Screening of the Potential Bioactivities of Pennyroyal (Mentha pulegium L.) Essential Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Essential Oil
2.2. Essential Oil Chemical Composition
2.3. Antioxidant Activity Evaluation
2.3.1. DPPH Free Radical Scavenging Assay
2.3.2. β-Carotene/Linoleic Acid System
2.4. Antimicrobial Activity Evaluation
2.4.1. Microorganisms and Culture Conditions
2.4.2. Disk Diffusion Assay
2.4.3. Resazurin Microtiter Method
2.5. Anti-Quorum Sensing Activity Evaluation
2.5.1. Biomonitor Strain
2.5.2. Disk Diffusion Assay
2.6. Anti-Inflammatory Activity Evaluation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition
3.2. Antioxidant Activity
3.3. Antimicrobial Activity
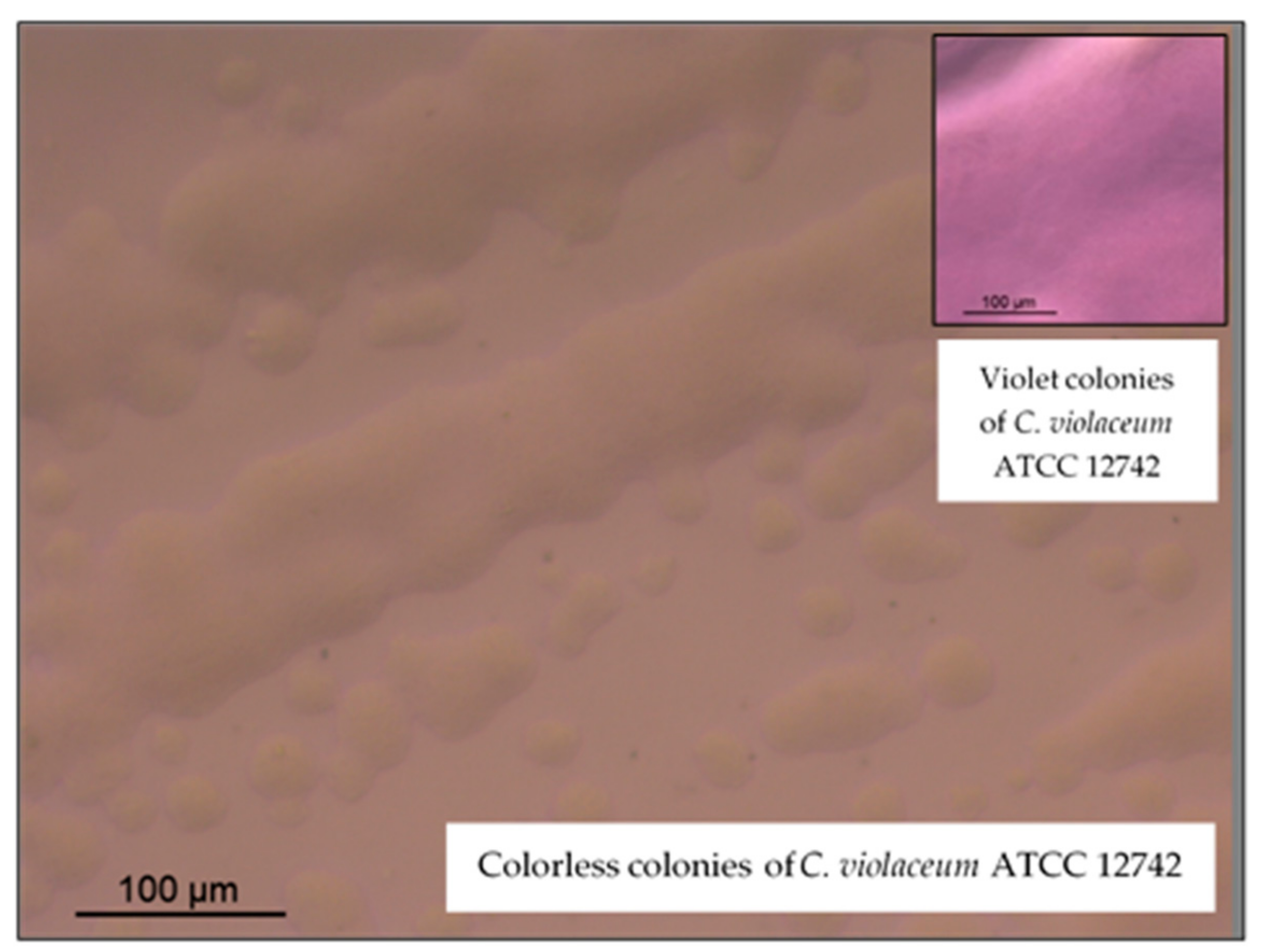
3.4. Anti-Quorum Sensing Activity
3.5. Anti-Inflammatory Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barbour, E.K.; Sharif, M.A.; Sagherian, V.K.; Habre, A.N.; Talhouk, R.S.; Talhouk, S.N. Screening of selected indigenous plants of Lebanon for antimicrobial activity. J. Ethnopharmacol. 2004, 93, 1–7. [Google Scholar] [CrossRef]
- Vieira, M.; Bessa, L.J.; Martins, M.R.; Arantes, S.; Teixeira, A.P.S.; Mendes, Â.; Costa, P.M.; Belo, A.D.F. Chemical Composition, Antibacterial, Antibiofilm and Synergistic Properties of Essential Oils from Eucalyptus globulus Labill. and Seven Mediterranean Aromatic Plants. Chem. Biodivers. 2017, 14, e1700006. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, B.; Marques, A.; Ramos, C.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind. Crops Prod. 2013, 43, 587–595. [Google Scholar] [CrossRef]
- Ahmadi, F.; Sadeghi, S.; Modarresi, M.; Abiri, R.; Mikaeli, A. Chemical composition, in vitro anti-microbial, antifungal and antioxidant activities of the essential oil and methanolic extract of Hymenocrater longiflorus Benth., of Iran. Food Chem. Toxicol. 2010, 48, 1137–1144. [Google Scholar] [CrossRef]
- Scandorieiro, S.; Camargo, L.C.; Lancheros, C.A.C.; Yamada-Ogatta, S.F.Y.; Nakamura, C.V.; Oliveira, A.G.; Andrade, C.G.T.J.; Duran, N.; Nakazato, G.; Kobayashi, R.K.T. Synergistic and additive effect of oregano essential oil and biological silver nanoparticles against multidrug-resistant bacterial strains. Front. Microbiol. 