Polyphenols and Organic Acids as Alternatives to Antimicrobials in Poultry Rearing: A Review
Abstract
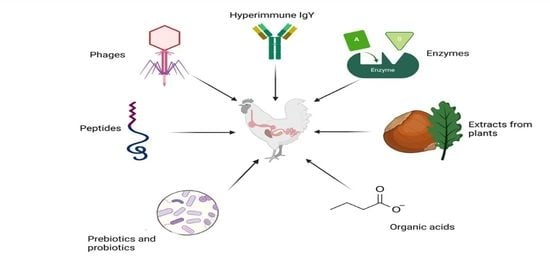
:1. Introduction
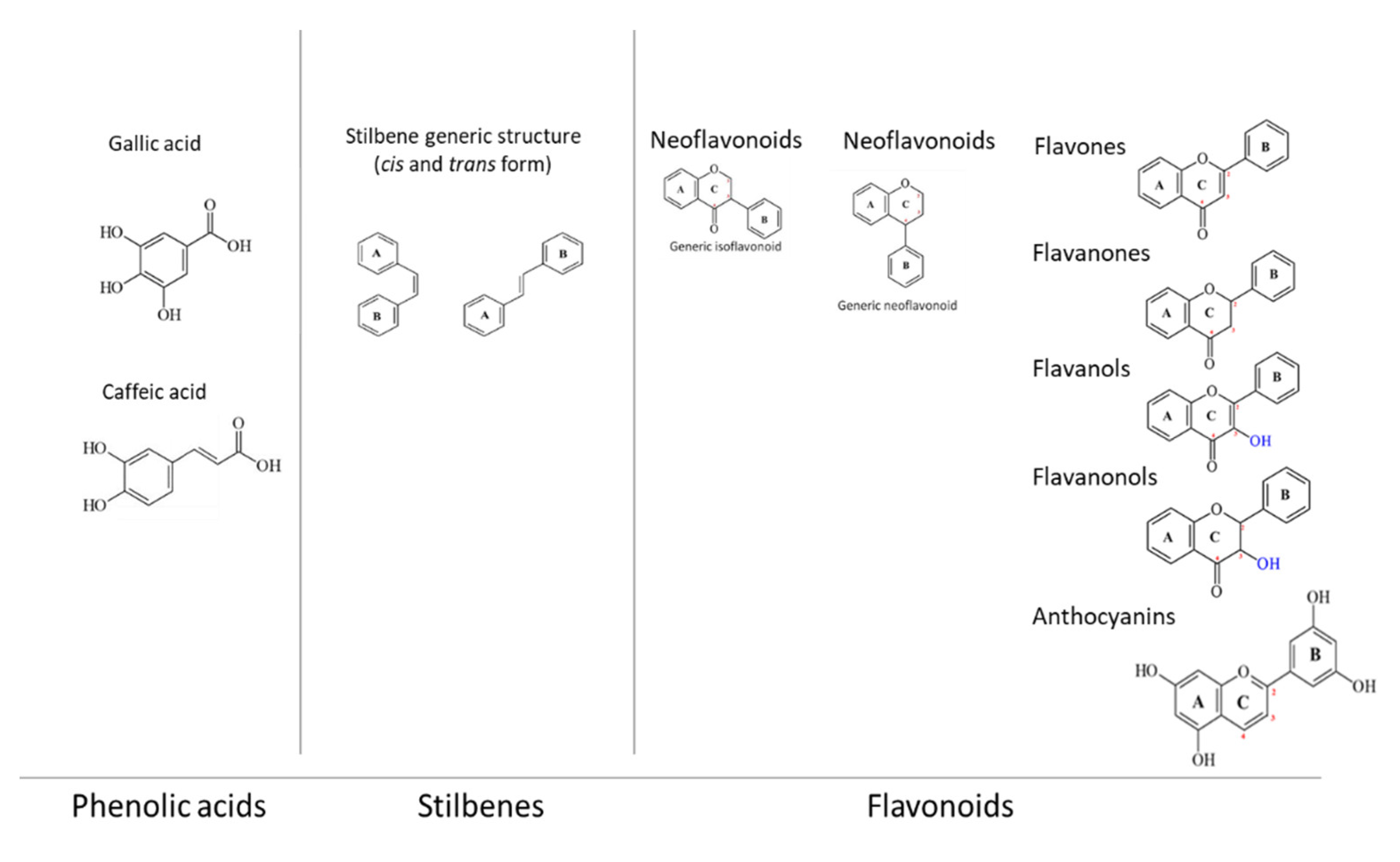
2. Polyphenols
2.1. Chemical Characteristics
2.2. Antimicrobial Activity
2.3. Poultry Feeding Application
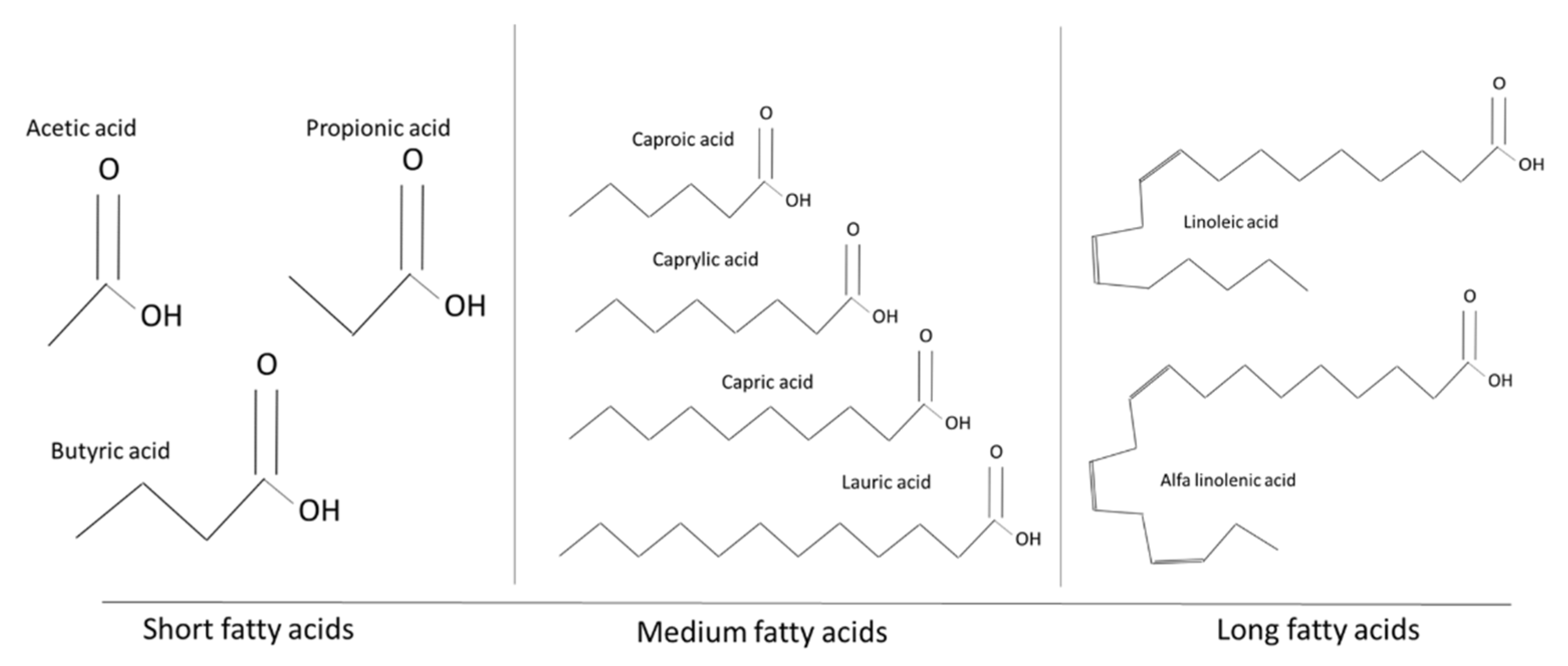
3. Organic Acids
3.1. Chemical Characteristics
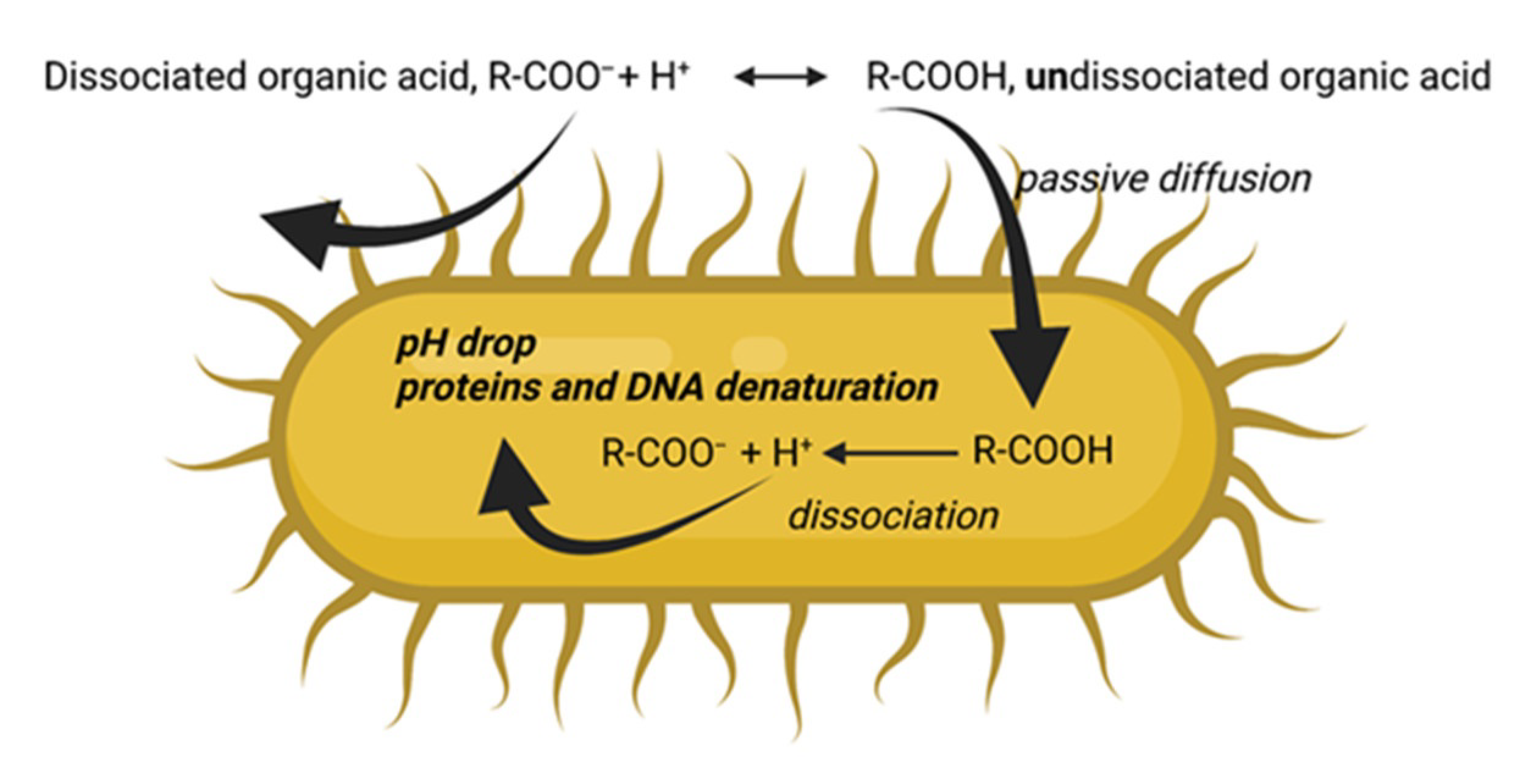
3.2. Antimicrobial Action
3.3. Poultry Feeding Application
4. Blends of Polyphenols and Organic Acids
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Haulisah, N.A.; Hassan, L.; Bejo, S.K.; Jajere, S.M.; Ahmad, N.I. High Levels of Antibiotic Resistance in Isolates from Diseased Livestock. Front. Vet. Sci. 2021, 8, 652351. [Google Scholar] [CrossRef]
- ECDC; EFSA; EMA. ECDC/EFSA/EMA Second Joint Report on the Integrated Analysis of the Consumption of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Humans and Food-Producing Animals. EFSA J. 2017, 15, 4872. