 2018 WSES/SIS-E Consensus Conference: Recommendations for the Management of Skin and Soft-Tissue Infections. World J. Emerg. Surg. 2018, 13, 58. [Google Scholar] [CrossRef]
- Kwak, Y.G.; Choi, S.-H.; Kim, T.; Park, S.Y.; Seo, S.-H.; Kim, M.B.; Choi, S.-H. Clinical Guidelines for the Antibiotic Treatment for Community-Acquired Skin and Soft Tissue Infection. Infect. Chemother. 2017, 49, 301. [Google Scholar] [CrossRef]
- Sunderkötter, C.; Becker, K.; Eckmann, C.; Graninger, W.; Kujath, P.; Schöfer, H. S2k Guidelines for Skin and Soft Tissue Infections Excerpts from the S2k Guidelines for “Calculated Initial Parenteral Treatment of Bacterial Infections in Adults—Update 2018”. J. Dtsch. Dermatol. Ges. 2019, 17, 345–369. [Google Scholar] [CrossRef]
- Kao, L.S.; Lew, D.F.; Arab, S.N.; Todd, S.R.; Awad, S.S.; Carrick, M.M.; Corneille, M.G.; Lally, K.P. Local Variations in the Epidemiology, Microbiology, and Outcome of Necrotizing Soft-Tissue Infections: A Multicenter Study. Am. J. Surg. 2011, 202, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Chia, L.; Crum-Cianflone, N.F. Emergence of Multi-Drug Resistant Organisms (MDROs) Causing Fournier’s Gangrene. J. Infect. 2018, 76, 38–43. [Google Scholar] [CrossRef]
- Gunaratne, D.A.; Tseros, E.A.; Hasan, Z.; Kudpaje, A.S.; Suruliraj, A.; Smith, M.C.; Riffat, F.; Palme, C.E. Cervical Necrotizing Fasciitis: Systematic Review and Analysis of 1235 Reported Cases from the Literature. Head Neck 2018, 40, 2094–2102. [Google Scholar] [CrossRef]
- Huang, T.-Y.; Peng, K.-T.; Hsiao, C.-T.; Fann, W.-C.; Tsai, Y.-H.; Li, Y.-Y.; Hung, C.-H.; Chuang, F.-Y.; Hsu, W.-H. Predictors for Gram-Negative Monomicrobial Necrotizing Fasciitis in Southern Taiwan. BMC Infect. Dis. 2020, 20, 60. [Google Scholar] [CrossRef]
- INFECT study group; Thänert, R.; Itzek, A.; Hoßmann, J.; Hamisch, D.; Madsen, M.B.; Hyldegaard, O.; Skrede, S.; Bruun, T.; Norrby-Teglund, A.; et al. Molecular Profiling of Tissue Biopsies Reveals Unique Signatures Associated with Streptococcal Necrotizing Soft Tissue Infections. Nat. Commun. 2019, 10, 3846. [Google Scholar] [CrossRef]
- Das, D.K.; Baker, M.G.; Venugopal, K. Risk Factors, Microbiological Findings and Outcomes of Necrotizing Fasciitis in New Zealand: A Retrospective Chart Review. BMC Infect. Dis. 2012, 12, 348. [Google Scholar] [CrossRef]
- Bodansky, D.M.S.; Begaj, I.; Evison, F.; Webber, M.; Woodman, C.B.; Tucker, O.N. A 16-Year Longitudinal Cohort Study of Incidence and Bacteriology of Necrotising Fasciitis in England. World J. Surg. 2020, 44, 2580–2591. [Google Scholar] [CrossRef]
- Miller, L.G.; Perdreau-Remington, F.; Rieg, G.; Mehdi, S.; Perlroth, J.; Bayer, A.S.; Tang, A.W.; Phung, T.O.; Spellberg, B. Necrotizing Fasciitis Caused by Community-Associated Methicillin-Resistant Staphylococcus Aureus in Los Angeles. N. Engl. J. Med. 2005, 352, 1445–1453. [Google Scholar] [CrossRef]
- Lee, T.C.; Carrick, M.M.; Scott, B.G.; Hodges, J.C.; Pham, H.Q. Incidence and Clinical Characteristics of Methicillin-Resistant Staphylococcus Aureus Necrotizing Fasciitis in a Large Urban Hospital. Am. J. Surg. 2007, 194, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Thy, M.; Tanaka, S.; Tran-Dinh, A.; Ribeiro, L.; Lortat-Jacob, B.; Donadio, J.; Zappella, N.; Ben-Rehouma, M.; Tashk, P.; Snauwaert, A.; et al. Dynamic Changes in Microbial Composition During Necrotizing Soft-Tissue Infections in ICU Patients. Front. Med. 2021, 7, 609497. [Google Scholar] [CrossRef] [PubMed]
- Bruun, T.; Rath, E.; Madsen, M.B.; Oppegaard, O.; Nekludov, M.; Arnell, P.; Karlsson, Y.; Babbar, A.; Bergey, F.; Itzek, A.; et al. Risk Factors and Predictors of Mortality in Streptococcal Necrotizing Soft-Tissue Infections: A Multicenter Prospective Study. Clin. Infect. Dis. 2020, 72, 293–300. [Google Scholar] [CrossRef]
- Bernigaud, C.; Chosidow, O. Are Swabs an Appropriate Way to Sample for Skin Microbiome Research? Br. J. Dermatol. 2019, 181, 444–445. [Google Scholar] [CrossRef]
