A New Nanocomposite Packaging Based on LASiS-Generated AgNPs for the Preservation of Apple Juice
Abstract
:1. Introduction
2. Results and Discussion
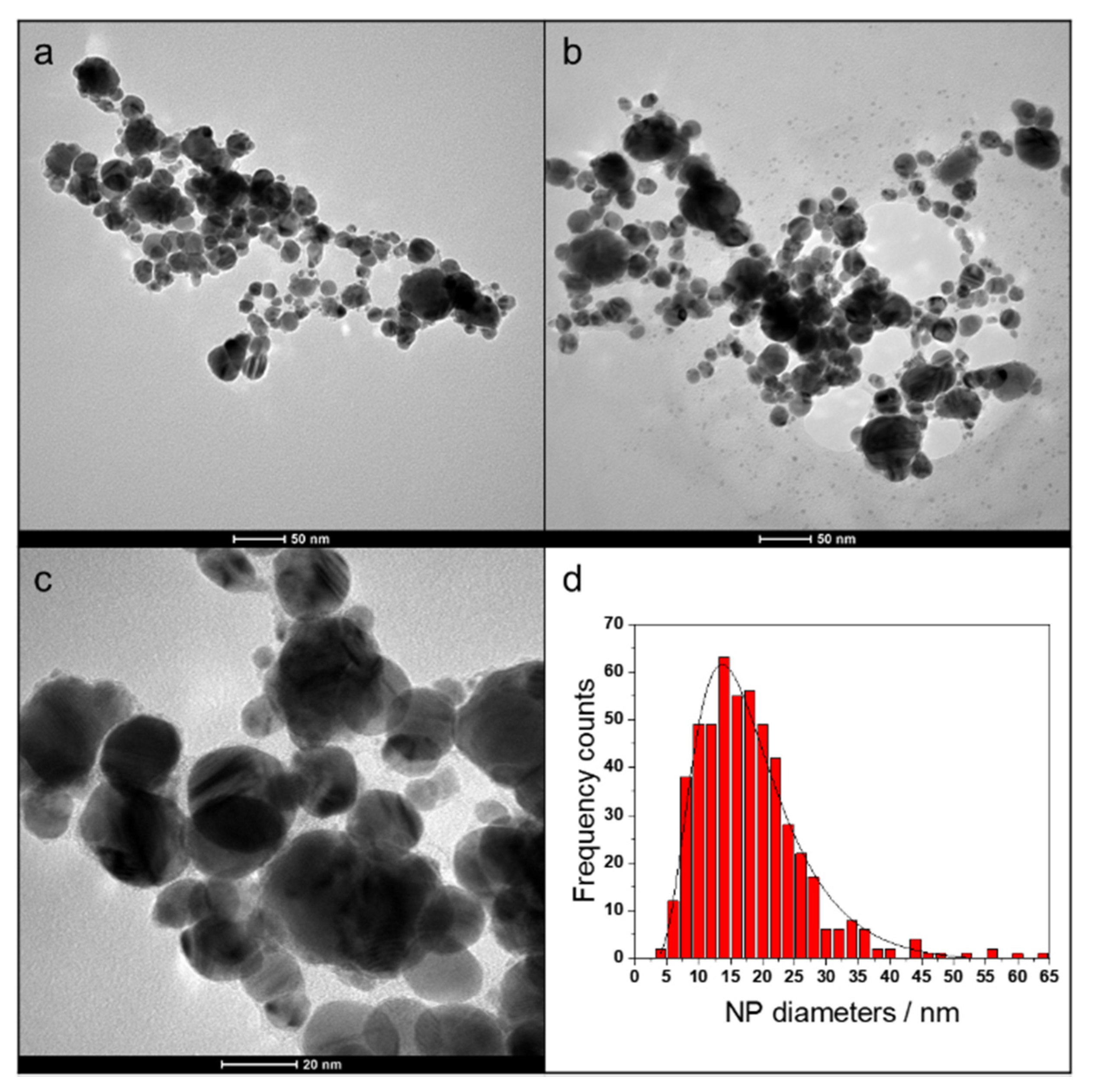
2.1. LASiS of AgNPs and Preparation of AgNPs-PHBV Composites
2.2. Kinetics of Silver Ions Release
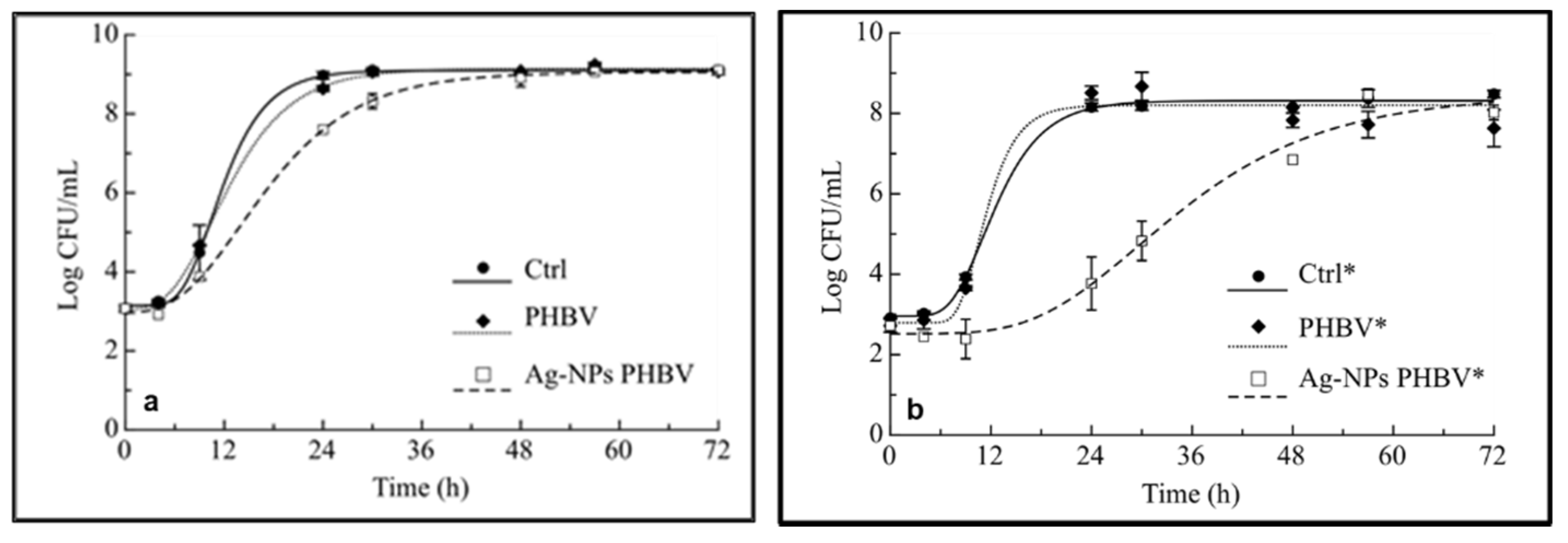
2.3. Antimicrobial Effects of AgNPs-PHBV
3. Materials and Methods
3.1. Materials
3.2. Production of AgNPs by Laser Ablation and Composite Preparation
3.3. Morphological and Spectroscopic Characterization
3.4. Determination of Silver Release
- -
- Step 1a: 110 °C in 1 s, hold time 45 s;
- -
- Step 1b: 130 °C in 15 s, hold time 90 s;
- -
- Step 2: 1200 °C in 10 s, hold time 20 s;
- -
- Step 3: 2000 °C in ~0 s, hold time 5 s;
- -
- Step 4: 2450 °C in 1 s, hold time 5 s.
