South Africa’s Best BARK Medicines Prescribed at the Johannesburg Muthi Markets for Skin, Gut, and Lung Infections: MIC’s and Brine Shrimp Lethality
Abstract
1. Introduction
2. Results and Discussion
2.1. Muthi Market Survey
2.2. Antimicrobial Outcomes
2.2.1. Erythrina lysistemon
2.2.2. Garcinia livingstonei
| Species | Bark Descriptions | Skin Ailments | Gastrointestinal Ailments | Respiratory Ailments | Other Uses of Bark | References |
|---|---|---|---|---|---|---|
| Albizia adianthifolia (Schumach.) W. Wight (Fabaceae) | OUTER BARK APPEARANCE Yellowish brown. Smooth in young stems; rough in mature trunk with irregular cracks. Flakes sometimes appear rectangular. On young stems, lenticels are small to medium-sized, roundish in shape, and scattered. INNER BARK APPEARANCE Yellow (10 YR 7/6) to strong brown (7.5 YR 5/68). Rough and fibrous. Fibers come off as threads. | Pounded bark is used as an aqueous lotion for eczema and other itchy skin complaints, as a body wash, or as a facial sauna. Bark is used in cosmetics, and for skin diseases and scabies. Bark is soaked in hot water and used as a wash for chickenpox | No traditional use | No traditional use | Bark is used for cleansing of blood, bronchitis, gynecological disorders; for epilepsy, gonorrhea, and eye sight problems. Powdered bark is used as a snuff for headaches and sinusitis | [13,14,40,43,71,72,73,74] |
| Bersama lucens (Hochst.) Szyszył. (Melianthaceae) | OUTER BARK APPEARANCE Brownish yellow (green layer underneath). Young stems smooth with tiny vertical fissures; green layer is present underneath periderm. Lenticels scattered, small to medium-sized, and arranged in horizontal (sometimes-vertical) rows. Mature bark is rough with dominant vertical cracks. Scales can be vertical and irregular. INNER BARK APPEARANCE Yellow (2.5 Y 7/6). Smooth and solid. | No traditional use | Bark is boiled and incorporated into herbal mixtures (imbiza), used as an emetic for stomach ailments | No traditional use | Bark is used for barrenness and impotence, to relieve menstrual pain and leprosy, and to calm nervous disorders and sexually transmitted infections. Powdered bark is taken as a snuff to treat congestive headaches, strokes, and apoplexy | [13,14,40,43,71,75,76] |
| Calodendrum capense (L. f.) Thunb. (Rutaceae) | OUTER BARK APPEARANCE Brownish grey. Smooth, even in mature trunk, with small shallow vertical cracks. In young stems, a green layer is present underneath the periderm. Lenticels are in vertical rows or scattered. INNER BARK APPEARANCE Very pale yellow (Munsell white diagram 2.5 Y 9/2). Smooth with visible patterns of rays in diagonal lines. | Bark is used as an ointment and moisturizer to remove pimples. Paste is applied facially as a skin lightener | No traditional use | No traditional use | No traditional use | [13,14,40,71,75,77] |
| Cinnamomum camphora (L.) J.Presl * (Lauraceae) | OUTER BARK APPEARANCE Dark reddish brown and light brown in cracks. Bark is rough and deeply furrowed. No lenticels. INNER BARK APPEARANCE Reddish yellow (7.5 YR 6/8). Very smooth (slippery) | Bark is used for skin ailments | Bark is used to relieve abdominal discomfort | Bark is used for colds and influenza | Bark is used as a perfume and to treat fever | [13,14,39,40,71,78] |
| Croton sylvaticus Hochst. (Euphorbiaceae) | OUTER BARK APPEARANCE Yellowish grey to brown. Smooth with green layer underneath. Lenticels small to medium with horizontal or vertical apertures; lenticels scattered or present inside cracks in mature bark samples. INNER BARK APPEARANCE Light yellowish brown (10 YR 6/4) to brownish yellow (10 YR 6/6). Smooth and solid. | Pounded decoction is used for wounds. Finely ground bark is rubbed into incisions on the skin as an irritant for inflammation | Decoction is used for abdominal disorders, digestive and intestinal complaints | The bark is used as a remedy taken orally to treat tuberculosis (TB). Powdered bark is applied topically for chest pain | Decoction is used for rheumatism, fever, inflammation, and uterine diseases | [13,14,39,43,79] |
| Cryptocarya latifolia Sond. (Lauraceae) | OUTER BARK APPEARANCE Brown (7.5 YR, 4/3). Smooth, lenticels small (<1 mm) and scattered. INNER BARK APPEARANCE Very dark brown (7.5 YR, 2.5/3). Smooth, sometimes with small ridges. | No traditional use | Powdered bark infusion is used for stomach cramps | Powdered bark mixed with crocodile fat and is used to treat chest ailments. Bark is also used to treat tuberculosis | Internal pains, allergy. An infusion is used orally for muscular cramps. Decoction is used as enemas for urinary tract diseases, uterine spasms, and menstrual pains | [13,14,43,80] |