- Groupe Francophone de Réanimation et Urgences Pédiatriques (GFRUP); Réseau Mères-Enfants de la Francophonie (RMEF); Dauger, S.; Blondé, R.; Brissaud, O.; Marcoux, M.-O.; Angoulvant, F.; Levy, M. Necrotizing Soft-Tissue Infections in Pediatric Intensive Care: A Prospective Multicenter Case-Series Study. Crit. Care 2021, 25, 139. [Google Scholar] [CrossRef]
- Kha, P.; Colot, J.; Gervolino, S.; Guerrier, G. Necrotizing Soft-Tissue Infections in New Caledonia: Epidemiology, Clinical Presentation, Microbiology, and Prognostic Factors. Asian J. Surg. 2017, 40, 290–294. [Google Scholar] [CrossRef][Green Version]
- Cornaglia, G.; Courcol, R.; Herrmann, J.-L.; Kahlmeter, G.; Peigue-Lafeuille, H.; Jordi, V. European Manual of Clinical Microbiology; European Society for Clinical Microbiology and Infections Diseases: Basel, Switzerland, 2012; ISBN 978-2-87805-026-4. [Google Scholar]
- Norrby-Teglund, A.; Svensson, M.; Skrede, S. (Eds.) Necrotizing Soft Tissue Infections: Clinical and Pathogenic Aspects; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2020; Volume 1294, ISBN 978-3-030-57615-8. [Google Scholar]
- Wilson, M.R.; Sample, H.A.; Zorn, K.C.; Arevalo, S.; Yu, G.; Neuhaus, J.; Federman, S.; Stryke, D.; Briggs, B.; Langelier, C.; et al. Clinical Metagenomic Sequencing for Diagnosis of Meningitis and Encephalitis. N. Engl. J. Med. 2019, 380, 2327–2340. [Google Scholar] [CrossRef]
- Thoendel, M.J.; Jeraldo, P.R.; Greenwood-Quaintance, K.E.; Yao, J.Z.; Chia, N.; Hanssen, A.D.; Abdel, M.P.; Patel, R. Identification of Prosthetic Joint Infection Pathogens Using a Shotgun Metagenomics Approach. Clin. Infect. Dis. 2018, 67, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Langelier, C.; Kalantar, K.L.; Moazed, F.; Wilson, M.R.; Crawford, E.D.; Deiss, T.; Belzer, A.; Bolourchi, S.; Caldera, S.; Fung, M.; et al. Integrating Host Response and Unbiased Microbe Detection for Lower Respiratory Tract Infection Diagnosis in Critically Ill Adults. Proc. Natl. Acad. Sci. USA 2018, 115, E12353–E12362. [Google Scholar] [CrossRef]
- Rodriguez, C.; Jary, A.; Hua, C.; Woerther, P.-L.; Bosc, R.; Desroches, M.; Sitterlé, E.; Gricourt, G.; De Prost, N.; Pawlotsky, J.-M.; et al. Pathogen Identification by Shotgun Metagenomics of Patients with Necrotizing Soft-Tissue Infections. Br. J. Dermatol. 2019, 62. [Google Scholar] [CrossRef] [PubMed]
- Charalampous, T.; Kay, G.L.; Richardson, H.; Aydin, A.; Baldan, R.; Jeanes, C.; Rae, D.; Grundy, S.; Turner, D.J.; Wain, J.; et al. Nanopore Metagenomics Enables Rapid Clinical Diagnosis of Bacterial Lower Respiratory Infection. Nat. Biotechnol. 2019, 37, 783–792. [Google Scholar] [CrossRef]
- Brindle, R.; Williams, O.M.; Barton, E.; Featherstone, P. Assessment of Antibiotic Treatment of Cellulitis and Erysipelas: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2019, 155, 1033. [Google Scholar] [CrossRef]
- The STIC Study Group; Vick-Fragoso, R.; Hernández-Oliva, G.; Cruz-Alcázar, J.; Amábile-Cuevas, C.F.; Arvis, P.; Reimnitz, P.; Bogner, J.R. Efficacy and Safety of Sequential Intravenous/Oral Moxifloxacin vs Intravenous/Oral Amoxicillin/Clavulanate for Complicated Skin and Skin Structure Infections. Infection 2009, 37, 407–417. [Google Scholar] [CrossRef]
- Eckmann, C.; Heizmann, W.; Bodmann, K.-F.; von Eiff, C.; Petrik, C.; Loeschmann, P.-A. Tigecycline in the Treatment of Patients with Necrotizing Skin and Soft Tissue Infections Due to Multiresistant Bacteria. Surg. Infect. 2015, 16, 618–625. [Google Scholar] [CrossRef]
- Stevens, D.L.; Gibbons, A.E.; Bergstrom, R.; Winn, V. The Eagle Effect Revisited: Efficacy of Clindamycin, Erythromycin, and Penicillin in the Treatment of Streptococcal Myositis. J. Infect. Dis. 1988, 158, 23–28. [Google Scholar] [CrossRef]
- Mascini, E.M.; Jansze, M.; Schouls, L.M.; Verhoef, J.; Van Dijk, H. Penicillin and Clindamycin Differentially Inhibit the Production of Pyrogenic Exotoxins A and B by Group A Streptococci. Int. J. Antimicrob. Agents 2001, 18, 395–398. [Google Scholar] [CrossRef]
