HPLC-PDA-ESI-MS/MS Profiling and Anti-Biofilm Potential of Eucalyptussideroxylon Flowers
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material and Extraction
2.2. Strains and Culture Conditions
2.3. Anti-Microbial Assay
2.4. Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
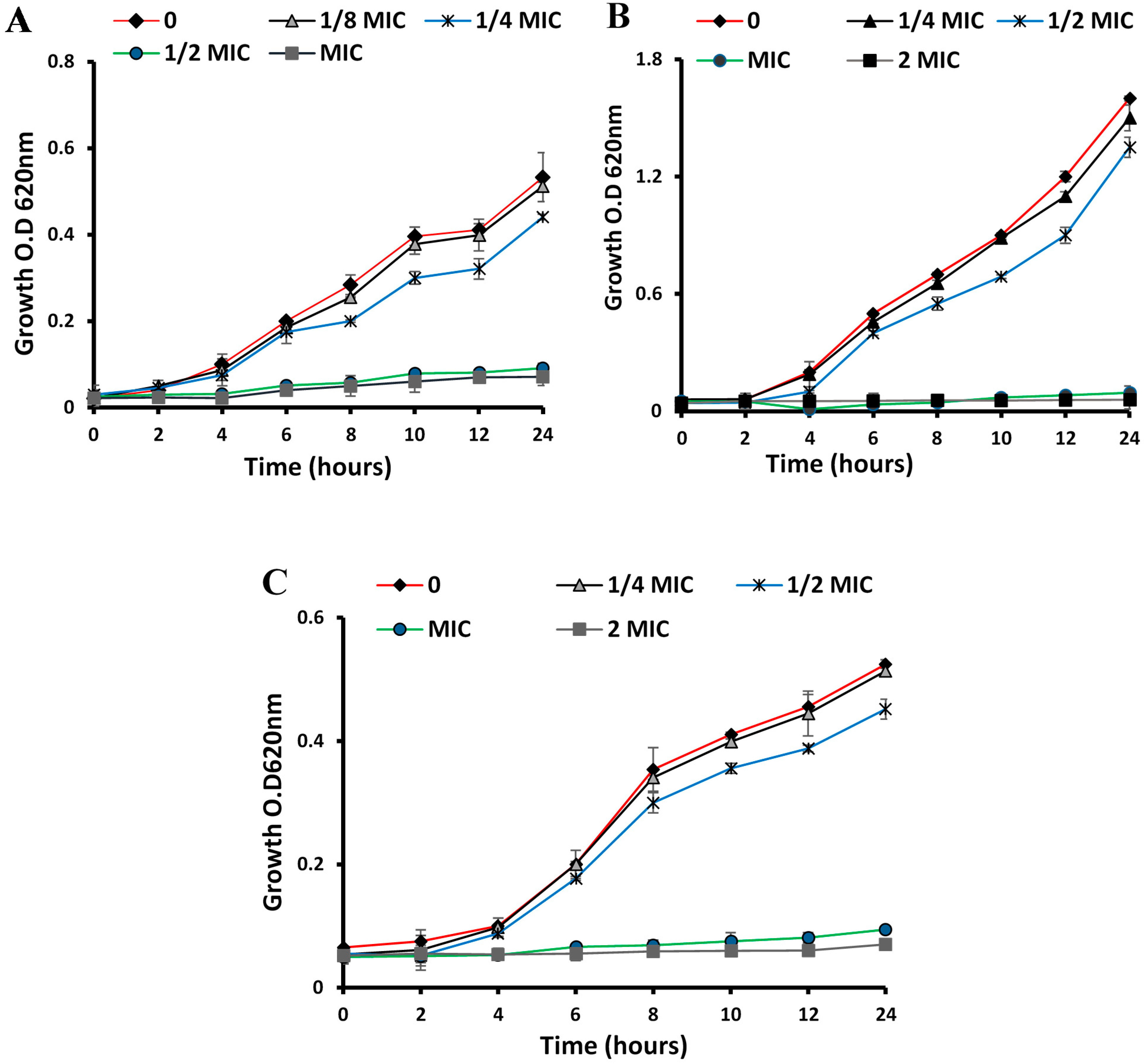
2.5. Time–Kill Curves
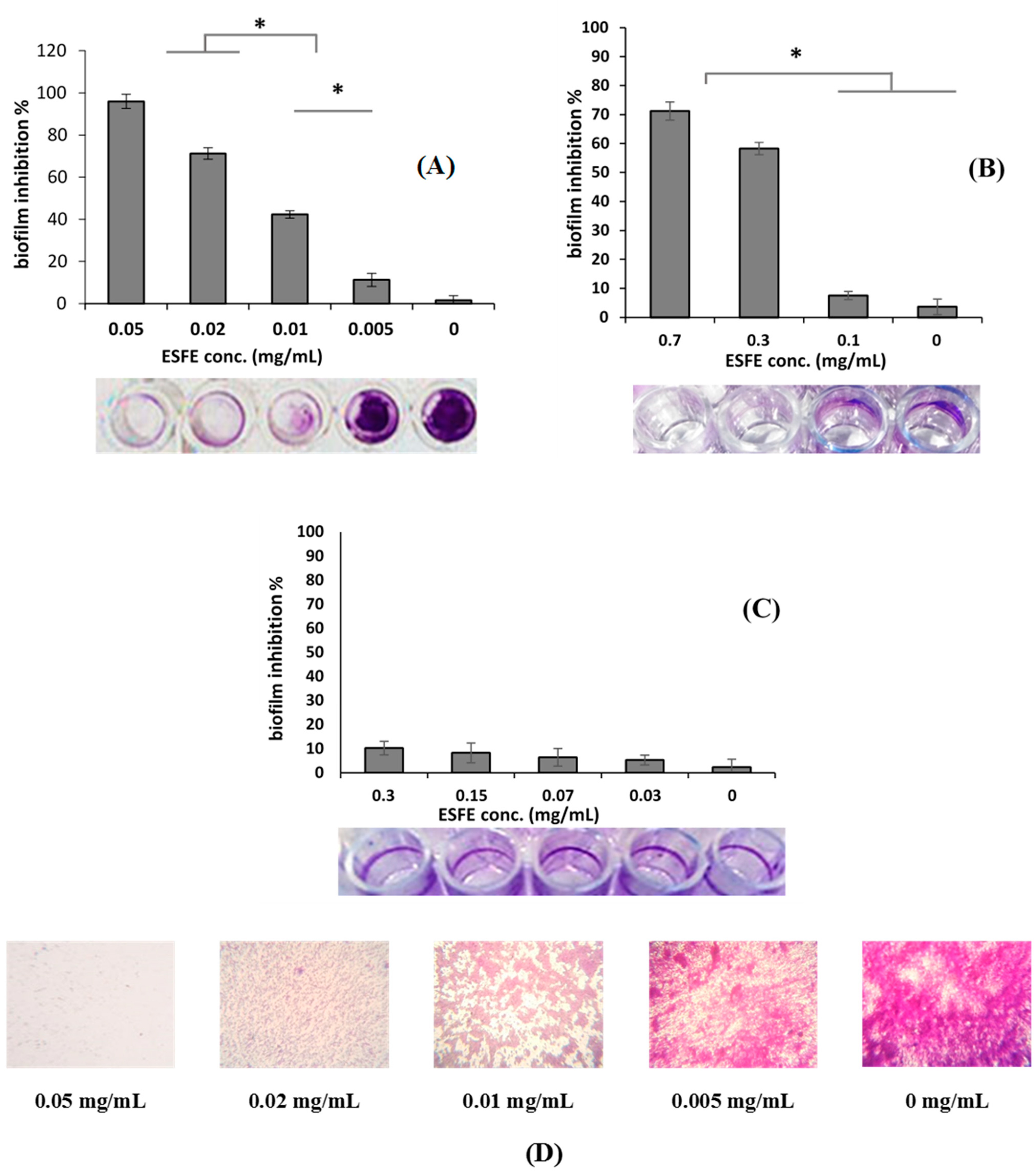
2.6. Biofilm Inhibition Assay and MBIC
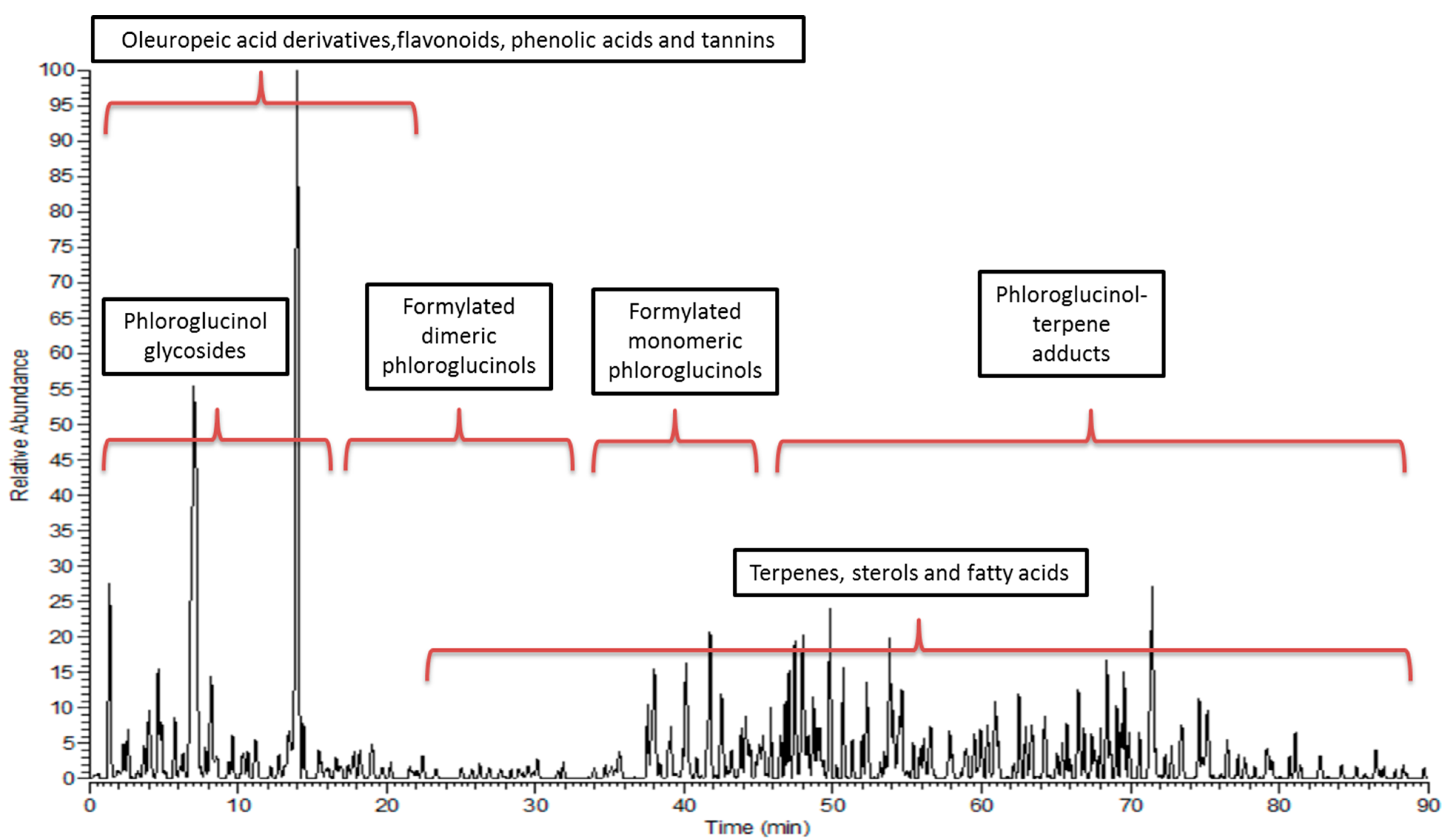
2.7. HPLC-PDA-ESI-MS/MS
2.8. Statistical Analyses
3. Results
3.1. Antibacterial Activities
3.1.1. Anti-Microbial, MIC and MBC of ESFE
3.1.2. Biofilm Inhibition Activity
3.2. Metabolic Profiling
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, F.; Pham, D.T.N.; Oloketuyi, S.F.; Manivasagan, P.; Oh, J.; Kim, Y.-M. Chitosan and Their Derivatives: Antibiofilm Drugs Against Pathogenic Bacteria. Colloids Surf. B Biointerfaces 2020, 185, 110627. [Google Scholar] [CrossRef]
- Desouky, S.E.; El-Gamal, M.S.; Mohammed, A.F.; Abu-Elghait, M.A. Determination of Some Virulence Factors in Staphylococus Spp. Isolated from Clinical Samples of Different Egyptian Patients. World Appl. Sci. J. 2014, 32, 731–740. [Google Scholar]
- Otto, M. Staphylococcal Biofilms. Microbiol. Spectr. 2019, 6, 699–711. [Google Scholar]
- Kaur, S.; Sharma, N.; Aanchal, A.G.; Sharma, A.; Sharma, A.; Sharma, V. Anti-biofilm Potential of Aqueous Eucalyptus Leaf Extract against Nosocomial Pathogens: Staphylococcus and Pseudomonas Aeruginosa. Pharm. Innov. J. 2018, 7, 425–432. [Google Scholar]
- Lu, L.; Hu, W.; Tian, Z.; Yuan, D.; Yi, G.; Zhou, Y.; Cheng, Q.; Zhu, J.; Li, M. Developing Natural Products as Potential Anti-Biofilm Agents. Chin. Med. 2019, 14, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Barriuso, J. Quorum Sensing Mechanisms in Fungi. AIMS Microbiol. 2015, 1, 37–47. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Ashour, R.M.; Okba, M.M.; Saber, F.R. Callistemon Citrinus Bioactive Metabolites as New Inhibitors of Methicillin-Resistant Staphylococcus Aureus Biofilm Formation. J. Ethnopharmacol. 2020, 254, 112669. [Google Scholar] [CrossRef]
- Mostafa, I.; Abbas, H.A.; Ashour, M.L.; Yasri, A.; El-Shazly, A.M.; Wink, M.; Sobeh, M. Polyphenols from Salix Tetrasperma Impair Virulence and Inhibit Quorum Sensing of Pseudomonas Aeruginosa. Molecules 2020, 25, 1341. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, G.A.; Ibrahim, S.R. Eucalyptone G, a New Phloroglucinol Derivative and Other Constituents from Eucalyptus Globulus Labill. Arkivoc 2007, 15, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Bhuyan, D.J.; Vuong, Q.V.; Chalmers, A.C.; van Altena, I.A.; Bowyer, M.C.; Scarlett, C.J. Phytochemical, Antibacterial and Antifungal Properties of an Aqueous Extract of Eucalyptus Microcorys Leaves. S. Afr. J. Bot. 2017, 112, 180–185. [Google Scholar] [CrossRef]
- Bailey, L.H. Manual of Cultivated Plants; The Macmillan Company: New York, NY, USA, 1958. [Google Scholar]
- Umberto Quattrocchi, F.L.S. World Dictionary of Plant Names; Harvard University and Harvard University Herbaria: Cambridge, MA, USA, 1999; Volume 2. [Google Scholar]