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines on Use of Medically Important Antimicrobials in Food-Producing Animals; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- European Commission. Ban on Antibiotics as Growth Promoters in Animal Feed Enters into Effect; European Commission: Brussels, Belgium, 2005. [Google Scholar]
- Communication from the Commission to the European Parliament and the Council. Action Plan against the Rising Threats from Antimicrobial Resistance; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- Salaheen, S.; Almario, J.A.; Biswas, D. Inhibition of Growth and Alteration of Host Cell Interactions of Pasteurella Multocida with Natural Byproducts. Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S0032579119323107?token=12F0C4DE36333F4BE72ED03EACE23B4296DE66D33822AB2C8E75530CBD83EB087AAC0420C6AB4F0A10F463748E9777C2&originRegion=eu-west-1&originCreation=20210512142458 (accessed on 12 May 2021).
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2017, 4, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Peric, L.; Zikic, D.; Lukic, M. Application of alternative growth promoters in broiler production. Biotehnol. Stoc. 2009, 25, 387–397. [Google Scholar] [CrossRef] [Green Version]
- Perricone, V.; Comi, M.; Giromini, C.; Rebucci, R.; Agazzi, A.; Savoini, G.; Bontempo, V. Green Tea and Pomegranate Extract Administered During Critical Moments of the Production Cycle Improves Blood Antiradical Activity and Alters Cecal Microbial Ecology of Broiler Chickens. Animals 2020, 10, 785. [Google Scholar] [CrossRef] [PubMed]
- Council of the European Union; European Parliament. Regulation (EU) 2019/4 of the European Parliament and of the Council of 11 December 2018 on the Manufacture, Placing on the Market and Use of Medicated Feed, Amending Regulation (EC) No 183/2005 of the European Parliament and of the Council and Repealing Council Directive 90/167/EEC. 2018. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32019R0004&from=EN (accessed on 19 August 2021).
- Council of the European Union; European Parliament. Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on Veterinary Medicinal Products and Repealing Directive 2001/82/EC. 2018. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32019R0006&from=EN (accessed on 19 August 2021).