- Sriskandan, S.; McKee, A.; Hall, L.; Cohen, J. Comparative Effects of Clindamycin and Ampicillin on Superantigenic Activity of Streptococcus Pyogenes. J. Antimicrob. Chemother. 1997, 40, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Sawai, J.; Hasegawa, T.; Kamimura, T.; Okamoto, A.; Ohmori, D.; Nosaka, N.; Yamada, K.; Torii, K.; Ohta, M. Growth Phase-Dependent Effect of Clindamycin on Production of Exoproteins by Streptococcus Pyogenes. Antimicrob. Agents Chemother. 2007, 51, 461–467. [Google Scholar] [CrossRef]
- Andreoni, F.; Zürcher, C.; Tarnutzer, A.; Schilcher, K.; Neff, A.; Keller, N.; Marques Maggio, E.; Poyart, C.; Schuepbach, R.A.; Zinkernagel, A.S. Clindamycin Affects Group A Streptococcus Virulence Factors and Improves Clinical Outcome. J. Infect. Dis. 2017, 215, 269–277. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eagle, H. Experimental Approach to the Problem of Treatment Failure with Penicillin. I. Group A Streptococcal Infection in Mice. Am. J. Med. 1952, 13, 389–399. [Google Scholar] [CrossRef]
- Villalón, P.; Sáez-Nieto, J.A.; Rubio-López, V.; Medina-Pascual, M.J.; Garrido, N.; Carrasco, G.; Pino-Rosa, S.; Valdezate, S. Invasive Streptococcus Pyogenes Disease in Spain: A Microbiological and Epidemiological Study Covering the Period 2007-2019. Eur. J. Clin. Microbiol. Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Fay, K.; Onukwube, J.; Chochua, S.; Schaffner, W.; Cieslak, P.; Lynfield, R.; Muse, A.; Smelser, C.; Harrison, L.H.; Farley, M.; et al. Patterns of Antibiotic Nonsusceptibility among Invasive Group A Streptococcus Infections—United States, 2006–2017. Clin. Infect. Dis. 2021, ciab575. [Google Scholar] [CrossRef] [PubMed]
- Carapetis, J.R.; Jacoby, P.; Carville, K.; Ang, S.-J.J.; Curtis, N.; Andrews, R. Effectiveness of Clindamycin and Intravenous Immunoglobulin, and Risk of Disease in Contacts, in Invasive Group a Streptococcal Infections. Clin. Infect. Dis. 2014, 59, 358–365. [Google Scholar] [CrossRef]
- Babiker, A.; Li, X.; Lai, Y.L.; Strich, J.R.; Warner, S.; Sarzynski, S.; Dekker, J.P.; Danner, R.L.; Kadri, S.S. Effectiveness of Adjunctive Clindamycin in β-Lactam Antibiotic-Treated Patients with Invasive β-Haemolytic Streptococcal Infections in US Hospitals: A Retrospective Multicentre Cohort Study. Lancet Infect. Dis. 2021, 21, 697–710. [Google Scholar] [CrossRef]
- Coyle, E.A.; Cha, R.; Rybak, M.J. Influences of Linezolid, Penicillin, and Clindamycin, Alone and in Combination, on Streptococcal Pyrogenic Exotoxin a Release. Antimicrob. Agents Chemother. 2003, 47, 1752–1755. [Google Scholar] [CrossRef]
- Siemens, N.; Chakrakodi, B.; Shambat, S.M.; Morgan, M.; Bergsten, H.; Hyldegaard, O.; Skrede, S.; Arnell, P.; Madsen, M.B.; Johansson, L.; et al. Biofilm in Group A Streptococcal Necrotizing Soft Tissue Infections. JCI Insight 2016, 1, e87882. [Google Scholar] [CrossRef]
- Hertzén, E.; Johansson, L.; Kansal, R.; Hecht, A.; Dahesh, S.; Janos, M.; Nizet, V.; Kotb, M.; Norrby-Teglund, A. Intracellular Streptococcus Pyogenes in Human Macrophages Display an Altered Gene Expression Profile. PLoS ONE 2012, 7, e35218. [Google Scholar] [CrossRef] [PubMed]
- Faraklas, I.; Yang, D.; Eggerstedt, M.; Zhai, Y.; Liebel, P.; Graves, G.; Dissanaike, S.; Mosier, M.; Cochran, A. A Multi-Center Review of Care Patterns and Outcomes in Necrotizing Soft Tissue Infections. Surg. Infect. 2016, 17, 773–778. [Google Scholar] [CrossRef]
- on behalf of the REACH study group; Garau, J.; Blasi, F.; Medina, J.; McBride, K.; Ostermann, H. Early Response to Antibiotic Treatment in European Patients Hospitalized with Complicated Skin and Soft Tissue Infections: Analysis of the REACH Study. BMC Infect. Dis. 2015, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A. Pharmacokinetic/Pharmacodynamic Parameters: Rationale for Antibacterial Dosing of Mice and Men. Clin. Infect. Dis. 1998, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Pereira, J.; Póvoa, P. Antibiotics in Critically Ill Patients: A Systematic Review of the Pharmacokinetics of β-Lactams. Crit. Care 2011, 15, R206. [Google Scholar] [CrossRef] [PubMed]