- Ashour, H.M. Antibacterial, Antifungal, and Anticancer Activities of Volatile Oils and Extracts from Stems, Leaves, and Flowers of Eucalyptus Sideroxylon and Eucalyptus Torquata. Cancer Biol. Ther. 2008, 7, 399–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elaissi, A.; Salah, K.H.; Mabrouk, S.; Larbi, K.M.; Chemli, R.; Harzallah-Skhiri, F. Antibacterial Activity and Chemical Composition of 20 Eucalyptus Species’ Essential Oils. Food Chem. 2011, 129, 1427–1434. [Google Scholar] [CrossRef]
- Okba, M.M.; El Gedaily, R.A.; Ashour, R.M. UPLC–PDA–ESI–qTOF-MS Profiling and Potent Anti-HSV-II Activity of Eucalyptus Sideroxylon Leaves. J. Chromatogr. B 2017, 1068, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Ashour, R.M.; Okba, M.M.; Menze, E.T.; El Gedaily, R.A. Eucalyptus Sideroxylon Bark Anti-inflammatory Potential, Its UPLC-PDA-ESI-qTOF-MS Profiling, and Isolation of a New Phloroglucinol. J. Chromatogr. Sci. 2019, 57, 565–574. [Google Scholar] [CrossRef]
- Santos, B.M.d.; Zibrandtsen, J.F.; Gunbilig, D.; Sørensen, M.; Cozzi, F.; Boughton, B.A.; Heskes, A.M.; Neilson, E.H.J. Quantification and Localization of Formylated Phloroglucinol Compounds (Fpcs) in Eucalyptus Species. Front. Plant Sci. 2019, 10, 186. [Google Scholar] [CrossRef]
- Hamed, A.; Abdel-Razek, A.S.; Araby, M.; Abu-Elghait, M.; El-Hosari, D.G.; Frese, M.; Soliman, H.S.; Stammler, H.G.; Sewald, N.; Shaaban, M. Meleagrin from Marine Fungus Emericella Dentata Nq45: Crystal Structure and Diverse Biological Activity Studies. Nat. Prod. Res. 2020, 1–9. [Google Scholar] [CrossRef]
- Stankov, S.; Fidan, H.; Stefanova, G.; Kostova, I.; Damyanova, S.; Dimitrova-Dyulgerova, I.; Ercisli, S.; Stoyanova, A. Chemical Composition and Antimicrobial Activity of Essential Oil from Aerial Part (Leaves and Fruit) of Eucalyptus gomphocephala DC. J. Essent. Oil Plants 2020, 23, 1–9. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; CLSI document M100-S20; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2011. [Google Scholar]
- Abdelhameed, R.M.; Abu-Elghait, M.; El-Shahat, M. Hybrid Three Mofs Composites (ZIF-67@ ZIF-8@ MIL-125-NH2): Enhancement the Biological and Visible-Light Photocatalytic Activity. J. Environ. Chem. Eng. 2020, 8, 104107. [Google Scholar] [CrossRef]
- Qian, W.; Liu, M.; Fu, Y.; Zhang, J.; Liu, W.; Li, J.; Li, X.; Li, Y.; Wang, T. Antimicrobial Mechanism of Luteolin against Staphylococcus Aureus and Listeria Monocytogenes and Its Antibiofilm Properties. Microb. Pathog. 2020, 142, 104056. [Google Scholar] [CrossRef] [PubMed]
- Desouky, S.E.; Hassan, S.E.; El-gamal, M.S.; Ragab, M.A.E.T.I.; Emam, M. Effect of Salvia Egypticae and Foeniculum Vulgara Extracts on Quorum Sensing and Biofilm Formation of Methicillin Resistant/Sensitive Staphylococcus Aureus Isolates. World J. Pharm. Med. Res. 2017, 3, 466–475. [Google Scholar]
- Černohorská, L.; Votava, M. Antibiotic Synergy against Biofilm-Forming Pseudomonas Aeruginosa. Folia Microbiol. 2008, 53, 57. [Google Scholar] [CrossRef]