- Choudhury, P.K.; Salem, A.Z.M.; Jena, R.; Kumar, S.; Singh, R.; Puniya, A.K. Rumen Microbiology: An overview. In Rumen Microbiology: From Evolution to Revolution; Puniya, A., Singh, R., Kamra, D., Eds.; Springer: New Delhi, India, 2015. [Google Scholar]
- Vasta, V.; Daghio, M.; Cappucci, A.; Buccioni, A.; Serra, A.; Viti, C.; Mele, M. Invited review: Plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: Experimental evidence and methodological approaches. J. Dairy Sci. 2019, 102, 3781–3804. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, S.P.; Perumal, S.S.; Balakrishnan, A. Scope of Hydrolysable Tannins as Possible Antimicrobial Agent. Phytother. Res. 2016, 30, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- El-Hack, M.E.A.; Alagawany, M.; Abdel-Moneim, A.-M.E.; Mohammed, N.G.; Khafaga, A.F.; Bin-Jumah, M.; Othman, S.I.; Allam, A.A.; Elnesr, S.S. Cinnamon (Cinnamomum zeylanicum) Oil as a Potential Alternative to Antibiotics in Poultry. Antibiotics 2020, 9, 210. [Google Scholar] [CrossRef]
- Alhotan, R.A.; Abudabos, A. Anticoccidial and antioxidant effects of plants derived polyphenol in broilers exposed to induced coccidiosis. Environ. Sci. Pollut. Res. 2019, 26, 14194–14199. [Google Scholar] [CrossRef]
- Dumari, M.A.; Sarir, H.; Fani Makki, O.; Afzali, N. Effect of Milk Thistle (Silybum marianum L.) on Biochemical Parameters and Immunity of Broiler Chicks Fed Aflatoxin B1 after Three Weeks. Iran. J. Toxicol. 2014, 8, 1098–1103. [Google Scholar]
- Adil, S.; Banday, T.; Bhat, G.A.; Mir, M.S.; Rehman, M. Effect of Dietary Supplementation of Organic Acids on Performance, Intestinal Histomorphology, and Serum Biochemistry of Broiler Chicken. Vet. Med. Int. 2010, 2010, 479485. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.H.; Iqbal, J. Recent advances in the role of organic acids in poultry nutrition. J. Appl. Anim. Res. 2015, 44, 359–369. [Google Scholar] [CrossRef]
- Akbar, M.A.; Tewatia, B.; Kumar, S. Effect of dietary supplementation of salts of organic acids on growth performance, carcass traits and meat composition of broilers. Int. J. Chem. Stud. 2019, 7, 2825–2828. [Google Scholar]
- FAO Headquarters. Proceedings of the Expert Meeting on How to Feed the World in 2050 Technical; FAO Headquarters: Rome, Italy, 2009; Available online: http://www.fao.org/3/ak542e/ak542e00.htm (accessed on 19 August 2021).
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Cutrim, C.S.; Cortez, M.A.S. A review on polyphenols: Classification, beneficial effects and their application in dairy products. Int. J. Dairy Technol. 2018, 71, 564–578. [Google Scholar] [CrossRef]
- Correddu, F.; Gaspa, G.; Pulina, G.; Nudda, A. Grape seed and linseed, alone and in combination, enhance unsaturated fatty acids in the milk of Sarda dairy sheep. J. Dairy Sci. 2016, 99, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basheer, L.; Kerem, Z. Interactions between CYP3A4 and Dietary Polyphenols. Oxidative Med. Cell. Longev. 2015, 2015, 854015. [Google Scholar] [CrossRef] [Green Version]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Correddu, F.; Lunesu, M.F.; Buffa, G.; Atzori, A.S.; Nudda, A.; Battacone, G.; Pulina, G. Can agro-industrial by-products rich in polyphenols be advantageously used in the feeding and nutrition of dairy small ruminants? Animals 2020, 10, 131. [Google Scholar] [CrossRef] [Green Version]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Phenols, Polyphenols and Tannins: An Overview. In Plant Secondary Metabolites; Blackwell Publishing Ltd.: Oxford, UK, 2007; pp. 1–24. ISBN 9781405125093. [Google Scholar]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 2010, 91, 24–37. [Google Scholar] [CrossRef]
- Armanini, E.H.; Boiago, M.M.; de Oliveira, P.V.; Roscamp, E.; Strapazzon, J.V.; de Lima, A.G.; Copetti, P.M.; Morsch, V.M.; de Oliveira, F.C.; Wagner, R.; et al. Inclusion of a phytogenic bend in broiler diet as a performance enhancer and anti-aflatoxin agent: Impacts on health, performance, and meat quality. Res. Vet. Sci. 2021, 137, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Akyildiz, S.; Denli, M. Application of plant extracts as feed additives in poultry nutrition. Anim. Sci. 2016, LIX, 71–74. [Google Scholar]
- Butler, L.G. Antinutritional effects of condensed and hydrolyzable tannins. In Plant Polyphenols; Springer: Boston, MA, USA, 1992; pp. 693–698. [Google Scholar] [CrossRef]