- Ulldemolins, M.; Roberts, J.A.; Wallis, S.C.; Rello, J.; Lipman, J. Flucloxacillin Dosing in Critically Ill Patients with Hypoalbuminaemia: Special Emphasis on Unbound Pharmacokinetics. J. Antimicrob. Chemother. 2010, 65, 1771–1778. [Google Scholar] [CrossRef]
- Joynt, G.M.; Lipman, J.; Gomersall, C.D.; Young, R.J.; Wong, E.L.; Gin, T. The Pharmacokinetics of Once-Daily Dosing of Ceftriaxone in Critically Ill Patients. J. Antimicrob. Chemother. 2001, 47, 421–429. [Google Scholar] [CrossRef]
- Taccone, F.S.; Laterre, P.-F.; Dugernier, T.; Spapen, H.; Delattre, I.; Wittebole, X.; De Backer, D.; Layeux, B.; Wallemacq, P.; Vincent, J.-L.; et al. Insufficient β-Lactam Concentrations in the Early Phase of Severe Sepsis and Septic Shock. Crit. Care 2010, 14, R126. [Google Scholar] [CrossRef] [PubMed]
- Delattre, I.K.; Taccone, F.S.; Jacobs, F.; Hites, M.; Dugernier, T.; Spapen, H.; Laterre, P.-F.; Wallemacq, P.E.; Van Bambeke, F.; Tulkens, P.M. Optimizing β-Lactams Treatment in Critically-Ill Patients Using Pharmacokinetics/Pharmacodynamics Targets: Are First Conventional Doses Effective? Expert Rev. Anti-Infect. Ther. 2017, 15, 677–688. [Google Scholar] [CrossRef]
- Joukhadar, C.; Frossard, M.; Mayer, B.X.; Brunner, M.; Klein, N.; Siostrzonek, P.; Eichler, H.G.; Müller, M. Impaired Target Site Penetration of Beta-Lactams May Account for Therapeutic Failure in Patients with Septic Shock. Crit. Care Med. 2001, 29, 385–391. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of Hypotension before Initiation of Effective Antimicrobial Therapy Is the Critical Determinant of Survival in Human Septic Shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.-M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining Antibiotic Levels in Intensive Care Unit Patients: Are Current -Lactam Antibiotic Doses Sufficient for Critically Ill Patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Sulaiman, H.; Mat-Nor, M.-B.; Rai, V.; Wong, K.K.; Hasan, M.S.; Abd Rahman, A.N.; Jamal, J.A.; Wallis, S.C.; Lipman, J.; et al. Beta-Lactam Infusion in Severe Sepsis (BLISS): A Prospective, Two-Centre, Open-Labelled Randomised Controlled Trial of Continuous versus Intermittent Beta-Lactam Infusion in Critically Ill Patients with Severe Sepsis. Intensive Care Med. 2016, 42, 1535–1545. [Google Scholar] [CrossRef]
- Mohr, J.F.; Wanger, A.; Rex, J.H. Pharmacokinetic/Pharmacodynamic Modeling Can Help Guide Targeted Antimicrobial Therapy for Nosocomial Gram-Negative Infections in Critically Ill Patients. Diagn. Microbiol. Infect. Dis. 2004, 48, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Carrié, C.; Petit, L.; d’Houdain, N.; Sauvage, N.; Cottenceau, V.; Lafitte, M.; Foumenteze, C.; Hisz, Q.; Menu, D.; Legeron, R.; et al. Association between Augmented Renal Clearance, Antibiotic Exposure and Clinical Outcome in Critically Ill Septic Patients Receiving High Doses of β-Lactams Administered by Continuous Infusion: A Prospective Observational Study. Int. J. Antimicrob. Agents 2018, 51, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Du, X.; Kuti, J.L.; Nicolau, D.P. Clinical Pharmacodynamics of Meropenem in Patients with Lower Respiratory Tract Infections. Antimicrob. Agents Chemother. 2007, 51, 1725–1730. [Google Scholar] [CrossRef]
- McKinnon, P.S.; Paladino, J.A.; Schentag, J.J. Evaluation of Area under the Inhibitory Curve (AUIC) and Time above the Minimum Inhibitory Concentration (T>MIC) as Predictors of Outcome for Cefepime and Ceftazidime in Serious Bacterial Infections. Int. J. Antimicrob. Agents 2008, 31, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Drusano, G.L. Antimicrobial Pharmacodynamics: Critical Interactions of “Bug and Drug”. Nat. Rev. Microbiol. 2004, 2, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Crandon, J.L.; Ariano, R.E.; Zelenitsky, S.A.; Nicasio, A.M.; Kuti, J.L.; Nicolau, D.P. Optimization of Meropenem Dosage in the Critically Ill Population Based on Renal Function. Intensive Care Med. 2011, 37, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Lodise, T.P.; Lomaestro, B.; Rodvold, K.A.; Danziger, L.H.; Drusano, G.L. Pharmacodynamic Profiling of Piperacillin in the Presence of Tazobactam in Patients through the Use of Population Pharmacokinetic Models and Monte Carlo Simulation. Antimicrob. Agents Chemother. 2004, 48, 4718–4724. [Google Scholar] [CrossRef]