- Sobeh, M.; Mahmoud, M.F.; Sabry, O.M.; Adel, R.; Dmirieh, M.; El-Shazly, A.M.; Wink, M. HPLC-PDA-MS/MS Characterization of Bioactive Secondary Metabolites from Turraea Fischeri Bark Extract and Its Antioxidant and Hepatoprotective Activities in Vivo. Molecules 2017, 22, 2089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Hawary, S.S.; Mubarek, M.M.; Lotfy, R.A.; Hassan, A.R.; Sobeh, M.; Okba, M.M. Validation of Antidiabetic Potential of Gymnocarpos decandrus Forssk. Nat. Prod. Res. 2020, 1–6. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Sobeh, M.; Badr, W.K.; Abdelfattah, M.A.; Ali, Z.Y.; El-Tantawy, M.E.; Rabeh, M.A.; Wink, M. HPLC-PDA-MS/MS Profiling of Secondary Metabolites from Opuntia Ficus-Indica Cladode, Peel and Fruit Pulp Extracts and Their Antioxidant, Neuroprotective Effect in Rats with Aluminum Chloride Induced Neurotoxicity. Saudi J. Biol. Sci. 2020, 27, 2829–2838. [Google Scholar] [CrossRef]
- Singh, I.P.; Sidana, J.; Bharate, S.B.; Foley, W.J. Phloroglucinol Compounds of Natural Origin: Synthetic Aspects. Nat. Prod. Rep. 2010, 27, 393–416. [Google Scholar] [CrossRef]
- Qin, X.-J.; Feng, M.-Y.; Liu, H.; Ni, W.; Rauwolf, T.; Porco, J.A., Jr.; Yan, H.; He, L.; Liu, H.-Y. Eucalyptusdimers A–C, Dimeric Phloroglucinol–Phellandrene Meroterpenoids from Eucalyptus robusta. Org. Lett. 2018, 20, 5066–5070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojas-Garbanzo, C.; Zimmermann, B.F.; Schulze-Kaysers, N.; Schieber, A. Characterization of Phenolic and Other Polar Compounds in Peel and Flesh of Pink Guava (Psidium Guajava L. Cv.‘Criolla’) by Ultra-High Performance Liquid Chromatography with Diode Array and Mass Spectrometric Detection. Food Res. Int. 2017, 100, 445–453. [Google Scholar] [CrossRef]
- Brezáni, V.; Šmejkal, K. Secondary Metabolites Isolated from the Genus Eucalyptus. Curr. Trends Med. Chem. 2013, 7, 65–75. [Google Scholar]
- Gurbuz, P.; Baran, M.Y.; Demirezer, L.O.; Guvenalp, Z.; Kuruuzum-Uz, A. Phenylacylated-Flavonoids from Peucedanum chryseum. Rev. Bras. Farmacogn. 2018, 28, 228–230. [Google Scholar] [CrossRef]
- Yamakoshi, Y.; Murata, M.; Shimizu, A.; Homma, S. Isolation and Characterization of Macrocarpals BG Antibacterial Compounds from Eucalyptus Macrocarpa. Biosci. Biotechnol. Biochem. 1992, 56, 1570–1576. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, T.; Takano, F.; Takata, T.; Niiyama, M.; Ohta, T. Bioactive Monoterpene Glycosides Conjugated with Gallic Acid from the Leaves of Eucalyptus Globulus. Phytochemistry 2008, 69, 747–753. [Google Scholar] [CrossRef]
- Ito, H.; Koreishi, M.; Tokuda, H.; Nishino, H.; Yoshida, T. Cypellocarpins A−C, Phenol Glycosides Esterified with Oleuropeic Acid, from Eucalyptus Cypellocarpa. J. Nat. Prod. 2000, 63, 1253–1257. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, H.; Xiao, W.; Yong, Z.-P.; Bai, N. High-Performance Liquid Chromatographic Fingerprint Analysis for Different Origins of Sea Buckthorn Berries. J. Chromatogr. A 2007, 1154, 250–259. [Google Scholar] [CrossRef] [PubMed]
- González-Burgos, E.; Liaudanskas, M.; Viškelis, J.; Žvikas, V.; Janulis, V.; Gómez-Serranillos, M.P. Antioxidant Activity, Neuroprotective Properties and Bioactive Constituents Analysis of Varying Polarity Extracts from Eucalyptus Globulus Leaves. J. Food Drug Anal. 2018, 26, 1293–1302. [Google Scholar] [CrossRef]
- Al-Sayed, E.; Martiskainen, O.; Bobrowska-Hägerstrand, M.; Sinkkonen, J.; Törnquist, K.; Pihlaja, K.; Ayoub, N.; Singab, A.-N. Phenolic Compounds from Eucalyptus Gomphocephala with Potential Cytotoxic and Antioxidant Activities. Nat. Prod. Commun. 2010, 5, 1934578X1000501025. [Google Scholar] [CrossRef] [Green Version]