- Redondo, L.; Dominguez, J.; Rabinovitz, B.; Redondo, E.; Miyakawa, M.F. Hydrolyzable and condensed tannins resistance in Clostridium perfringens. Anaerobe 2015, 34, 139–145. [Google Scholar] [CrossRef]
- Dos Santos, A.F.; Da Silva, A.S.; Galli, G.M.; Paglia, E.B.; Dacoreggio, M.V.; Kempka, A.P.; Souza, C.F.; Baldissera, M.D.; da Rosa, G.; Boiago, M.M.; et al. Addition of yellow strawberry guava leaf extract in the diet of laying hens had antimicrobial and antioxidant effect capable of improving egg quality. Biocatal. Agric. Biotechnol. 2020, 29, 101788. [Google Scholar] [CrossRef]
- Hidanah, S.; Sabdoningrum, E.K.; Anam, M.; Arif, A.; Nur, A.; Ansori, M.; Hasanah, T.P.; Viola, L.; Widaya, A. Sambiloto (Andrographis paniculata) Extract Improves the Performance of Animal Model Infected with Escherichia coli. Indian J. Forensic Med. Toxicol. 2020, 14, 3491–3496. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, H.; Du, E.; Jin, F.; Zheng, C.; Fan, Q.; Zhao, N.; Guo, W.; Zhang, W.; Huang, S.; et al. Effects of magnolol on egg production, egg quality, antioxidant capacity, and intestinal health of laying hens in the late phase of the laying cycle. Poult. Sci. 2020, 100, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Balenović, M.; Savić, V.; Janječić, Z.; Popović, M.; Šimpraga, B.; Carović-Stanko, K.; Bedeković, D.; Zelenika, T.A. Immunomodulatory and antimicrobial effects of selected herbs on laying hens. Vet. Arh. 2018, 88, 673–686. [Google Scholar] [CrossRef]
- Ramirez, S.Y.; Peñuela-Sierra, L.M.; Ospina, M.A. Effects of oregano (Lippia origanoides) essential oil supplementation on the performance, egg quality, and intestinal morphometry of Isa Brown laying hens. Vet. World 2021, 14, 595–602. [Google Scholar] [CrossRef]
- Oyeleke, A.M.; Adeyemi, O.A.; Egbeyale, L.T.; Sobayo, R.A. Antimicrobial, immunomodulatory and hepatomodulatory effects of aqueous extracts of Petiveria alliacea root and leaf on growing pullets. Span. J. Agric. Res. 2021, 19, e0502. [Google Scholar] [CrossRef]
- Branciari, R.; Ranucci, D.; Ortenzi, R.; Roila, R.; Trabalza-Marinucci, M.; Servili, M.; Papa, P.; Galarini, R.; Valiani, A. Dietary Administration of Olive Mill Wastewater Extract Reduces Campylobacter spp. Prevalence in Broiler Chickens. Sustainability 2016, 8, 837. [Google Scholar] [CrossRef] [Green Version]
- Galli, G.M.; Gerbet, R.R.; Griss, L.G.; Fortuoso, B.F.; Petrolli, T.G.; Boiago, M.M.; Souza, C.F.; Baldissera, M.D.; Mesadri, J.; Wagner, R.; et al. Combination of herbal components (curcumin, carvacrol, thymol, cinnamaldehyde) in broiler chicken feed: Impacts on response parameters, performance, fatty acid profiles, meat quality and control of coccidia and bacteria. Microb. Pathog. 2020, 139, 103916. [Google Scholar] [CrossRef]
- Galli, G.M.; Griss, L.G.; Boiago, M.M.; Petrolli, T.G.; Glombowsky, P.; Bissacotti, B.F.; Copetti, P.M.; da Silva, A.D.; Schetinger, M.R.; Sareta, L.; et al. Effects of curcumin and yucca extract addition in feed of broilers on microorganism control (anticoccidial and antibacterial), health, performance and meat quality. Res. Vet. Sci. 2020, 132, 156–166. [Google Scholar] [CrossRef]
- Sanchez, S.D.; Dsouza, D.; Biswas, D.; Hanning, I. Botanical alternatives to antibiotics for use in organic poultry production. Poult. Sci. 2015, 94, 1419–1430. [Google Scholar] [CrossRef]
- Matin, A.; Islam, A.; Khatun, M.M. Prevalence of colibacillosis in chickens in greater Mymensingh district of Bangladesh. Vet. World 2017, 10, 29–33. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Zhou, S.; Yang, X.; Chen, Q.; He, Y.; Huang, W. Magnolol Protects Against Oxidative Stress-Mediated Neural Cell Damage by Modulating Mitochondrial Dysfunction and PI3K/Akt Signaling. J. Mol. Neurosci. 2013, 50, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Z.; Huang, X.; Shi, W.; Zhang, R.; Chen, M.; Huang, H.; Wu, L. Insights on the Multifunctional Activities of Magnolol. BioMed Res. Int. 2019, 2019, 1847130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranaware, A.M.; Banik, K.; Deshpande, V.; Padmavathi, G.; Roy, N.K.; Sethi, G.; Fan, L.; Kumar, A.P.; Kunnumakkara, A.B. Magnolol: A Neolignan from the Magnolia Family for the Prevention and Treatment of Cancer. Int. J. Mol. Sci. 2018, 19, 2362. [Google Scholar] [CrossRef] [Green Version]