- Lomaestro, B.M.; Drusano, G.L. Pharmacodynamic Evaluation of Extending the Administration Time of Meropenem Using a Monte Carlo Simulation. Antimicrob. Agents Chemother. 2005, 49, 461–463. [Google Scholar] [CrossRef]
- Nicolau, D.P.; McNabb, J.; Lacy, M.K.; Quintiliani, R.; Nightingale, C.H. Continuous versus Intermittent Administration of Ceftazidime in Intensive Care Unit Patients with Nosocomial Pneumonia. Int. J. Antimicrob. Agents 2001, 17, 497–504. [Google Scholar] [CrossRef]
- Roberts, J.A.; Kirkpatrick, C.M.J.; Roberts, M.S.; Dalley, A.J.; Lipman, J. First-Dose and Steady-State Population Pharmacokinetics and Pharmacodynamics of Piperacillin by Continuous or Intermittent Dosing in Critically Ill Patients with Sepsis. Int. J. Antimicrob. Agents 2010, 35, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Abdul-Aziz, M.-H.; Davis, J.S.; Dulhunty, J.M.; Cotta, M.O.; Myburgh, J.; Bellomo, R.; Lipman, J. Continuous versus Intermittent β-Lactam Infusion in Severe Sepsis. A Meta-Analysis of Individual Patient Data from Randomized Trials. Am. J. Respir. Crit. Care Med. 2016, 194, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Vardakas, K.Z.; Voulgaris, G.L.; Maliaros, A.; Samonis, G.; Falagas, M.E. Prolonged versus Short-Term Intravenous Infusion of Antipseudomonal β-Lactams for Patients with Sepsis: A Systematic Review and Meta-Analysis of Randomised Trials. Lancet Infect. Dis. 2018, 18, 108–120. [Google Scholar] [CrossRef]
- Rhodes, N.J.; Liu, J.; O’Donnell, J.N.; Dulhunty, J.M.; Abdul-Aziz, M.H.; Berko, P.Y.; Nadler, B.; Lipman, J.; Roberts, J.A. Prolonged Infusion Piperacillin-Tazobactam Decreases Mortality and Improves Outcomes in Severely Ill Patients: Results of a Systematic Review and Meta-Analysis*. Crit. Care Med. 2018, 46, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Longuet, P.; Lecapitaine, A.L.; Cassard, B.; Batista, R.; Gauzit, R.; Lesprit, P.; Haddad, R.; Vanjak, D.; Diamantis, S. Groupe des référents en infectiologie d’Île-de-France (GRIF) Preparing and Administering Injectable Antibiotics: How to Avoid Playing God. Med. Mal. Infect. 2016, 46, 242–268. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial Therapeutic Drug Monitoring in Critically Ill Adult Patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- Blackman, A.L.; Jarugula, P.; Nicolau, D.P.; Chui, S.H.; Joshi, M.; Heil, E.L.; Gopalakrishnan, M. Evaluation of Linezolid Pharmacokinetics in Critically Ill Obese Patients with Severe Skin and Soft Tissue Infections. Antimicrob. Agents Chemother. 2021, 65. [Google Scholar] [CrossRef]
- Pea, F. Practical Concept of Pharmacokinetics/Pharmacodynamics in the Management of Skin and Soft Tissue Infections. Curr. Opin. Infect. Dis. 2016, 29, 153–159. [Google Scholar] [CrossRef]
- Barbour, A.; Scaglione, F.; Derendorf, H. Class-Dependent Relevance of Tissue Distribution in the Interpretation of Anti-Infective Pharmacokinetic/Pharmacodynamic Indices. Int. J. Antimicrob. Agents 2010, 35, 431–438. [Google Scholar] [CrossRef]
- Sahre, M.; Sabarinath, S.; Grant, M.; Seubert, C.; DeAnda, C.; Prokocimer, P.; Derendorf, H. Skin and Soft Tissue Concentrations of Tedizolid (Formerly Torezolid), a Novel Oxazolidinone, Following a Single Oral Dose in Healthy Volunteers. Int. J. Antimicrob. Agents 2012, 40, 51–54. [Google Scholar] [CrossRef]
- Stein, G.E.; Smith, C.L.; Missavage, A.; Saunders, J.P.; Nicolau, D.P.; Battjes, S.M.; Kepros, J.P. Tigecycline Penetration into Skin and Soft Tissue. Surg. Infect. 2011, 12, 465–467. [Google Scholar] [CrossRef]
- Bielecka-Grzela, S.; Klimowicz, A. Application of Cutaneous Microdialysis to Evaluate Metronidazole and Its Main Metabolite Concentrations in the Skin after a Single Oral Dose. J. Clin. Pharm. Ther. 2003, 28, 465–469. [Google Scholar] [CrossRef]