- Saraf, I.; Marsh, K.J.; Vir, S.; Foley, W.J.; Singh, I.P. Quantitative Analysis of Various B-ring Unsubstituted and Substituted Flavonoids in Ten Australian Species of Eucalyptus. Nat. Prod. Commun. 2017, 12, 1934578X1701201109. [Google Scholar] [CrossRef] [Green Version]
- Wollenweber, E.; Kohorst, G. Epicuticular Leaf Flavonoids from Eucalyptus Species and from Kalmia Latifolia. Zeitschrift Nat. C 1981, 36, 913–915. [Google Scholar] [CrossRef]
- Ghareeb, M.A.; Sobeh, M.; El-Maadawy, W.H.; Mohammed, H.S.; Khalil, H.; Botros, S.; Wink, M. Chemical Profiling of Polyphenolics in Eucalyptus Globulus and Evaluation of Its Hepato–Renal Protective Potential against Cyclophosphamide Induced Toxicity in Mice. Antioxidants 2019, 8, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC–DAD–ESI-MS/MS Screening of Bioactive Components from Rhus Coriaria L. (Sumac) Fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.-W.; Guo, Q.-M. Studies on Chemical Constituents in Fruits of Eucalyptus Globulus. China J. Chin. Mater. Med. 2007, 32, 496–500. [Google Scholar]
- Jamal, A.; Yaacob, W.; Din, L.B. A Chemical Study on Phyllanthus Reticulatus. J. Phys. Sci. 2008, 19, 45–50. [Google Scholar]
- Moore, B.D.; Wallis, I.R.; Palá-Paúl, J.; Brophy, J.J.; Willis, R.H.; Foley, W.J. Antiherbivore Chemistry of Eucalyptus—Cues and Deterrents for Marsupial Folivores. J. Chem. Ecol. 2004, 30, 1743–1769. [Google Scholar] [CrossRef] [PubMed]
- Eyles, A.; Davies, N.W.; Mohammed, C. Novel Detection of Formylated Phloroglucinol Compounds (Fpcs) in the Wound Wood of Eucalyptus Globulus and E. Nitens. J. Chem. Ecol. 2003, 29, 881–898. [Google Scholar] [CrossRef] [PubMed]
- Ablajan, K.; Abliz, Z.; Shang, X.Y.; He, J.M.; Zhang, R.P.; Shi, J.G. Structural Characterization of Flavonol 3, 7-Di-O-Glycosides and Determination of the Glycosylation Position by Using Negative Ion Electrospray Ionization Tandem Mass Spectrometry. J. Mass Spectrom. 2006, 41, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.-W.; Zhang, Y.-J.; Wang, Y.-F.; Lai, C.-C.; Yang, C.-R. Eucalmaidins A−E, (+)-Oleuropeic Acid Derivatives from the Fresh Leaves of Eucalyptus Maideni. J. Nat. Prod. 2009, 72, 1608–1611. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.D.; Brodbelt, J.S. Determination of the Glycosylation Site of Flavonoid Monoglucosides by Metal Complexation and Tandem Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2004, 15, 1287–1299. [Google Scholar] [CrossRef] [Green Version]
- Fathoni, A.; Saepudin, E.; Cahyana, A.; Rahayu, D.; Haib, J. Identification of Nonvolatile Compounds in Clove (Syzygium aromaticum) from Manado. AIP Conference Proceedings. 2017. Available online: https://aip.scitation.org/doi/abs/10.1063/1.4991183 (accessed on 18 June 2021).
- Tsiri, D.; Aligiannis, N.; Graikou, K.; Spyropoulos, C.; Chinou, I. Triterpenoids from Eucalyptus Camaldulensis Dehnh. Tissue Cultures. Helv. Chim. Acta 2008, 91, 2110–2114. [Google Scholar] [CrossRef]
- Wheelan, P.; Zirrolli, J.A.; Murphy, R.C. Low-Energy Fast Atom Bombardment Tandem Mass Spectrometry of Monohydroxy Substituted Unsaturated Fatty Acids. Biol. Mass Spectrom. 1993, 22, 465–473. [Google Scholar] [CrossRef]
- Kerwin, J.L.; Wiens, A.M.; Ericsson, L.H. Identification of Fatty Acids by Electrospray Mass Spectrometry and Tandem Mass Spectrometry. J. Mass Spectrom. 1996, 31, 184–192. [Google Scholar] [CrossRef]