| Dombeya rotundifolia (Hochst.) Planch. (Malvaceae) | OUTER BARK APPEARANCE Grey to dark brown. Rough with irregular cracks. INNER BARK APPEARANCE Reddish yellow (5 YR 7/6). Fibrous but smooth. Fibees peel off as long strips. | Decoction is used to steam “ukufutha” for itchy skin problems. Inflexible fibers of the bark are used to cover wounds. | Bark or wood infusions are taken orally or as enemas for intestinal ulcers, stomach complaints, and diarrhea | No traditional use | Bark is mixed with banana seeds (Momordica balsamina) to treat heart diseases. Bark is used for headache, palpitations, nausea, abortion, and irregular menstruation | [13,14,40,43,71,81] |
| Ekebergia capensis Sparrm. (Meliaceae) | OUTER BARK APPEARANCE Light grey with yellowish lenticels in young stems. Grey-brown in mature stems. Bark is relatively smooth in young stems with tiny vertical fissures; lenticels are in vertical rows 2 to 20 mm apart. Bark is rough in mature trunk with irregular cracks. INNER BARK APPEARANCE Strong brown (7.5 YR 5/8; 7.5 YR 4/4). Relatively smooth. Looks fibrous, but solid. | Ground bark is mixed with flour in water to form a poultice to treat abscesses and boils. Hot infusion is used as a wash for pimples | Powdered bark and roots are mixed to make tea in order to treat gastritis | Decoction is taken as emetics for respiratory and chest complaints | Bark is used to treat malaria, heartburn, and sexually transmitted diseases. Infusion is taken as an emetic for purifying blood | [13,14,39,40,43,71,82] |
| Elaeodendron transvaalensis (Burtt Davy) R. H. Archer (Celastraceae) | OUTER BARK APPEARANCE Grey with prominent orange color underneath the periderm. Bark is relatively smooth with vertical fissures. Lenticels are not visible. INNER BARK APPEARANCE Pinkish white (5 YR 8/2). Smooth and solid. | The bark is used for skin rashes and skin infections | Decoction is taken orally or as an enema for stomach complaints and internal wounds. Decoction and/or powdered bark is licked to treat diarrhea, intestinal worms, and stomach cramps | No traditional use | Bark is used to treat fever, piles, kidney, and bladder infections; to relieve body pain and for heavy menstruation | [13,14,40,43,71,78,83,84] |
| Erythrina lysistemon Hutch. (Fabaceae) | OUTER BARK APPEARANCE Grey brown, green underneath the superficial layer (in very old trunks greenish yellow). Relatively smooth with longitudinal grooves and scattered knob-like projections with hooked pointed thorns. Lenticels are prominent in vertical rows. INNER BARK APPEARANCE Yellow (10 YR 7/8). Smooth with visible rays. | The bark is used as a poultice for swellings and abscesses. Ash from burnt bark is used to disinfect wounds | Infusion is used for internal wounds, allergies, and stomach ulcers, and used as an emetic for food poisoning | No traditional use | Bark is used to facilitate childbirth or taken as tea to relieve labor pains, for toothache, and as a mouthwash | [13,14,40,43,71,73,85,86] |
| Erythrophleum lasianthum Corbishley (Fabaceae) | OUTER BARK APPEARANCE Bark is grey on the superficial part and becomes strong brown underneath and is dark red further down. Smooth in young stems and rough with irregular (mostly vertical) cracks in mature trunk. INNER BARK APPEARANCE The bark may be grey or dark brown. Smooth with visible stripes that are vertical or sometimes twisted. | No traditional use | Decoction is taken for intestinal spasms, abdominal pains, and as a strong purgative and an anthelmintic | Powdered bark is used for colds | Powdered bark is snuffed to relieve headache. Bark is used in ethnoveterinary medicine | [13,14,40,43,71] |
| Garcinia livingstonei T. Anderson (Clusiaceae) | OUTER BARK APPEARANCE Grey, but orange underneath superficial layer. Smooth in younger stems. Rough, with prominent vertical cracks in mature stem. The bark resembles a mosaic appearance. INNER BARK APPEARANCE Yellowish red (5 YR 5/8). Relatively smooth, saturated with many small dots (exudates) that are dark reddish in color. | The bark is used for cosmetics purposes as a skin lightener | Bark is used for treatment of internal parasites and diarrhea | Stem is used to treat coughs | Bark is used to treat fever | [13,14,39,50,87] |