- Lanao, J.M.; Navarro, A.S.; Dominguez-Gil, A.; Tabernero, J.M.; Rodriguez, I.C.; Lopez, A.G. Amikacin Concentrations in Serum and Blister Fluid in Healthy Volunteers and in Patients with Renal Impairment. J. Antimicrob. Chemother. 1983, 12, 481–488. [Google Scholar] [CrossRef]
- Simon, P.; Petroff, D.; Busse, D.; Heyne, J.; Girrbach, F.; Dietrich, A.; Kratzer, A.; Zeitlinger, M.; Kloft, C.; Kees, F.; et al. Meropenem Plasma and Interstitial Soft Tissue Concentrations in Obese and Nonobese Patients-A Controlled Clinical Trial. Antibiotics 2020, 9, 931. [Google Scholar] [CrossRef]
- Kiang, T.K.L.; Häfeli, U.O.; Ensom, M.H.H. A Comprehensive Review on the Pharmacokinetics of Antibiotics in Interstitial Fluid Spaces in Humans: Implications on Dosing and Clinical Pharmacokinetic Monitoring. Clin. Pharm. 2014, 53, 695–730. [Google Scholar] [CrossRef] [PubMed]
- Maglio, D.; Teng, R.; Thyrum, P.T.; Nightingale, C.H.; Nicolau, D.P. Pharmacokinetic Profile of Meropenem, Administered at 500 Milligrams Every 8 Hours, in Plasma and Cantharidin-Induced Skin Blister Fluid. Antimicrob. Agents Chemother. 2003, 47, 1771–1773. [Google Scholar] [CrossRef]
- Ong, C.T.; Kuti, J.L.; Nicolau, D.P. Pharmacodynamic Modeling of Imipenem-Cilastatin, Meropenem, and Piperacillin-Tazobactam for Empiric Therapy of Skin and Soft Tissue Infections: A Report from the OPTAMA Program. Surg. Infect. 2005, 6, 419–426. [Google Scholar] [CrossRef] [PubMed]
- So, W.; Kuti, J.L.; Nicolau, D.P. Population Pharmacokinetics of Cefazolin in Serum and Tissue for Patients with Complicated Skin and Soft Tissue Infections (CSSTI). Infect. Dis. Ther. 2014, 3, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Cristinacce, A.; Wright, J.G.; Macpherson, M.; Iaconis, J.; Das, S. Comparing Probability of Target Attainment against Staphylococcus Aureus for Ceftaroline Fosamil, Vancomycin, Daptomycin, Linezolid, and Ceftriaxone in Complicated Skin and Soft Tissue Infection Using Pharmacokinetic/Pharmacodynamic Models. Diagn. Microbiol. Infect. Dis. 2021, 99, 115292. [Google Scholar] [CrossRef] [PubMed]
- Takesue, Y.; Mikamo, H.; Kusachi, S.; Watanabe, S.; Takahashi, K.; Yoshinari, T.; Ishii, M.; Aikawa, N. Correlation between Pharmacokinetic/Pharmacodynamic Indices and Clinical Outcomes in Japanese Patients with Skin and Soft Tissue Infections Treated with Daptomycin: Analysis of a Phase III Study. Diagn. Microbiol. Infect. Dis. 2015, 83, 77–81. [Google Scholar] [CrossRef][Green Version]
- Lozano-Alonso, S.; Linares-Palomino, J.P.; Vera-Arroyo, B.; Bravo-Molina, A.; Hernández-Quero, J.; Ros-Díe, E. Evaluación de la capacidad de difusión tisular de antibióticos en isquemia de miembros inferiores. Enferm. Infecc. Y Microbiol. Clínica 2016, 34, 477–483. [Google Scholar] [CrossRef]
- Grillon, A.; Argemi, X.; Gaudias, J.; Ronde-Ousteau, C.; Boeri, C.; Jenny, J.-Y.; Hansmann, Y.; Lefebvre, N.; Jehl, F. Bone Penetration of Daptomycin in Diabetic Patients with Bacterial Foot Infections. Int. J. Infect. Dis. 2019, 85, 127–131. [Google Scholar] [CrossRef]
- Stein, G.E.; Throckmorton, J.K.; Scharmen, A.E.; Weiss, W.J.; Prokai, L.; Smith, C.L.; Havlichek, D.H. Tissue Penetration and Antimicrobial Activity of Standard- and High-Dose Trimethoprim/Sulfamethoxazole and Linezolid in Patients with Diabetic Foot Infection. J. Antimicrob. Chemother. 2013, 68, 2852–2858. [Google Scholar] [CrossRef] [PubMed]
- Wiskirchen, D.E.; Shepard, A.; Kuti, J.L.; Nicolau, D.P. Determination of Tissue Penetration and Pharmacokinetics of Linezolid in Patients with Diabetic Foot Infections Using in Vivo Microdialysis. Antimicrob. Agents Chemother. 2011, 55, 4170–4175. [Google Scholar] [CrossRef] [PubMed]
- Traunmüller, F.; Schintler, M.V.; Metzler, J.; Spendel, S.; Mauric, O.; Popovic, M.; Konz, K.H.; Scharnagl, E.; Joukhadar, C. Soft Tissue and Bone Penetration Abilities of Daptomycin in Diabetic Patients with Bacterial Foot Infections. J. Antimicrob. Chemother. 2010, 65, 1252–1257. [Google Scholar] [CrossRef]