- Guyard-Nicodème, M.; Keita, A.; Quesne, S.; Amelot, M.; Poezevara, T.; Le Berre, B.; Sánchez, J.; Vesseur, P.; Martín, Á.; Medel, P.; et al. Efficacy of feed additives against Campylobacter in live broilers during the entire rearing period. Poult. Sci. 2016, 95, 298–305. [Google Scholar] [CrossRef]
- Roila, R.; Ranucci, D.; Valiani, A.; Galarini, R.; Servili, M.; Branciari, R. Antimicrobial and anti-biofilm activity of olive oil by-products against Campylobacter spp. isolated from chicken meat. Acta Sci. Pol. Technol. Aliment. 2015, 18, 43–52. [Google Scholar] [CrossRef]
- Lee, N.-K.; Jung, B.S.; Na, D.S.; Yu, H.H.; Kim, J.-S.; Paik, H.-D. The impact of antimicrobial effect of chestnut inner shell extracts against Campylobacter jejuni in chicken meat. LWT 2016, 65, 746–750. [Google Scholar] [CrossRef]
- Silván, J.M.; Mingo, E.; Hidalgo, M.; de Pascual-Teresa, S.; Carrascosa, A.V.; Martinez-Rodriguez, A.J. Antibacterial activity of a grape seed extract and its fractions against Campylobacter spp. Food Control 2012, 29, 25–31. [Google Scholar] [CrossRef]
- Nm, J.; Joseph, A.; Maliakel, B.; Im, K. Dietary addition of a standardized extract of turmeric (TurmaFEEDTM) improves growth performance and carcass quality of broilers. J. Anim. Sci. Technol. 2018, 60, 8. [Google Scholar] [CrossRef] [Green Version]
- Galli, G.M.; Da Silva, A.S.; Biazus, A.H.; Reis, J.H.; Boiago, M.M.; Topazio, J.P.; Migliorini, M.J.; Guarda, N.S.; Moresco, R.N.; Ourique, A.F.; et al. Feed addition of curcumin to laying hens showed anticoccidial effect, and improved egg quality and animal health. Res. Vet. Sci. 2018, 118, 101–106. [Google Scholar] [CrossRef]
- Van Der Wielen, P.W.J.J.; Biesterveld, S.; Notermans, S.; Hofstra, H.; Urlings, B.A.P.; Van Knapen, F. Role of Volatile Fatty Acids in Development of the Cecal Microflora in Broiler Chickens during Growth. Appl. Environ. Microbiol. 2000, 66, 2536–2540. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, S.; Yahyavi, M.; Zare, D.N. Influence of Dietary Organic Acids Supplementation on Reproductive Performance of Freshwater Angelfish (Pterophyllum scalare). Glob. Vet. 2014, 13, 373–377. [Google Scholar] [CrossRef]
- Us, V.; Sheoran, N.; Shunthwal, J.; Akbar, M.; Tewatia, B. Effect of Supplementation of Salts of Organic Acids on Serum and Haematological Parameters of Broilers. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 4211–4218. [Google Scholar] [CrossRef]
- Hsiao, C.-P.; Siebert, K.J. Modeling the inhibitory effects of organic acids on bacteria. Int. J. Food Microbiol. 1999, 47, 189–201. [Google Scholar] [CrossRef]
- Dierick, N.A.; Decuypere, J.A.; Molly, K.; Van Beek, E.; Vanderbeke, E. The combined use of triacylglycerols containing medium-chainfatty acids (MCFAs) and exogenous lipolytic enzymes as alternative for nutritional antibiotics in piglet nutrition: I. In vitro screening of the release of MCFAs from selected fat sources by selected exogenous lipolytic enzymes under simulated pig gastric conditions and their effects on the gut flora of piglets. Livest. Prod. Sci. 2002, 75, 129–142. [Google Scholar]
- Hermans, D.; Martel, A.; Van Deun, K.; Verlinden, M.; Van Immerseel, F.; Garmyn, A.; Messens, W.; Heyndrickx, M.; Haesebrouck, F.; Pasmans, F. Intestinal mucus protects Campylobacter jejuni in the ceca of colonized broiler chickens against the bactericidal effects of medium-chain fatty acids. Poult. Sci. 2010, 89, 1144–1155. [Google Scholar] [CrossRef]
- Huyghebaert, G.; Ducatelle, R.; Van Immerseel, F. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011, 187, 182–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannelli, F.; Minieri, S.; Tosi, G.; Secci, G.; Daghio, M.; Massi, P.; Fiorentini, L.; Galigani, I.; Lancini, S.; Rapaccini, S.; et al. Effect of Chestnut Tannins and Short Chain Fatty Acids as Anti-Microbials and as Feeding Supplements in Broilers Rearing and Meat Quality. Animals 2019, 9, 659. [Google Scholar] [CrossRef] [Green Version]
- Galli, G.M.; Aniecevski, E.; Petrolli, T.G.; da Rosa, G.; Boiago, M.M.; Simões, C.A.; Wagner, R.; Copetti, P.M.; Morsch, V.M.; Araujo, D.N.; et al. Growth performance and meat quality of broilers fed with microencapsulated organic acids. Anim. Feed. Sci. Technol. 2021, 271, 114706. [Google Scholar] [CrossRef]