- Scott, E.N.; Gescher, A.J.; Steward, W.P.; Brown, K. Development of Dietary Phytochemical Chemopreventive Agents: Biomarkers and Choice of Dose for Early Clinical Trials. Cancer Prev. Res. 2009, 2, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Mulyaningsih, S.; Sporer, F.; Reichling, J.; Wink, M. Antibacterial Activity of Essential Oils from Eucalyptus and of Selected Components against Multidrug-Resistant Bacterial Pathogens. Pharm. Biol. 2011, 49, 893–899. [Google Scholar] [CrossRef]
- Ghalem, B.R.; Mohamed, B. Antibacterial Activity of Leaf Essential Oils of Eucalyptus Globulus and Eucalyptus Camaldulensis. Afr. J. Pharm. Pharmacol. 2008, 2, 211–215. [Google Scholar]
- Barbosa, J.P.; de Oliveira, T.R.; Puppin, D.d.G.P.B.; Teixeira, A.L.; Boni, G.C.; de Feiria, S.N.B.; Höfling, J.F. Anti-Candida Activity of Essential Oils from Eucalyptus Species. A Preliminary Study. Oral Health 2018, 8, 1–5. [Google Scholar]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus Aureus Clinical Strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vikram, A.; Jayaprakasha, G.K.; Jesudhasan, P.; Pillai, S.; Patil, B. Suppression of Bacterial Cell–Cell Signalling, Biofilm Formation and Type Iii Secretion System by Citrus Flavonoids. J. Appl. Microbiol. 2010, 109, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Sarkisian, S.A.; Janssen, M.; Matta, H.; Henry, G.; LaPlante, K.L.; Rowley, D.C. Inhibition of Bacterial Growth and Biofilm Production by Constituents from Hypericum spp. Phytother. Res. 2012, 26, 1012–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monte, J.; Abreu, A.C.; Borges, A.; Simões, L.C.; Simões, M. Antimicrobial Activity of Selected Phytochemicals against Escherichia Coli and Staphylococcus Aureus and Their Biofilms. Pathogens 2014, 3, 473–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.-G.; Lee, J.-H.; Gwon, G.; Kim, S.-I.; Park, J.G.; Lee, J. Essential Oils and Eugenols Inhibit Biofilm Formation and the Virulence of Escherichia Coli O157: H7. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Shou, Q.; Smith, J.E.; Mon, H.; Brkljača, Z.; Smith, A.-S.; Smith, D.M.; Griesser, H.J.; Wohlmuth, H. Rhodomyrtals A–D, Four Unusual Phloroglucinol-Sesquiterpene Adducts from Rhodomyrtus Psidioides. RSC Adv. 2014, 4, 13514–13517. [Google Scholar] [CrossRef] [Green Version]
- Hou, A.; Liu, Y.; Lin, Z.W.; Sun, H. Eucaglobulin, a New Complex of Gallotannin and Monoterpene from Eucalyptus Globulus. Chin. Chem. Lett. 1998, 9, 541–543. [Google Scholar]
- Hou, A.-J.; Liu, Y.-Z.; Yang, H.; Lin, Z.-W.; Sun, H.-D. Hydrolyzable Tannins and Related Polyphenols from Eucalyptus Globulus. J. Asian Nat. Prod. Res. 2000, 2, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Boulekbache-Makhlouf, L.; Meudec, E.; Chibane, M.; Mazauric, J.-P.; Slimani, S.; Henry, M.; Cheynier, V.; Madani, K. Analysis by High-Performance Liquid Chromatography Diode Array Detection Mass Spectrometry of Phenolic Compounds in Fruit of Eucalyptus Globulus Cultivated in Algeria. J. Agric. Food Chem. 2010, 58, 12615–12624. [Google Scholar] [CrossRef]
- Miranda, I.; Lima, L.; Quilhó, T.; Knapic, S.; Pereira, H. The Bark of Eucalyptus Sideroxylon as a Source of Phenolic Extracts with Anti-Oxidant Properties. Ind. Crop. Prod. 2016, 82, 81–87. [Google Scholar] [CrossRef]