| Harpephyllum caffrum Bernh. (Anacardiaceae) | OUTER BARK APPEARANCE Brownish in young stems and grey in mature bark. Young stems smooth with vertical fissures and scattered lenticels. Bark from mature stem is rough with dominant vertical cracks forming rectangular to irregular scales. INNER BARK APPEARANCE Red and smooth. | Decoction is used as a wash for skin ailments such as acne and eczema. The bark is used to purify the skin for cosmetic purposes by means of facial steam bath and to reduce pimples | Powdered bark is used licked “khotha” for treatment of food poisoning | The bark of H. caffrum is mixed with bark of Schotia brachypetala and Syzygium cordatum and ground into powder, boiled, and taken as an emetic to treat respiratory complaints | Powdered burnt bark is rubbed into scarification, around sprains and fractures. Bark is used for diabetes; decoctions are taken as emetics to purify blood and strengthen the body | [13,14,39,40,43,71,77,78] |
| Kigelia africana (Lam.) Benth. (Bignoniaceae) | OUTER BARK APPEARANCE Greyish brown. Relatively smooth with vertical fissures, no visible lenticels. Mature trunk can be with rectangular or irregular scales. INNER BARK APPEARANCE Dark brown (7.5 YR 3/3). Smooth, sometimes with visible rays. | Stem and twigs are used to treat sores, wounds, and snakebites | Ground bark decoctions are administered as enemas for stomach ailments in children. The bark is used for ulcers while the stem and twigs are used for dysentery | Bark is used to treat pneumonia | Bark is used for syphilis, Human Immunodeficiency Virus (HIV), gonorrhea, and rheumatism. Decoction is used as a mouthwash to relieve pain and inflammation caused by toothache | [13,14,40,71,72,88] |
| Ocotea bullata (Burch.) E. Mey. in Drége (Lauraceae) | OUTER BARK APPEARANCE Grey to brown. Relatively smooth and even in mature trunks. Roundish or slightly elongated dark lenticels scattered on young stems. INNER BARK APPEARANCE Brown (7.5 YR 4/4) to dark brown (7.5 YR 3/2). Relatively smooth with vertical ridges. | No traditional use | The bark is used as a general tonic and for diarrhea in children. Bark is also used for a “bleeding stomach” | No traditional use | Bark is used for nervous disorders. Powdered bark is mixed with Croton gratissimus and Zingiber officinale root for urinary complaints. Bark powder is snuffed or smoked for headache | [13,14,39,40,43,71] |
| Peltophorum africanum Sond. (Fabaceae) | OUTER BARK APPEARANCE Light grey to black and becomes strong brown and yellowish underneath. Smooth in young stems, rough with vertical cracks and horizontal fissures in medium-sized stems, and very rough with irregular cracks in old mature trunks. Lenticels are present on smooth parts and their remains. INNER BARK APPEARANCE Brown (7.5 YR 4/6) to red (2.5 YR 3/6). Fibrous, fibres flexible when wet. | Decoction is used to treat wounds | Decoction is used to treat diarrhea, dysentery, intestinal parasites, and ulcers, and as a general tonic. Fresh bark is chewed to treat colic | Bark is used for coughs, sore throats, and tuberculosis | Bark and roots are used for sterility, backache, and sexually transmitted infections | [13,14,39,42,78,89] |
| Pittosporum viridiflorum Sims (Pittosporaceae) | OUTER BARK APPEARANCE Greyish brown. Relatively smooth. Lenticels small to medium-sized with horizontal apertures scattered or in distinct horizontal rows. INNER BARK APPEARANCE Pale brown (10 YR 6/3). Very smooth and solid. | No traditional use | Decoction is used as emetics or enemas for stomach problems | Bark is used to treat chest complaints | Decoction is used for malaria and cancer, to induce febrile complaints, and for back pains. Powdered bark is used for toothache. Used as a chewing stick to manage oral fungal infections in HIV patients | [13,14,39,40,71,78,90] |
| Prunus africana (Hook.f.) Kalkman (Rosaceae) | OUTER BARK APPEARANCE Grey to black or dark brown to black. Very rough, with various irregular vertical and horizontal cracks, forming irregular scales. No lenticels. INNER BARK APPEARANCE Dark red (2.5 YR 3/6) or strong brown (7.5 YR 4/6). Fibrous and brittle. | The bark is used for the treatment of allergies and for wound dressing | Bark is used for stomachache | Infusion is administered orally to treat colds and influenza. The bark is used for chest pain | Decoction is used for intercostal pains, urinary tract disorders, kidney diseases, malaria, and inflammation. Bark is active against prostatic hypertrophy | [13,14,39,40,43,71,82,91] |