3.5. Antimicrobial Activity of AgNP-PHBV Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guan, Q.-F.; Yang, H.-B.; Han, Z.-M.; Ling, Z.-C.; Yu, S.-H. An All-Natural Bioinspired Structural Material for Plastic Replacement. Nat. Commun. 2020, 11, 5401. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Eldin, R.E.S.; Serea, E.S.A.; Gomaa, N.M.; AboElmagd, G.M.; Salem, S.A.; Elsayed, Z.A.; Edrees, A.; Shams-Eldin, E.; Shalan, A.E. Advances in Nanotechnology and Antibacterial Properties of Biodegradable Food Packaging Materials. RSC Adv. 2020, 10, 20467–20484. [Google Scholar] [CrossRef]
- Thakur, S.; Chaudhary, J.; Sharma, B.; Verma, A.; Tamulevicius, S.; Thakur, V.K. Sustainability of Bioplastics: Opportunities and Challenges. Curr. Opin. Green Sustain. Chem. 2018, 13, 68–75. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Sun, B.; Zhang, D.; Chen, G.; Yang, X.; Yao, J. Reinforcement of Biodegradable Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) with Cellulose Nanocrystal/Silver Nanohybrids as Bifunctional Nanofillers. J. Mater. Chem. B 2014, 2, 8479–8489. [Google Scholar] [CrossRef] [PubMed]
- Castro-Mayorga, J.L.; Martínez-Abad, A.; Fabra, M.J.; Olivera, C.; Reis, M.; Lagarón, J.M. Stabilization of Antimicrobial Silver Nanoparticles by a Polyhydroxyalkanoate Obtained from Mixed Bacterial Culture. Int. J. Biol. Macromol. 2014, 71, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, A.; Kim, H.-Y.; Kim, Y.-R. Advances in the Applications of Polyhydroxyalkanoate Nanoparticles for Novel Drug Delivery System. BioMed Res. Int. 2013, 2013, 581684. [Google Scholar] [CrossRef]
- Tepha, Inc. Receives FDA Clearance for First Medical Device Derived from New Class of Biopolymers. Available online: https://www.meddeviceonline.com/doc/tepha-inc-receives-fda-clearance-for-first-me-0001 (accessed on 24 February 2021).
- Raho, S.; Carofiglio, V.E.; Montemurro, M.; Miceli, V.; Centrone, D.; Stufano, P.; Schioppa, M.; Pontonio, E.; Rizzello, C.G. Production of the Polyhydroxyalkanoate PHBV from Ricotta Cheese Exhausted Whey by Haloferax Mediterranei Fermentation. Foods 2020, 9, 1459. [Google Scholar] [CrossRef]
- Sportelli, M.; Izzi, M.; Volpe, A.; Clemente, M.; Picca, R.; Ancona, A.; Lugarà, P.; Palazzo, G.; Cioffi, N. The Pros and Cons of the Use of Laser Ablation Synthesis for the Production of Silver Nano-Antimicrobials. Antibiotics 2018, 7, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarfraz, J.; Gulin-Sarfraz, T.; Nilsen-Nygaard, J.; Pettersen, M.K. Nanocomposites for Food Packaging Applications: An Overview. Nanomaterials 2021, 11, 10. [Google Scholar] [CrossRef]