- Bergan, T.; Kalager, T.; Hellum, K.B.; Solberg, C.O. Penetration of Cefotaxime and Desacetylcefotaxime into Skin Blister Fluid. J. Antimicrob. Chemother. 1982, 10, 193–196. [Google Scholar] [CrossRef]
- So, W.; Kuti, J.L.; Shepard, A.; Nugent, J.; Nicolau, D.P. Tissue Penetration and Exposure of Cefepime in Patients with Diabetic Foot Infections. Int. J. Antimicrob. Agents 2016, 47, 247–248. [Google Scholar] [CrossRef]
- Raymakers, J.T.; Houben, A.J.; van der Heyden, J.J.; Tordoir, J.H.; Kitslaar, P.J.; Schaper, N.C. The Effect of Diabetes and Severe Ischaemia on the Penetration of Ceftazidime into Tissues of the Limb. Diabet. Med. 2001, 18, 229–234. [Google Scholar] [CrossRef]
- Kerin, M.J.; Greenstein, D.; Chisholm, E.M.; Sheehan, S.J.; Kester, R.C. Is Antibiotic Penetration Compromised in the Ischaemic Tissues of Patients Undergoing Amputation? Ann. R. Coll. Surg. Engl. 1992, 74, 274–276. [Google Scholar]
- Skhirtladze, K.; Hutschala, D.; Fleck, T.; Thalhammer, F.; Ehrlich, M.; Vukovich, T.; Müller, M.; Tschernko, E.M. Impaired Target Site Penetration of Vancomycin in Diabetic Patients Following Cardiac Surgery. Antimicrob. Agents Chemother. 2006, 50, 1372–1375. [Google Scholar] [CrossRef]
- Safdar, N.; Andes, D.; Craig, W.A. In Vivo Pharmacodynamic Activity of Daptomycin. Antimicrob. Agents Chemother. 2004, 48, 63–68. [Google Scholar] [CrossRef]
- Andes, D.; van Ogtrop, M.L.; Peng, J.; Craig, W.A. In Vivo Pharmacodynamics of a New Oxazolidinone (Linezolid). Antimicrob. Agents Chemother. 2002, 46, 3484–3489. [Google Scholar] [CrossRef]
- Berger, S.A.; Barza, M.; Haher, J.; McFarland, J.J.; Louie, S.; Kane, A. Penetration of Clindamycin into Decubitus Ulcers. Antimicrob. Agents Chemother. 1978, 14, 498–499. [Google Scholar] [CrossRef] [PubMed]
- Bassi, C.; Pederzoli, P.; Vesentini, S.; Falconi, M.; Bonora, A.; Abbas, H.; Benini, A.; Bertazzoni, E.M. Behavior of Antibiotics during Human Necrotizing Pancreatitis. Antimicrob. Agents Chemother. 1994, 38, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Minelli, E.B.; Benini, A.; Muner, A.; Bassi, C.; Abbas, H.; Pederzoli, P. Pefloxacin Penetration into Human Necrotic Pancreatic Tissue. J. Antimicrob. Chemother. 1996, 38, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Foitzik, T.; Hotz, H.G.; Kinzig, M.; Sörgel, F.; Buhr, H.J. Influence of Changes in Pancreatic Tissue Morphology and Capillary Blood Flow on Antibiotic Tissue Concentrations in the Pancreas during the Progression of Acute Pancreatitis. Gut 1997, 40, 526–530. [Google Scholar] [CrossRef]
- Maguire, C.; Agrawal, D.; Daley, M.J.; Douglass, E.; Rose, D.T. Rethinking Carbapenems: A Pharmacokinetic Approach for Antimicrobial Selection in Infected Necrotizing Pancreatitis. Ann. Pharmacother. 2021, 55, 902–913. [Google Scholar] [CrossRef]
- Gowland Hopkins, N.F.; Jamieson, C.W. Antibiotic Concentration in the Exudate of Venous Ulcers: The Prediction of Ulcer Healing Rate. Br. J. Surg. 1983, 70, 532–534. [Google Scholar] [CrossRef]
- Mangum, L.C.; Garcia, G.R.; Akers, K.S.; Wenke, J.C. Duration of Extremity Tourniquet Application Profoundly Impacts Soft-Tissue Antibiotic Exposure in a Rat Model of Ischemia-Reperfusion Injury. Injury 2019, 50, 2203–2214. [Google Scholar] [CrossRef]
- Duckworth, C.; Fisher, J.F.; Carter, S.A.; Newman, C.L.; Cogburn, C.; Nesbit, R.R.; Wray, C.H. Tissue Penetration of Clindamycin in Diabetic Foot Infections. J. Antimicrob. Chemother. 1993, 31, 581–584. [Google Scholar] [CrossRef]
- Stainton, S.M.; Monogue, M.L.; Baummer-Carr, A.; Shepard, A.K.; Nugent, J.F.; Kuti, J.L.; Nicolau, D.P. Comparative Assessment of Tedizolid Pharmacokinetics and Tissue Penetration between Diabetic Patients with Wound Infections and Healthy Volunteers via In Vivo Microdialysis. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Monogue, M.L.; Stainton, S.M.; Baummer-Carr, A.; Shepard, A.K.; Nugent, J.F.; Kuti, J.L.; Nicolau, D.P. Pharmacokinetics and Tissue Penetration of Ceftolozane-Tazobactam in Diabetic Patients with Lower Limb Infections and Healthy Adult Volunteers. Antimicrob. Agents Chemother. 2017, 61, e01449-17. [Google Scholar] [CrossRef]