| Pterocelastrus rostratus Walp. (Celastraceae) | OUTER BARK APPEARANCE Greyish brown. Bark is smooth with small scattered yellowish lenticels. INNER BARK APPEARANCE Dark reddish brown (2.5 YR 3/3). Smooth with solid fibrous appearance. Fibers brittle break if pulled. | No traditional use | No traditional use | Decoction is taken as emetics for respiratory ailments, chest block, and wheezing chest | Powdered bark mixed with that of Rapanea melanophloeos is added in warm water and taken orally to relieve general body pains | [13,14,43] |
| Rapanea melanophloeos (L.) Mez (Primulaceae) | OUTER BARK APPEARANCE Greyish brown (10 YR 5/2) and reddish towards the inner surface. Relatively smooth with vertical fissures in young and rough with irregular cracks in mature trunk. INNER BARK APPEARANCE Reddish black (5 R 2.5/1). Relatively smooth in touch with visible rays, which is lighter in color than ground tissues. Often vertical cracks occur in samples. | Bark is used as a skin lightener and to treat wounds | Bark is used for acidity and stomach ailments. Decoction is taken for hematemesis and stomachache | Fresh pieces of dried powdered bark are chewed to relieve sore throats | The bark is used for strengthening the heart, and for muscular pains and fever | [13,14,39,40,43,58,71,73] |
| Rauvolfia caffra Sond. (Apocynaceae) | OUTER BARK APPEARANCE Yellow to light brown. Smooth in young stems, rough with short vertical cracks, and horizontal lines on mature trunk. Periderm is spongy to the touch (such as polystyrene). Lenticels are present, even in mature bark from the trunk. They are medium in size and scattered. INNER BARK APPEARANCE Yellowish brown (10 YR 5/6). Relatively smooth and spongy to the touch. Sometimes with visible rays. | Bark is applied topically for measles, urticaria, and other skin rashes. Infusion is used to kill maggots in wounds | Decoction is used for abdominal complaints | The bark is used to treat pneumonia and chewed to relieve coughs | Decoction is used for fever, as a tranquilizer for hysteria, and for insomnia and malaria. It is used for general body swelling, rheumatism, and uterine complaints | [13,14,39,40,42,43,71,73] |
| Schotia brachypetala Sond. (Fabaceae) | OUTER BARK APPEARANCE Grey brown. Smooth in young stems, coarse with vertical or rarely horizontal fissures. No lenticels are visible. INNER BARK APPEARANCE Light red (2.5 YR 6/8). Smooth with brittle fibres. | Infusion is taken as emetics for pimples. The bark is also used topically as a wash and to reduce swellings on the body | Powdered bark is used for food poisoning. The bark is soaked in cold water to make “ukhamba” (medication) used as an enema to clean bleeding stomach | Powdered bark of S. brachypetala is mixed with bark of H. caffrum and Syzygium cordatum, put to boil, and used as an emetic to treat respiratory complaints | Decoction is taken for heartburn and a “hangover headache”. Bark is used to treat nervous and cardiac conditions | [13,14,39,40,43,71] |
| Sclerocarya birrea (A. Rich.) Hochst. (Anacardiaceae) | OUTER BARK APPEARANCE Grey to black. Smooth in young stems. Trunk and old branches with irregular or sometimes roundish scales; mature old trunk with strong and prominent vertical cracks. No lenticels present. INNER BARK APPEARANCE Dark reddish brown (5 YR 3/3). Smooth, with papery strips or layers that peel off easily. | Inner bark is boiled and applied as poultice for smallpox, skin ulcers, and skin complaints | Decoctions are administered as enemas for treatment of diarrhea, abdominal complaints, and other stomach ailments | No traditional use | Used for malaria, fever, gonorrhea, headache, toothache, backache, infertility, and to strengthen the heart and to treat diabetes mellitus | [13,14,39,40,42,43,71] |
| Securidaca longepedunculata Fresen. (Polygalaceae) | OUTER BARK APPEARANCE Brown (7.5 YR 4/3). Relatively smooth. Lenticels are present. (Chopped sample) INNER BARK APPEARANCE Yellow (10 YR 8/6) and smooth. | The root or rootbark is used topically for wounds and sores | No traditional use | Rootbark and stembark decoction is used to treat coughs, chest complaints, and tuberculosis | Used for epilepsy. Decoction is used to treat gonorrhea, syphilis, and meningitis | [39,40,71,88] |