- Ding, Y.; Dou, C.; Chang, S.; Xie, Z.; Yu, D.-G.; Liu, Y.; Shao, J. Core–Shell Eudragit S100 Nanofibers Prepared via Triaxial Electrospinning to Provide a Colon-Targeted Extended Drug Release. Polymers 2020, 12, 2034. [Google Scholar] [CrossRef]
- Wang, M.; Li, D.; Li, J.; Li, S.; Chen, Z.; Yu, D.-G.; Liu, Z.; Guo, J.Z. Electrospun Janus Zein–PVP Nanofibers Provide a Two-Stage Controlled Release of Poorly Water-Soluble Drugs. Mater. Des. 2020, 196, 109075. [Google Scholar] [CrossRef]
- Kang, S.; He, Y.; Yu, D.-G.; Li, W.; Wang, K. Drug–Zein@lipid Hybrid Nanoparticles: Electrospraying Preparation and Drug Extended Release Application. Colloids Surf. B Biointerfaces 2021, 201, 111629. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Kang, S.-X.; Yang, Y.-Y.; Yu, D.-G. Electrospun Functional Nanofiber Membrane for Antibiotic Removal in Water: Review. Polymers 2021, 13, 226. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Izzi, M.; Volpe, A.; Clemente, M.; Picca, R.A.; Ancona, A.; Cioffi, N. Novel Polyethylene Oxide Coatings Implementing Ultra-Stable Laser-Ablated Silver Nanoparticles. Appl. Surf. Sci. 2020, 507, 145156. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Clemente, M.; Izzi, M.; Volpe, A.; Ancona, A.; Picca, R.A.; Palazzo, G.; Cioffi, N. Exceptionally Stable Silver Nanoparticles Synthesized by Laser Ablation in Alcoholic Organic Solvent. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 148–158. [Google Scholar] [CrossRef]
- Streubel, R.; Barcikowski, S.; Gökce, B. Continuous Multigram Nanoparticle Synthesis by High-Power, High-Repetition-Rate Ultrafast Laser Ablation in Liquids. Opt. Lett. 2016, 41, 1486. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Izzi, M.; Volpe, A.; Lacivita, V.; Clemente, M.; Di Franco, C.; Conte, A.; Del Nobile, M.A.; Ancona, A.; Cioffi, N. A New Nanocomposite Based on LASiS-Generated CuNPs as a Preservation System for Fruit Salads. Food Packag. Shelf Life 2019, 22, 100422. [Google Scholar] [CrossRef]
- Xiang, H.; Wang, S.; Wang, R.; Zhou, Z.; Peng, C.; Zhu, M. Synthesis and Characterization of an Environmentally Friendly PHBV/PEG Copolymer Network as a Phase Change Material. Sci. China Chem. 2013, 56, 716–723. [Google Scholar] [CrossRef]
- Conte, A.; Longano, D.; Costa, C.; Ditaranto, N.; Ancona, A.; Cioffi, N.; Scrocco, C.; Sabbatini, L.; Contò, F.; Del Nobile, M.A. A Novel Preservation Technique Applied to Fiordilatte Cheese. Innov. Food Sci. Emerg. Technol. 2013, 19, 158–165. [Google Scholar] [CrossRef]