2016, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Luís, Â.; Sousa, S.; Wackerlig, J.; Dobusch, D.; Duarte, A.P.; Pereira, L.; Domingues, F. Star anise (Illicium verum Hook. f.) essential oil: Antioxidant properties and antibacterial activity against Acinetobacter baumannii. Flavour Fragr. J. 2019, 34, 260–270. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Sporer, F.; Reichling, J.; Wink, M. Antibacterial activity of essential oils from Eucalyptus and of selected components against multidrug-resistant bacterial pathogens. Pharm. Biol. 2011, 49, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Chusri, S.; Villanueva, I.; Voravuthikunchai, S.P.; Davies, J. Enhancing antibiotic activity: A strategy to control Acinetobacter infections. J. Antimicrob. Chemother. 2009, 64, 1203–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truchado, P.; Giménez-Bastida, J.-A.; Larrosa, M.; Castro-Ibáñez, I.; Espín, J.C.; Tomás-Barbéran, F.A.; García-Conesa, M.T.; Allende, A. Inhibition of Quorum Sensing (QS) in Yersinia enterocolitica by an Orange Extract Rich in Glycosylated Flavanones. J. Agric. Food Chem. 2012, 60, 8885–8894. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Batista, I.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. European pennyroyal (Mentha pulegium) from Portugal: Chemical composition of essential oil and antioxidant and antimicrobial properties of extracts and essential oil. Ind. Crops Prod. 2012, 36, 81–87. [Google Scholar] [CrossRef]
- Luís, Â.; Ramos, A.; Domingues, F. Pullulan Films Containing Rockrose Essential Oil for Potential Food Packaging Applications. Antibiotics 2020, 9, 681. [Google Scholar] [CrossRef]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Luís, Â.; Neiva, D.; Pereira, H.; Gominho, J.; Domingues, F.; Duarte, A.P. Stumps of Eucalyptus globulus as a Source of Antioxidant and Antimicrobial Polyphenols. Molecules 2014, 19, 16428–16446. [Google Scholar] [CrossRef] [Green Version]
- Luís, Â.; Breitenfeld, L.; Ferreira, S.; Duarte, A.P.; Domingues, F. Antimicrobial, antibiofilm and cytotoxic activities of Hakea sericea Schrader extracts. Pharmacogn. Mag. 2014, 10, S6–S13. [Google Scholar] [PubMed] [Green Version]