- Luís, Â.; Silva, F.; Sousa, S.; Duarte, A.P.; Domingues, F. Antistaphylococcal and Biofilm Inhibitory Activities of Gallic, Caffeic, and Chlorogenic Acids. Biofouling 2014, 30, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Bakkiyaraj, D.; Nandhini, J.R.; Malathy, B.; Pandian, S.K. The Anti-Biofilm Potential of Pomegranate (Punica Granatum L.) Extract Against Human Bacterial and Fungal Pathogens. Biofouling 2013, 29, 929–937. [Google Scholar] [CrossRef]
- Quave, C.L.; Estévez-Carmona, M.; Compadre, C.M.; Hobby, G.; Hendrickson, H.; Beenken, K.E.; Smeltzer, M.S. Ellagic Acid Derivatives from Rubus Ulmifolius Inhibit Staphylococcus Aureus Biofilm Formation and Improve Response to Antibiotics. PLoS ONE 2012, 7, e28737. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.-S.; Oh, J.-S.; Kang, I.-C.; Hong, S.-J.; Choi, C.-H. Inhibitory Effect of Methyl Gallate and Gallic Acid on Oral Bacteria. J. Microbiol. 2008, 46, 744–750. [Google Scholar] [CrossRef]
- Shao, D.; Li, J.; Li, J.; Tang, R.; Liu, L.; Shi, J.; Huang, Q.; Yang, H. Inhibition of Gallic Acid on the Growth and Biofilm Formation of Escherichia Coli and Streptococcus Mutans. J. Food Sci. 2015, 80, M1299–M1305. [Google Scholar] [CrossRef]
- Hancock, V.; Dahl, M.; Vejborg, R.M.; Klemm, P. Dietary Plant Components Ellagic Acid and Tannic Acid Inhibit Escherichia Coli Biofilm Formation. J. Med. Microbiol. 2010, 59, 496–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Strain | ESFE (mg/mL) | Gentamicin (10 µg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 20 | 10 | 5 | 2.5 | 1.25 | 0.6 | 0.12 | |||
| Gram positive | MSSA | +++ | +++ | +++ | +++ | ++ | + | − | +++ |
| MRSA | +++ | +++ | +++ | +++ | ++ | + | − | +++ | |
| B. subtilis | +++ | +++ | +++ | ++ | ++ | + | − | +++ | |
| Gram negative | E. coli | +++ | ++ | ++ | + | − | − | − | +++ |
| P. aeruginosa | +++ | +++ | ++ | ++ | − | − | − | +++ | |
| Yeast | C. albicans | ++ | ++ | + | − | − | − | − | ++ |
| Strain | ESFE (mg/mL) | ||
|---|---|---|---|
| MIC | MBC | ||
| Gram positive | MSSA | 0.5 | 1.0 |
| MRSA | 0.5 | 1.0 | |
| B. subtilis | 1.2 | 2.5 | |
| Gram negative | E. coli | 1.2 | 2.5 |
| P. aeruginosa | 1.2 | 2.5 | |
| Yeast | C. albicans | 3 | 6 |
| No. | Identification | Rt (min) | [M−H]− | Main Fragments | Ref. |
|---|---|---|---|---|---|
| Phloroglucinol | |||||
| Formylated monomeric phloroglucinols | |||||
| 1 | Jensenone | 27.98 | 265 | 249, 193, 165, 149 | |
| 2 | Grandinol | 37.98 | 251 | 236, 167 | [28] |
| 3 | Homograndinol | 40.73 | 265 | 250, 207 | |
| Formylated dimeric phloroglucinols | |||||
| 4 | Dehydro-eucalyptusdimer C | 7.72, 10.88 | 725 | 563, 441, 423, 361, 207 | [29] |
| 5 | Eucalyptusdimer A/B | 10.28 | 713 | 609, 503, 489, 457, 207 | [29] |
| 6 | Sideroxylonal A/B/C | 4.66, 8.41, 10.96 | 499 | 471, 453, 423, 207, 165 | [15] |
| 7 | Loxophlebal A | 8.97 | 471 | 281, 249, 207 | [28] |
| 8 | Eucalyprobusone A | 27.96 | 459 | 319, 251, 249, 209, 181 | [29] |
| Phloroglucinol glycosides | |||||
| 9 | Myrciaphenone B | 1.47 | 481 | 331, 319, 301, 183, 163 | [30] |
| 10 | Eucalmainoside A | 6.30 | 301 | 257, 229, 183, 177, 169 | [31] |
| 11 | Eucalmainoside C/Myrciaphenone A | 15.35 | 329 | 229, 183, 171, 169, 167 | [32] |