- WHO. World Health Organization WHO—Chemical Hazards in Drinking-Water: Silver. Available online: http://www.who.int/ (accessed on 22 March 2021).
- Istiqola, A.; Syafiuddin, A. A Review of Silver Nanoparticles in Food Packaging Technologies: Regulation, Methods, Properties, Migration, and Future Challenges. J. Chin. Chem. Soc. 2020, 67, 1942–1956. [Google Scholar] [CrossRef]
- EFSA Guidance on Risk Assessment of the Application of Nanoscience and Nanotechnologies in the Food and Feed Chain: Part 1, Human and Animal Health. Available online: https://www.efsa.europa.eu/it/efsajournal/pub/5327 (accessed on 16 June 2021).
- Thapar, P.; Garcha, S. Incidence and Characterization of Pseudomonas Species Isolated from Spoilt Fresh Produce. IJEB 2017, 55, 372–376. [Google Scholar]
- Quintieri, L.; Fanelli, F.; Caputo, L. Antibiotic Resistant Pseudomonas Spp. Spoilers in Fresh Dairy Products: An Underestimated Risk and the Control Strategies. Foods 2019, 8, 372. [Google Scholar] [CrossRef] [Green Version]
- Moodley, J.S.; Krishna, S.B.N.; Pillay, K.; Sershen; Govender, P. Green Synthesis of Silver Nanoparticles from Moringa Oleifera Leaf Extracts and Its Antimicrobial Potential. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 015011. [Google Scholar] [CrossRef] [Green Version]
- Souza, V.G.L.; Fernando, A.L. Nanoparticles in Food Packaging: Biodegradability and Potential Migration to Food—A Review. Food Packag. Shelf Life 2016, 8, 63–70. [Google Scholar] [CrossRef]
- Dairi, N.; Ferfera-Harrar, H.; Ramos, M.; Garrigós, M.C. Cellulose Acetate/AgNPs-Organoclay and/or Thymol Nano-Biocomposite Films with Combined Antimicrobial/Antioxidant Properties for Active Food Packaging Use. Int. J. Biol. Macromol. 2019, 121, 508–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanmani, P.; Rhim, J.-W. Physical, Mechanical and Antimicrobial Properties of Gelatin Based Active Nanocomposite Films Containing AgNPs and Nanoclay. Food Hydrocoll. 2014, 35, 644–652. [Google Scholar] [CrossRef]
- Rescignano, N.; Hernandez, R.; Lopez, L.D.; Calvillo, I.; Kenny, J.M.; Mijangos, C. Preparation of Alginate Hydrogels Containing Silver Nanoparticles: A Facile Approach for Antibacterial Applications. Polym. Int. 2016, 65, 921–926. [Google Scholar] [CrossRef]