- Luís, Â.; Ramos, A.; Domingues, F. Pullulan–Apple Fiber Biocomposite Films: Optical, Mechanical, Barrier, Antioxidant and Antibacterial Properties. Polymers 2021, 13, 870. [Google Scholar] [CrossRef] [PubMed]
- Luís, Â.; Pereira, L.; Domingues, F.; Ramos, A. Development of a carboxymethyl xylan film containing licorice essential oil with antioxidant properties to inhibit the growth of foodborne pathogens. LWT-Food Sci. Technol. 2019, 111, 218–225. [Google Scholar] [CrossRef]
- Luís, Â.; Neiva, D.M.; Pereira, H.; Gominho, J.; Domingues, F.; Duarte, A.P. Bioassay-guided fractionation, GC–MS identification and in vitro evaluation of antioxidant and antimicrobial activities of bioactive compounds from Eucalyptus globulus stump wood methanolic extract. Ind. Crops Prod. 2016, 91, 97–103. [Google Scholar] [CrossRef]
- Luís, Â.; Duarte, A.P.; Pereira, L.; Domingues, F. Chemical Profiling and Evaluation of Antioxidant and Anti-Microbial Properties of Selected Commercial Essential Oils: A Comparative Study. Medicines 2017, 4, 36. [Google Scholar] [CrossRef] [Green Version]
- Kamkar, A.; Javan, A.J.; Asadi, F.; Kamalinejad, M. The antioxidative effect of Iranian Mentha pulegium extracts and essential oil in sunflower oil. Food Chem. Toxicol 2010, 48, 1796–1800. [Google Scholar] [CrossRef]
- Piras, A.; Porcedda, S.; Falconieri, D.; Maxia, A.; Gonçalves, M.; Cavaleiro, C.; Salgueiro, L. Antifungal activity of essential oil from Mentha spicata L. and Mentha pulegium L. growing wild in Sardinia island (Italy). Nat. Prod. Res. 2021, 35, 993–999. [Google Scholar] [CrossRef]
- Mahboubi, M.; Haghi, G. Antimicrobial activity and chemical composition of Mentha pulegium L. essential oil. J. Ethnopharmacol. 2008, 119, 325–327. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, Q.; Lv, H.; Wang, F.; Liu, R.; Zeng, N. Effect of pulegone on the NLPR3 inflammasome during inflammatory activation of THP-1 cells. Exp. Ther. Med. 2019, 19, 1304–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheraif, K.; Bakchiche, B.; Gherib, A.; Bardaweel, S.K.; Ayvaz, M.Ç.; Flamini, G.; Ascrizzi, R.; Ghareeb, M.A. Chemical composition, antioxidant, anti-tyrosinase, anti-cholinesterase and cytotoxic activities of essential oils of six Algerian plants. Molecules 2020, 25, 1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blosser, R.S.; Gray, K.M. Extraction of violacein from Chromobacterium violaceum provides a new quantitative bioassay for N-acyl homoserine lactone autoinducers. J. Microbiol. Methods 2000, 40, 47–55. [Google Scholar] [CrossRef]