| 12 | Eucalmainoside B | 19.61 | 315 | 301, 249, 183, 169, 151 | [31] |
| Phloroglucinol-terpene adducts (phloroglucinol meroterpenoids) | |||||
| 13 | Macrocarpal E/Eucalyptone/Eucalyptals B/E | 44.71, 63.04 | 485 | 471, 409, 439, 373, 207 | [15] |
| 14 | Macrocarpal J/I | 39.69 | 489 | 471, 324, 249, 207 | |
| 15 | Eucalrobusone R/O | 35.84 | 469 | 423, 249, 207 | |
| 16 | (Iso)leptospermone | 43.56 | 265 | 250, 207, 112 | |
| 17 | Macrocarpal A/B/D/K/H/L-Eucalyptin A/B | 45.30, 47.87 | 471 | 469, 453, 385, 249, 207 | |
| 18 | Eucalyptal A/C/Eucalrobusone D | 49.55 | 467 | 453, 249, 207 | |
| 19 | Eucalyptone G | 58.44 | 675 | 453, 397, 250, 207 | [9] |
| 20 | Macrocarpal C/G | 61.95, 62.99 | 453 | 428, 407, 250, 207, 165 | [33] |
| 21 | Euglobals G1-G12/R | 63.39, 63.79 | 385 | 249, 207 | [15] |
| Oleuropeic acid derivatives | |||||
| 22 | Galloylglucose | 2.01 | 331 | 331, 313, 169 | [34] |
| 23 | Globulusin A | 4.08 | 483 | 313, 353, 183, 169, 151 | |
| 24 | Cypellocarpin B | 22.66 | 537 | 453, 385, 209, 183, 191 | [35] |
| 25 | Cypellocarpin C (Camaldulenside) | 24.67 | 519 | 353, 335, 245, 205, 183 | |
| 26 | Eucalmaidin D/Cypellogin A/B | 17.78, 23.54 | 629 | 519, 469, 463, 301, 183 | [15] |
| 27 | Globulusin B/Eucaglobulin/Cypellocarpin A | 28.27 | 497 | 437, 331, 313, 183, 169 | [34] |
| 28 | Dihydrocypellocarpine C | 29.10 | 521 | 489,441, 353, 279, 160 | [35] |
| Flavonoids and flavonoid glycosides | |||||
| 29 | Quercetin O-sophoroside | 7.97 | 625 | 463, 301, 271, 151 | [36] |
| 30 | Quercetin rutinoside (Rutin) | 10.28 | 609 | 463, 301, 271 | [31] |
| 31 | Quercetin O-arabinopyranoside-gallate | 11.92 | 585 | 301, 269 | [37] |
| 32 | Hydroxytetramethoxy-flavone-O-glucopyranoside | 12.05 | 519 | 447, 353, 335, 205 | [15] |
| 33 | Isorhamnetin O-rutinoside (Narcissin) | 12.50 | 623 | 315, 300, 285, 271, 255 | [37] |
| 34 | Quercetin O-glucopyranoside-gallate | 13.55 | 615 | 301, 271 | [38] |
| 35 | Luteolin O-rutinoside (Scolymoside) | 16.80 | 593 | 429, 285 | [37] |
| 36 | Quercetin O-arabinofuranoside/Quercetin O-arabinopyranoside | 19.61, 24.67 | 433 | 301, 271 | |
| 37 | Homoorientin (Isoorientin) | 20.95 | 447 | 315 | |
| 38 | Quercetin O-glucopyranoside | 22.24 | 463 | 301, 271, 151 | |
| 39 | Quercetin O-rhamnoside | 22.34 | 447 | 301, 271 | |
| 40 | Kaempherol O-glucopyranoside/Luteolin O- glucopyranoside | 25.59 | 447 | 285, 255 | |
| 41 | Trimethoxykaempferol | 26.53 | 327 | 309, 283, 255 | [9] |
| 42 | Isorhamnetin | 27.55 | 315 | 300, 285, 151, 107 | [37] |
| 43 | Desmethyl eucalyptin | 31.92 | 311 | 297, 293, 267, 249 | [39] |
| 44 | Sideroxylin | 38.17 | 311 | 296, 249, 207 | [40] |
| Phenolic acids | |||||
| 45 | Gallic acid | 2.01 | 169 | 169, 125 | [28] |
| 46 | Chlorogenic/Neochlorogenic acid | 2.21 2.25 | 353 | 233, 191 | [37] |
| 47 | Ferulic acid | 28.13 | 193 | 165 | |
| Gallic acid derivatives | |||||
| 48 | O-galloyl-O-HHDP-glucose | 1.54 | 633 | 463, 301, 275, 169 | [41] |
| 49 | Tri-O-galloylglucose | 2.45 | 635 | 483, 477, 465, 169 | [41] |
| 50 | Epicatechin gallate | 3.21 | 441 | 271, 169 | [37] |
| 51 | Tellimagradin I | 3.35 | 785 | 634, 617, 301, 169 | [28] |
| 52 | Coumaroyl-digalloylhexoside | 5.07 | 629 | 463, 459, 313, 169 | [42] |
| 53 | Sinapaldehyde | 14.32 | 207 | 179, 161 | [28] |
| Ellagic acid derivatives | |||||