- Koomanachai, P.; Keel, R.A.; Johnson-Arbor, K.K.; Suecof, L.A.; Nicolau, D.P.; Kuti, J.L. Linezolid Penetration into Wound Tissue of Two Diabetic Patients before and after Hyperbaric Oxygen Therapy. Undersea Hyperb. Med. 2011, 38, 11–16. [Google Scholar]
- Boselli, E.; Breilh, D.; Rimmelé, T.; Guillaume, C.; Xuereb, F.; Saux, M.-C.; Bouvet, L.; Chassard, D.; Allaouchiche, B. Alveolar Concentrations of Piperacillin/Tazobactam Administered in Continuous Infusion to Patients with Ventilator-Associated Pneumonia. Crit. Care Med. 2008, 36, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Skopnik, H.; Heimann, G. Pharmacokinetics of Antimicrobial Drugs in the Cerebrospinal Fluid. Pediatr. Pharmacol. 1983, 3, 313–320. [Google Scholar]
- Murali, S.; Pillai, A.V.; Ramachandran, R. Efficacy of Colistimethate Sodium as Local Application in Necrotising Fasciitis. BMJ Case Rep. 2019, 12, e232354. [Google Scholar] [CrossRef]
- González-López, J.; Macías-García, F.; Lariño-Noia, J.; Domínguez-Muñoz, J.E. Theoretical Approach to Local Infusion of Antibiotics for Infected Pancreatic Necrosis. Pancreatology 2016, 16, 719–725. [Google Scholar] [CrossRef]
- Urbina, T.; Hua, C.; Woerther, P.-L.; Mekontso Dessap, A.; Chosidow, O.; de Prost, N. Early Identification of Patients at High Risk of Group A Streptococcus-Associated Necrotizing Skin and Soft Tissue Infections: A Retrospective Cohort Study. Crit. Care 2019, 23, 417. [Google Scholar] [CrossRef]
- Willis, R.N.; Guidry, C.A.; Horn, C.B.; Gilsdorf, D.; Davies, S.W.; Dietch, Z.C.; Sawyer, R.G. Predictors of Monomicrobial Necrotizing Soft Tissue Infections. Surg. Infect. 2015, 16, 533–537. [Google Scholar] [CrossRef]
- Brook, I.; Frazier, E.H. Clinical and Microbiological Features of Necrotizing Fasciitis. J. Clin. Microbiol. 1995, 33, 2382–2387. [Google Scholar] [CrossRef]
- Wattel, F.; Mathieu, D.; Neut, C.; Dubreuil, L.; Cesari, J.-F.; Favory, R. Necrotizing soft tissue infections: Role of the localization for the antibiotic management. Bull. Acad. Natl. Med. 2004, 188, 473–486. [Google Scholar] [PubMed]
| Type of Pathogens | Primary | Secondary | Polymicrobial | Commensals |
|---|---|---|---|---|
| Pathogenicity | May cause NSTI in patients without known risk factors | May cause infection in patients with risk factors | Rarely pathogen in the absence of a primary or secondary pathogen | Do not cause NSTI although sometimes identified with other pathogens |
| Species | Group A Streptococcus Staphylococcus aureus Vibrio vulnificus Clostridium perfringens | Other Streptococcus (group B, C, G, anginosus) Pneumococcus Haemophilus influenzae Neisseria meningitidis Enterobacteriaceae Nonfermenting gram-negative bacilli Other anaerobes (Bacteroides, Prevotella, Fusobacterium) | Enterococcus | Bacillus Corynebacterium Micrococcus Coagulase negative Staphylococci |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbina, T.; Razazi, K.; Ourghanlian, C.; Woerther, P.-L.; Chosidow, O.; Lepeule, R.; de Prost, N. Antibiotics in Necrotizing Soft Tissue Infections. Antibiotics 2021, 10, 1104. https://doi.org/10.3390/antibiotics10091104
Urbina T, Razazi K, Ourghanlian C, Woerther P-L, Chosidow O, Lepeule R, de Prost N. Antibiotics in Necrotizing Soft Tissue Infections. Antibiotics. 2021; 10(9):1104. https://doi.org/10.3390/antibiotics10091104
Chicago/Turabian StyleUrbina, Tomas, Keyvan Razazi, Clément Ourghanlian, Paul-Louis Woerther, Olivier Chosidow, Raphaël Lepeule, and Nicolas de Prost. 2021. "Antibiotics in Necrotizing Soft Tissue Infections" Antibiotics 10, no. 9: 1104. https://doi.org/10.3390/antibiotics10091104
APA StyleUrbina, T., Razazi, K., Ourghanlian, C., Woerther, P.-L., Chosidow, O., Lepeule, R., & de Prost, N. (2021). Antibiotics in Necrotizing Soft Tissue Infections. Antibiotics, 10(9), 1104. https://doi.org/10.3390/antibiotics10091104