- Zheng, X.; Feyaerts, A.F.; Dijck, P.V.; Bossier, P. Inhibitory activity of essential oils against Vibrio campbellii and Vibrio parahaemolyticus. Microorganisms 2020, 8, 1946. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Direito, R.; Lima, A.; Mota, J.; Gonçalves, M.; Duarte, M.P.; Solas, J.; Peniche, B.F.; Fernandes, A.; Pinto, R.; et al. Reduction of inflammation and colon injury by a Pennyroyal phenolic extract in experimental inflammatory bowel disease in mice. Biomed. Pharmacother. 2019, 118, 109351. [Google Scholar] [CrossRef] [PubMed]
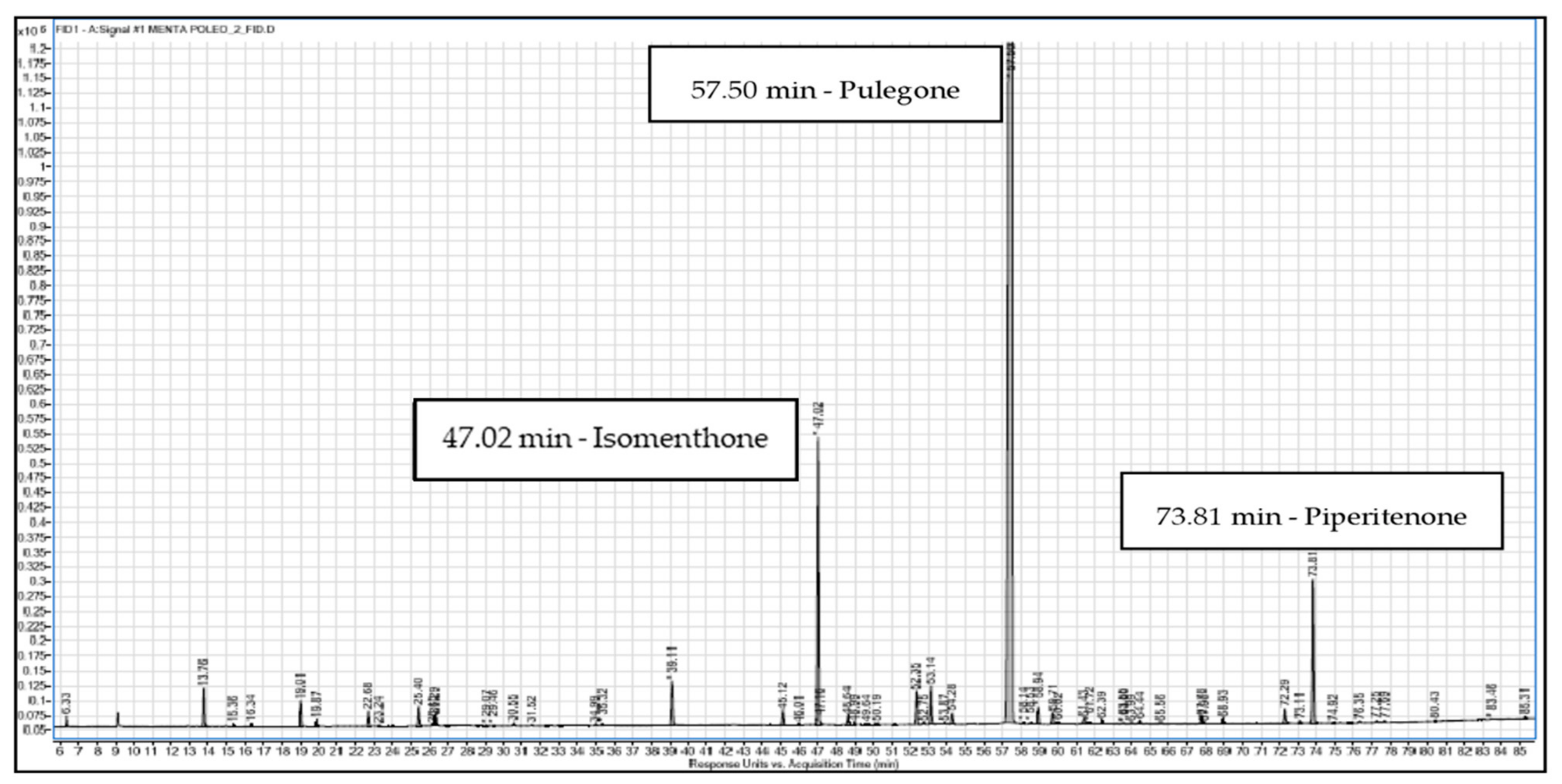
| Retention Time (min) | Area | m/z Predicted * | Compounds | Relative % |
|---|---|---|---|---|
| 13.76 | 285,520.68 | 136.13 | α-Pinene | 0.50 |
| 15.36 | 13,893.17 | 100.09 | 2,5-Diethyltetrahydrofuran | 0.02 |
| 19.01 | 209,964.64 | 136.13 | β-Pinene | 0.37 |
| 19.87 | 54,199.40 | 136.13 | Sabinene | 0.09 |
| 22.68 | 115,020.48 | 136.13 | β-Myrcene | 0.20 |
| 23.24 | 9218.27 | 136.13 | p-Mentha-1(7),8-diene | 0.02 |
| 25.40 | 157,870.05 | 136.13 | Limonene | 0.27 |
| 26.29 | 92,843.29 | 154.14 | 1,8-Cineole | 0.16 |
| 29.07 | 5981.18 | 136.13 | γ-Terpinene | 0.01 |
| 29.46 | 5959.16 | 128.12 | 3-Octanone | 0.01 |
| 30.55 | 15,695.05 | 164.34 | p-Cymene | 0.03 |
| 31.52 | 6537.28 | 136.13 | α-Terpinolene | 0.01 |
| 34.99 | 13,341.94 | 112.09 | 3-Methyl-Cyclohexanone | 0.02 |
| 35.32 | 14,539.98 | 172.15 | Octan-3-yl Acetate | 0.03 |
| 39.11 | 432,642.07 | 130.14 | 3-Octanol | 0.75 |
| 45.12 | 131,858.94 | 154.14 | Menthone | 0.23 |
| 46.01 | 12,943.67 | 150.11 | Menthofuran | 0.02 |
| 47.02 | 2,646,297.16 | 154.14 | Isomenthone | 4.60 |
| 48.64 | 95,801.61 | 152.12 | Camphor | 0.17 |
| 48.99 | 13,603.15 | 204.19 | β-Bourbonene | 0.02 |
| 49.64 | 9923.48 | 154.14 | Linalool | 0.02 |
| 50.19 | 9195.44 | 204.19 | β-Cubebene | 0.02 |
| 52.35 | 309,997.70 | 152.12 | cis-Isopulegone | 0.54 |
| 53.14 | 359,242.06 | 152.12 | trans-Isopulegone | 0.62 |
| 53.87 | 20,479.81 | 154.14 | Terpinen-4-ol | 0.04 |