- Incoronato, A.L.; Buonocore, G.G.; Conte, A.; Lavorgna, M.; Nobile, M.A.D. Active Systems Based on Silver-Montmorillonite Nanoparticles Embedded into Bio-Based Polymer Matrices for Packaging Applications. J. Food Prot. 2010, 73, 2256–2262. [Google Scholar] [CrossRef] [PubMed]
- Jost, V.; Kopitzky, R. Blending of Polyhydroxybutyrate-Co-Valerate with Polylactic Acid for Packaging Applications—Reflections on Miscibility and Effects on the Mechanical and Barrier Properties. Chem. Biochem. Eng. Q. 2015, 29, 221–246. [Google Scholar] [CrossRef]
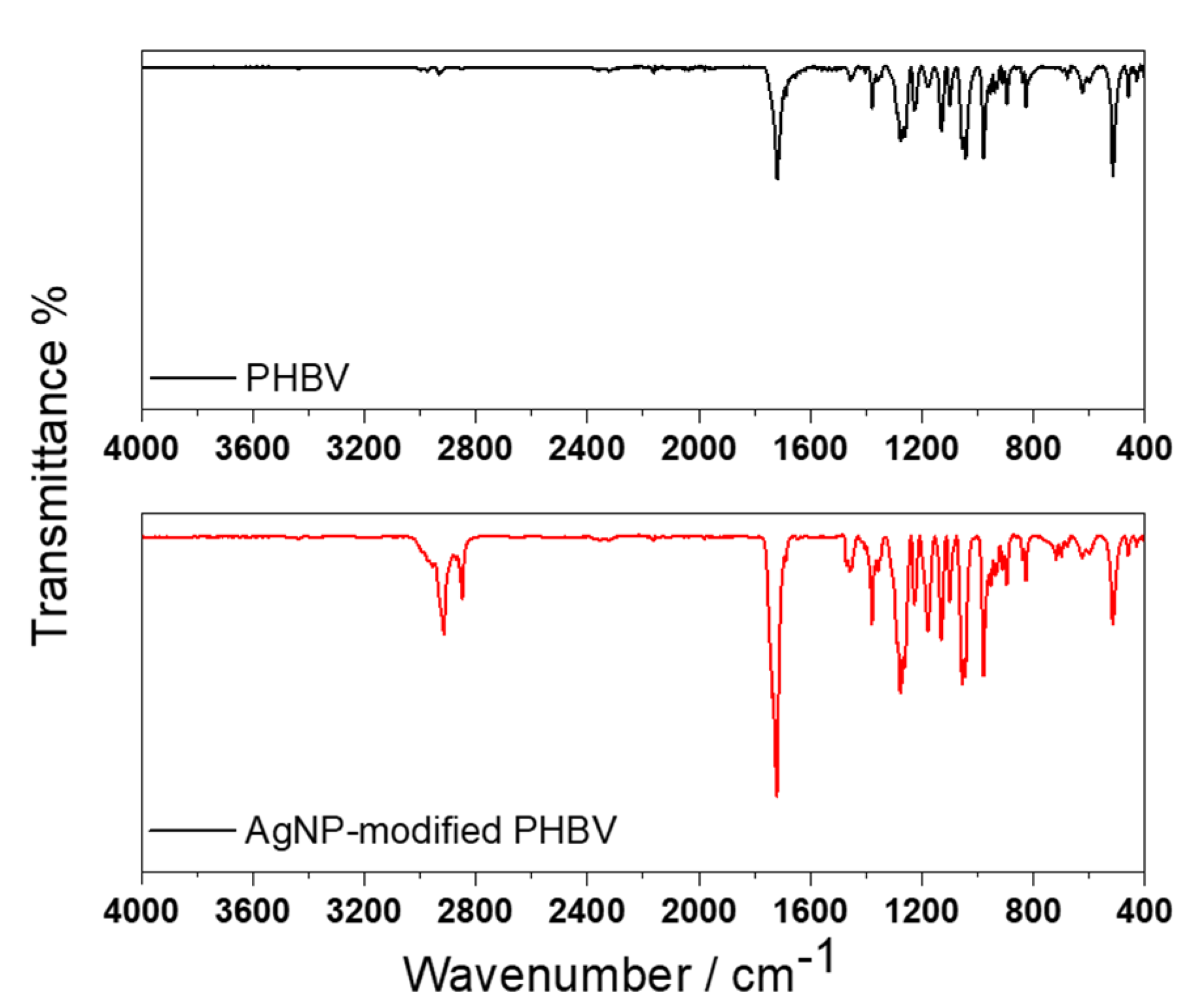

| Attributions | Spectral Regions (cm−1) |
|---|---|
| νs (CH, CH2), νa (CH, CH2) | 2975–2930 |
| νs (C=O) | 1718 |
| νs (C-C) | 1450–1380 |
| νs (C-O) | 1130 |
| δ (C-C) | 970–824 |
| Samples | A (log CFU/mL) | μmax (Δlog CFU/mL/h) | λ (h) | t* (h) |
|---|---|---|---|---|
| Ctrl | 5.9 ± 0.06 a | 0.60 ± 0.08c | 6.8 ± 0.35 b | 11.55 ± 0.36 a |
| Bare PHBV | 6.1 ± 0.09 a | 0.43 ± 0.02 b | 5.2 ± 0.39 a | 12.2 ± 0.27 a |
| AgNPs-PHBV | 6.1 ± 0.13 a | 0.29 ± 0.01 a | 6.4 ± 0.82 b | 16.8 ± 0.43 b |
| Ctrl* | 5.4 ± 0.12 A | 0.51 ± 0.1 B | 7.2 ± 0.56 A | 13.28 ± 0.9 A |
| Bare PHBV* | 5.4 ± 0.28 A | 0.79 ± 0.25 B | 8.0 ± 0.63 A | 12.12 ± 0.52 A |
| AgNPs-PHBV* | 5.9 ± 0.65 A | 0.18 ± 0.04 A | 17.23 ± 3.86 B | 37.33 ± 2.52 B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sportelli, M.C.; Ancona, A.; Volpe, A.; Gaudiuso, C.; Lavicita, V.; Miceli, V.; Conte, A.; Del Nobile, M.A.; Cioffi, N. A New Nanocomposite Packaging Based on LASiS-Generated AgNPs for the Preservation of Apple Juice. Antibiotics 2021, 10, 760. https://doi.org/10.3390/antibiotics10070760
Sportelli MC, Ancona A, Volpe A, Gaudiuso C, Lavicita V, Miceli V, Conte A, Del Nobile MA, Cioffi N. A New Nanocomposite Packaging Based on LASiS-Generated AgNPs for the Preservation of Apple Juice. Antibiotics. 2021; 10(7):760. https://doi.org/10.3390/antibiotics10070760
Chicago/Turabian StyleSportelli, Maria Chiara, Antonio Ancona, Annalisa Volpe, Caterina Gaudiuso, Valentina Lavicita, Valerio Miceli, Amalia Conte, Matteo Alessandro Del Nobile, and Nicola Cioffi. 2021. "A New Nanocomposite Packaging Based on LASiS-Generated AgNPs for the Preservation of Apple Juice" Antibiotics 10, no. 7: 760. https://doi.org/10.3390/antibiotics10070760
APA StyleSportelli, M. C., Ancona, A., Volpe, A., Gaudiuso, C., Lavicita, V., Miceli, V., Conte, A., Del Nobile, M. A., & Cioffi, N. (2021). A New Nanocomposite Packaging Based on LASiS-Generated AgNPs for the Preservation of Apple Juice. Antibiotics, 10(7), 760. https://doi.org/10.3390/antibiotics10070760