| 54 | Ellagic acid | 2.17 | 301 | 273, 257, 229 | [37] |
| 55 | Methylellagic acid acetyl hexoside | 4.53 | 503 | 373, 315, 313, 183 | [43] |
| 56 | Ellagic acid deoxyhexoside | 5.00 | 447 | 315, 301, 261, 185 | [16] |
| 57 | Dimethylellagic acid hexoside | 1.51 | 475 | 327, 301 | [28] |
| 58 | Methylellagic acid | 5.86 | 315 | 300, 269, 180 | [16] |
| 59 | Dimethyl ellagic acid | 11.42 | 329 | 315, 163 | |
| 60 | Trimethyl ellagic acid | 11.74 | 343 | 328, 315, 249 | |
| Terpenes | |||||
| 61 | Hydroxy-O-acetylhydroshengmanol-O-xylopyranoside | 38.11 | 695 | 649, 533, 520, 225 | [15] |
| 62 | Asiatic acid lactone | 39.88 | 485 | 403, 433, 251, 207 | |
| 63 | Betulin | 40.14, 42.48, 49 | 443 | 443, 399, 165 | |
| 64 | Hydroxy ursolic/betulinic acid | 45.30, 47.93, 59.77 | 471 | 453, 427, 380 | |
| 65 | Euscaphic/asiatic/arjunolic acid | 45.56 | 487 | 469, 453, 423, 207 | |
| 66 | Trihydroxy-oxoursenoic acid | 46.63 | 473 | 454, 375, 311 | |
| 67 | Nor-ursene-diol | 47.20 | 427 | 301, 297, 207 | |
| 68 | Acetyl ursolic/Acetyl oleanolic acid/Acetobetulinic acid | 48.33 | 497 | 485, 249, 207 | |
| 69 | O-Coumaroyl maslinic/Alhitolic acid | 48.40, 48.53, 49.22 | 617 | 574, 471, 455, 453, 249 | |
| 70 | Eucalyptic (eucalyptolic) acid | 49.27 | 647 | 632, 617, 497, 485, 397 | |
| 71 | Lupeol acetate | 57.17 | 467 | 439, 249, 209 | [44] |
| 72 | Eucalyptanoic acid | 57.62 | 453 | 249, 207 | [15] |
| 73 | Bryocoumaric acid | 61.91 | 599 | 555, 469, 437, 385, 249 | |
| 74 | O-coumaroyl tormentic acid | 62.04 | 633 | 471, 453, 207 | |
| 75 | Ursolic/Oleanolic/betulinic acid | 62.78, 65.38 | 455 | 398, 251, 249, 207 | |
| 76 | 4-Methoxycinnamoyloleanolic acid methyl ester | 63.51 | 629 | 614, 585, 485, 249 | |
| 77 | Ursolic acid lactone | 63.55 | 453 | 325 | |
| 78 | Nor triterpene | 64.45 | 453 | 385, 249 | |
| Fatty acids | |||||
| 79 | Trihydroxy octadecenoic acid | 50.23, 67.72 | 329 | 311, 293, 275, 229 | [15] |
| 80 | Hydroxy tetracosanoic acid | 50.27, 57.44 | 383 | 363, 326, 309, 272 | |
| 81 | Hydroxy octadecadienoic acid | 82.44 | 295 | 277, 171 | |
| Miscellaneous | |||||
| 82 | Vomifoliol | 25.61 | 223 | 208, 139 | [28] |
| 83 | Withanolide A | 65.63 | 469 | 425, 249, 205 | [15] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okba, M.M.; El-Shiekh, R.A.; Abu-Elghait, M.; Sobeh, M.; Ashour, R.M.S. HPLC-PDA-ESI-MS/MS Profiling and Anti-Biofilm Potential of Eucalyptussideroxylon Flowers. Antibiotics 2021, 10, 761. https://doi.org/10.3390/antibiotics10070761
Okba MM, El-Shiekh RA, Abu-Elghait M, Sobeh M, Ashour RMS. HPLC-PDA-ESI-MS/MS Profiling and Anti-Biofilm Potential of Eucalyptussideroxylon Flowers. Antibiotics. 2021; 10(7):761. https://doi.org/10.3390/antibiotics10070761
Chicago/Turabian StyleOkba, Mona M., Riham A. El-Shiekh, Mohammed Abu-Elghait, Mansour Sobeh, and Rehab M. S. Ashour. 2021. "HPLC-PDA-ESI-MS/MS Profiling and Anti-Biofilm Potential of Eucalyptussideroxylon Flowers" Antibiotics 10, no. 7: 761. https://doi.org/10.3390/antibiotics10070761
APA StyleOkba, M. M., El-Shiekh, R. A., Abu-Elghait, M., Sobeh, M., & Ashour, R. M. S. (2021). HPLC-PDA-ESI-MS/MS Profiling and Anti-Biofilm Potential of Eucalyptussideroxylon Flowers. Antibiotics, 10(7), 761. https://doi.org/10.3390/antibiotics10070761