| 54.28 | 108,658.39 | 204.19 + 168.15 | trans-β-Caryophyllene + p-Menth-3-en-8-ol | 0.19 |
| 57.50 | 49,804,964.30 | 152.12 | Pulegone | 86.64 |
| 58.94 | 160,304.10 | 204.19 | α-Humulene | 0.28 |
| 59.71 | 100,746.80 | 154.14 | α-Terpineol | 0.18 |
| 60.02 | 18,054.61 | 202.17 | Dehydro-Aromadendrene | 0.03 |
| 61.43 | 68,102.24 | 204.19 | D-Germacrene | 0.12 |
| 61.72 | 24,673.92 | 168.12 | cis-Piperitone Epoxide | 0.04 |
| 62.39 | 40,006.84 | 152.12 | Piperitone | 0.07 |
| 63.60 | 6533.06 | 156.15 | Citronellol | 0.01 |
| 63.99 | 11,083.13 | 204.19 | Δ-Cadinene | 0.02 |
| 65.56 | 6698.69 | 152.12 | Myrtenol | 0.01 |
| 67.78 | 74505.24 | 148.09 | Anethole | 0.13 |
| 67.90 | 12,588.66 | 168.12 | trans-Piperitone Epoxide | 0.02 |
| 73.81 | 1,485,580.23 | 150.11 | Piperitenone | 2.58 |
| 76.35 | 16,187.02 | 148.09 | Shisofuran | 0.03 |
| 83.46 | 5906.44 | 150.11 | Thymol Isomer | 0.01 |
| 85.31 | 21,831.71 | 150.11 | Carvacrol | 0.04 |
| Total identified | 43 compounds | 99.17 | ||
| Method | Parameters | Pennyroyal EO a | Gallic Acid b | BHT c | p-Values |
|---|---|---|---|---|---|
| DPPH | IC50 (%, v/v) | 1.29 ± 0.10 | 0.25 ± 0.02 | - | 0.002 ab,* |
| AAI | 4.02 ± 0.57 | 23.47 ± 0.34 | - | <0.001 ab,* | |
| Antioxidant Activity | Very Strong | Very Strong | - | - | |
| β-Carotene/Linoleic Acid | IC50 (%, v/v) | 1.15 ± 0.01 | - | 7.70 ± 0.62 | 0.003 ac,* |
| Microorganisms | Pennyroyal EO (15 µL/disk) a | Tetracycline (30 µg/disk) b | Amphotericin B (25 µg/disk) c | p-Values |
|---|---|---|---|---|
| S. aureus ATCC 25923 | 17.01 ± 1.22 | 30.32 ± 0.51 | - | <0.001 ab,* |
| L. monocytogenes LMG 16779 | 18.76 ± 0.47 | 18.31 ± 0.67 | - | 0.401 ab |
| E. faecalis ATCC 29212 | 10.73 ± 0.15 | 25.21 ± 0.65 | - | <0.001 ab,* |
| B. cereus ATCC 11778 | 19.53 ± 1.64 | 30.01 ± 0.80 | - | 0.002 ab,* |
| E. coli ATCC 25922 | 17.06 ± 0.76 | 23.36 ± 0.56 | - | 0.001 ab,* |
| A. baumannii LMG 1025 | 34.84 ± 0.66 | 25.67 ± 0.33 | - | <0.001 ab,* |
| A. baumannii LMG 1041 | 37.75 ± 2.18 | 27.48 ± 0.29 | - | 0.013 ab,* |
| S. Typhimurium ATCC 13311 | 12.54 ± 1.22 | 28.53 ± 0.49 | - | 0.001 ab,* |
| P. aeruginosa ATCC 27853 | 8.02 ± 1.07 | 11.59 ± 0.64 | - | 0.013 ab,* |
| C. albicans ATCC 90028 | 17.17 ± 0.86 | - | 20.31 ± 0.58 | 0.009 ac,* |