- Aljumaah, M.R.; Alkhulaifi, M.M.; Abudabos, A.M.; Alabdullatifb, A.; El-Mubarak, A.H.; Al Suliman, A.R.; Stanley, D. Organic acid blend supplementation increases butyrate and acetate production in Salmonella enterica serovar Typhimurium challenged broilers. PLoS ONE 2020, 15, e0232831. [Google Scholar] [CrossRef]
- Fortuoso, B.F.; dos Reis, J.H.; Gebert, R.R.; Barreta, M.; Griss, L.G.; Casagrande, R.A.; de Cristo, T.G.; Santiani, F.; Campigotto, G.; Rampazzo, L.; et al. Glycerol monolaurate in the diet of broiler chickens replacing conventional antimicrobials: Impact on health, performance and meat quality. Microb. Pathog. 2019, 129, 161–167. [Google Scholar] [CrossRef]
- Dauksiene, A.; Ruzauskas, M.; Gruzauskas, R.; Zavistanaviciute, P.; Starkute, V.; Lele, V.; Klupsaite, D.; Klementaviciute, J.; Bartkiene, E. A Comparison Study of the Caecum Microbial Profiles, Productivity and Production Quality of Broiler Chickens Fed Supplements Based on Medium Chain Fatty and Organic Acids. Animals 2021, 11, 610. [Google Scholar] [CrossRef]
- Vermeulen, K.; Verspreet, J.; Courtin, C.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Reduced particle size wheat bran is butyrogenic and lowers Salmonella colonization, when added to poultry feed. Vet. Microbiol. 2017, 198, 64–71. [Google Scholar] [CrossRef]
- Islam, R.; Hassan, Y.I.; Das, Q.; Lepp, D.; Hernandez, M.; Godfrey, D.V.; Orban, S.; Ross, K.; Delaquis, P.; Diarra, M.S. Dietary organic cranberry pomace influences multiple blood biochemical parameters and cecal microbiota in pasture-raised broiler chickens. J. Funct. Foods 2020, 72, 104053. [Google Scholar] [CrossRef]
- Pearlin, B.V.; Muthuvel, S.; Govidasamy, P.; Villavan, M.; Alagawany, M.; Farag, M.R.; Dhama, K.; Gopi, M. Role of acidifiers in livestock nutrition and health: A review. J. Anim. Physiol. Anim. Nutr. 2019, 104, 558–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yudiarti, T.; Isroli, I.; Yunianto, V.D. Nutritive and antioxidative properties of some selected agro-industrial by-products fermented with the fungus Chrysonillia crassa as alternative feedstuffs for poultry. J. Phys. Conf. Ser. 2020, 1524, 012145. [Google Scholar] [CrossRef]
- Galán, J.E.; Curtiss, R. Expression of Salmonella typhimurium genes required for invasion is regulated by changes in DNA supercoiling. Infect. Immun. 1990, 58, 1879–1885. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Moreno, E.; Gonzalez-Alvarado, J.; González-Sánchez, D.; Lázaro, R.; Mateos, G. Effects of type and particle size of dietary fiber on growth performance and digestive traits of broilers from 1 to 21 days of age. Poult. Sci. 2010, 89, 2197–2212. [Google Scholar] [CrossRef]
- Rezaei, M.; Torshizi, M.K.; Rouzbehan, Y. The influence of different levels of micronized insoluble fiber on broiler performance and litter moisture. Poult. Sci. 2011, 90, 2008–2012. [Google Scholar] [CrossRef]
- Cerisuelo, A.; Marín, C.; Sánchez-Vizcaíno, F.; Gomez, E.; de la Fuente, J.M.; Durán, R.; Fernández, C. The impact of a specific blend of essential oil components and sodium butyrate in feed on growth performance and Salmonella counts in experimentally challenged broilers. Poult. Sci. 2014, 93, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.; Massi, P.; Antongiovanni, M.; Buccioni, A.; Minieri, S.; Marenchino, L.; Mele, M. Efficacy Test of a Hydrolysable Tannin Extract Against Necrotic Enteritis in Challenged Broiler Chickens. Ital. J. Anim. Sci. 2013, 12, e62. [Google Scholar] [CrossRef]
- Gemede, H.F.; Ratta, N.; Gemede, H.F.; Ratta, N. Antinutritional Factors in Plant Foods: Potential Health Benefits and Adverse Effects. Int. J. Nutr. Food Sci. 2014, 3, 284. [Google Scholar] [CrossRef] [Green Version]
- Piluzza, G.; Sulas, L.; Bullitta, S. Tannins in forage plants and their role in animal husbandry and environmental sustainability: A review. Grass Forage Sci. 2013, 69, 32–48. [Google Scholar] [CrossRef]
- Timbermont, L.; Lanckriet, A.; Dewulf, J.; Nollet, N.; Schwarzer, K.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Control of Clostridium perfringens-induced necrotic enteritis in broilers by target-released butyric acid, fatty acids and essential oils. Avian Pathol. 2010, 39, 117–121. [Google Scholar] [CrossRef] [Green Version]

| Poliphenols | Quantity | Effects | References |
|---|---|---|---|
| Hydrolizable tannins | n.a | Bactericidial against Clostridium perfringens | Redondo et al., 2015 [39] |
| Condensed tannins | n.a. | Bacteriostatic against Clostridium perfringens | Redondo et al., 2015 [39] |
| Flavonoids | <0.2 g/kg | Antimicrobial against Salmonella spp. and Escherichia coli | Dos Santos et al., 2020 [40] |
| Andrographolide flavonoid and tannins | 0.3% | Antibacterial against Escherichia coli | Hidanah et al., 2020 [41] |
| Magnolol | 200 mg/kg | Antimicrobial against Escherichia coli | Chen et al., 2020 [42] |
| Flavonoids | 30 g/kg | Antimicrobial against Escherichia coli | Balenović et al., 2018 [43] |
| Thymol and carvacrol | 8% and 4.9% | Antimicrobial | Ramirez et al., 2021 [44] |
| Tannins, flavonoids and phenols | 0.45 g/L | Anticoccidial against Eimeria oocyst antimicrobial | Oyeleke et al., 2021 [45] |
| Polyphenols | 0.263 g/kg; 0.556 g/kg | Antimicrobial against Campylobacter spp. | Branciari et al., 2016 [46] |
| Curcumin, thymol, cinnamaldehyde and carvacrol | 50 mg/kg and 100 mg/kg | Anticoccidial against Eimeria oocyst and antibacterial against Escherichia coli | Galli et al., 2020a [47] |
| Curcumin, resveratrol, yuccaloids | 100 mg/kg and 250 mg/kg | Antimicrobial and anticoccidial against Eimeria | Galli et al., 2020b [48] |
| Curcumin, thymol, cinnamaldehyde and carvacrol | 50 mg/kg and 100 mg/kg | Anticoccidial against Eimeria oocyst and antibacterial against Escherichia coli | Galli et al., 2020a [47] |
| Organic Acid | Quantity | Effects | References |
|---|---|---|---|
| Glycerol-monolaurate | 300 mg/kg | Antimicrobial against Escherichia coli and anticoccidial against Eimeria oocyst | Fortuoso et al., 2019 [70] |
| Short and medium fatty acids | 3 g/kg | Antimicrobial against Salmonella enterica | Aljumaah et al., 2020 [69] |
| Short and medium fatty acids | 0.20% | Antibacterial against Enteroccocus | Dauksiene et al., 2021 [71] |
| Fatty acids produced by wheat bran fermentation | 1% with 280 µm particle size | Antimicrobial against Salmonella | Vermeulen et al., 2017 [72] |
| Long-chain fatty acids by cranberry pomace fermentation | αlinolenic acid 21% and linoleic acid 39.7% | Improvement of immunologic response against infectious bursal disease virus (IBDV) and Newcastle disease virus (NDV) | Islam et al., 2020 [73] |
| Blends of Polyphenols and Organic Acids | Quantity | Effects | References |
|---|---|---|---|
| Chestnut tannin extract and SN1 monoglycerides (a mix of organic acids from C4:0 to C12:0) | 2 g/kg and 1 g/kg; 1 g/kg and 2 g/kg | Antimicrobial against Clostridium perfringens, Salmonella typhymurium, Escherichia coli and Campylobacter jejuni | Mannelli et al., 2019 [67] |
| Glycerol-monolaurate with curcumin and cinnamaldehyde | 297 mg/kg, 276 mg/kg, and 156 mg/kg | Against Eimeria oocysts viability | Galli et al., 2021 [68] |
| Thymol and sodium butyrate | 50 mg/kg and 1 g/kg | Against Salmonella counts | Cerisuelo et al., 2014 [79] |
| Commercial blend | n.a. | Antimicrobial against Campylobacter | Guyard-Nicodème et al., 2016 [54] |
| Citric, fumaric, sorbic, and malic acids | 250 mg/kg, 500 mg/kg, and 1000 mg/kg | Antimicrobial Eimeria and Escherichia coli | Armanini et al., 2021 [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scicutella, F.; Mannelli, F.; Daghio, M.; Viti, C.; Buccioni, A. Polyphenols and Organic Acids as Alternatives to Antimicrobials in Poultry Rearing: A Review. Antibiotics 2021, 10, 1010. https://doi.org/10.3390/antibiotics10081010
Scicutella F, Mannelli F, Daghio M, Viti C, Buccioni A. Polyphenols and Organic Acids as Alternatives to Antimicrobials in Poultry Rearing: A Review. Antibiotics. 2021; 10(8):1010. https://doi.org/10.3390/antibiotics10081010
Chicago/Turabian StyleScicutella, Federica, Federica Mannelli, Matteo Daghio, Carlo Viti, and Arianna Buccioni. 2021. "Polyphenols and Organic Acids as Alternatives to Antimicrobials in Poultry Rearing: A Review" Antibiotics 10, no. 8: 1010. https://doi.org/10.3390/antibiotics10081010
APA StyleScicutella, F., Mannelli, F., Daghio, M., Viti, C., & Buccioni, A. (2021). Polyphenols and Organic Acids as Alternatives to Antimicrobials in Poultry Rearing: A Review. Antibiotics, 10(8), 1010. https://doi.org/10.3390/antibiotics10081010