| C. tropicalis ATCC 750 | 18.45 ± 0.74 | - | 21.49 ± 0.47 | 0.006 ac,* |
| Microorganisms | Pennyroyal EO (%, v/v) a | Tetracycline (µg/mL) b | Amphotericin B (µg/mL) c | p-Values |
|---|---|---|---|---|
| S. aureus ATCC 25923 | 8 | 0.06 | - | <0.001 ab,* <0.001 ac,* |
| L. monocytogenes LMG 16779 | 8 | 0.06 | - | <0.001 ab,* <0.001 ac,* |
| E. faecalis ATCC 29212 | 4 | 0.06 | - | <0.001 ab,* <0.001 ac,* |
| B. cereus ATCC 11778 | 8 | 0.06 | - | <0.001 ab,* <0.001 ac,* |
| E. coli ATCC 25922 | 8 | 0.06 | - | <0.001 ab,* <0.001 ac,* |
| A. baumannii LMG 1025 | 2 | 0.06 | - | <0.001 ab,* <0.001 ac,* |
| A. baumannii LMG 1041 | 2 | 0.06 | - | <0.001 ab,* <0.001 ac,* |
| S. Typhimurium ATCC 13311 | 32 | 0.24 | - | <0.001 ab,* <0.001 ac,* |
| P. aeruginosa ATCC 27853 | 16 | 0.24 | - | <0.001 ab,* <0.001 ac,* |
| C. albicans ATCC 90028 | 16 | - | 0.24 | <0.001 ab,* <0.001 ac,* |
| C. tropicalis ATCC 750 | 16 | - | 0.48 | <0.001 ab,* <0.001 ac,* |
| Samples | Diameters of Inhibition of the Violacein Pigment Production (mm) |
|---|---|
| Pennyroyal EO (15 µL/disk) a | 89.97 ± 1.22 |
| DMSO (15 µL/disk) b | 0.00 |
| Resveratrol (5 µg/disk) c | 8.59 ± 0.25 |
| p-Values | <0.001 ab,* <0.001 ac,* |
| Samples | Anti-Inflammatory Activity—IC50 (%, v/v) |
|---|---|
| Pennyroyal EO a | 88.31 ± 1.37 |
| DMSO b | >100 |
| Acetylsalicylic acid c | 89.47 ± 2.64 |
| p-Values | 0.005 ab,* 0.548 ac |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luís, Â.; Domingues, F. Screening of the Potential Bioactivities of Pennyroyal (Mentha pulegium L.) Essential Oil. Antibiotics 2021, 10, 1266. https://doi.org/10.3390/antibiotics10101266
Luís Â, Domingues F. Screening of the Potential Bioactivities of Pennyroyal (Mentha pulegium L.) Essential Oil. Antibiotics. 2021; 10(10):1266. https://doi.org/10.3390/antibiotics10101266
Chicago/Turabian StyleLuís, Ângelo, and Fernanda Domingues. 2021. "Screening of the Potential Bioactivities of Pennyroyal (Mentha pulegium L.) Essential Oil" Antibiotics 10, no. 10: 1266. https://doi.org/10.3390/antibiotics10101266
APA StyleLuís, Â., & Domingues, F. (2021). Screening of the Potential Bioactivities of Pennyroyal (Mentha pulegium L.) Essential Oil. Antibiotics, 10(10), 1266. https://doi.org/10.3390/antibiotics10101266