| Strychnos henningsii Gilg (Loganiaceae) | OUTER BARK APPEARANCE Greyish brown (lot of lichens, which can make the bark mottled). Bark smooth to rough with very irregular, scattered flakes. Small lenticels scattered or sometimes bigger lenticels in vertically elongated groups. INNER BARK APPEARANCE Yellowish brown (10 YR 5/4). Relatively smooth, sometimes with numerous black marks of mould. | The bark is used to treat schistosomiasis and snakebites | The bark is used as an anthelmintic, a health tonic; to treat schistosomiasis; used as an emetic to treat food poisoning. Bark is also chewed for stomach complaints | No traditional use | Powdered bark is taken with cold water for nausea. Bark is used for malaria, dysmenorrhea, erectile dysfunction, and diabetes mellitus | [13,14,40,43,71,92] |
| Syzygium cordatum Hochst. ex Krauss (Myrtaceae) | OUTER BARK APPEARANCE Grey to dark brown. Smooth in young stems. Mature bark is rough with irregular cracks. No lenticels. INNER BARK APPEARANCE Red (10 R 5/8). Fibrous but smooth. Fibers are flexible when wet. | The bark is used to treat wounds. Bark paste is applied topically for treatment of blisters, pimples, or acne and eczema | The bark is used for internal stomach wounds, diarrhea, and stomach complaints | Powdered bark of S. cordatum, S. brachypetala, and H. caffrum are boiled in water and taken as an emetic to treat respiratory complaints. Decoction is used to treat tuberculosis | Powdered bark is licked (khotha) for body pains. Bark is used for headaches, amenorrhoea, and inflammation | [13,14,40,42,71,73,78,93] |
| Trichilia emetica Vahl (Meliaceae) | OUTER BARK APPEARANCE Outer color not useful for identification of this bark, because it can be very mottled due to lichens and moss. Grey-brown, sometimes yellowish. Smooth in young stems. Mature trunk relatively smooth to course, with tiny vertical fissures. No lenticels on mature trunk. INNER BARK APPEARANCE Dark reddish brown (2.5 YR 3/3). Relatively smooth. | Powdered bark is mixed with petroleum jelly and applied on wounds between fingers and toes. Bark is used to promote wound healing | Decoction is used for stomach and intestinal complaints. Infusions are used for dysentery and intestinal worms. Bark infusion of T. emetica and Spirostachys africana is used for constipation | No traditional use | Bark is used to treat malaria. Infusions are used for rectal ulceration in children | [13,14,39,40,71] |
| Vachellia natalitia (E.Mey.) Kyal. and Boatwr. (Fabaceae) | OUTER BARK APPEARANCE Mottled: light grey in the superficial layer and brown to light brown towards the inner parts. Rough, irregularly fissured, no lenticels. INNER BARK APPEARANCE Yellow (10 YR 8/6). Fibrous (fibers are flexible). | Decoction is taken orally for wounds and is used as an emetic for pimples. Bark is used for cosmetic applications | Ground bark infusion is used for stomachache, diarrhea, and dysentery | Bark is mixed with leaves to make tea for coughs and colds | Infusion is used for hemorrhage, conjunctivitis, and as an antidote for cattle poisoning. Bark and roots are boiled together and used as a mouthwash | [13,14,40,71,73] |
| Vachellia robusta (Burch.) Kyalangalilwa and Boatwright subsp. robusta (Fabaceae) | OUTER BARK APPEARANCE Grey to brown. Smooth to rough. Vertical to irregular cracks in old bark. Lenticels only on smooth bark, in horizontal rows. INNER BARK APPEARANCE Light red (2.5 YR 5/8). Fibrous: strong elastic fibers, separated by thin threads. Visible patterns of radial rays. | Bark is used for the treatment of skin disorders | No traditional use | Steam of boiled water from bark is inhaled for chest complaints | Decoction is used for treatment of menstrual pains and sexually transmitted infections | [13,14,40] |
| Warburgia salutaris (G. Bertol.) Chiov. (Canellaceae) | OUTER BARK APPEARANCE Grey to dark brown. Smooth in young branches. Mature bark is rough, brittle with deep irregular cracks. Flakes irregular in shape or vertically elongated. INNER BARK APPEARANCE Pale yellowish pink (7.5 YR -/2). Smooth, sometimes with visible papery layers or may look fibrous, but the fibery layers are very brittle. | Powdered bark is mixed with fats and applied on skin to treat sores and skin eruptions | Purgatives and for stomach ulcers. Bark is used for constipation, stomachache, and other gastrointestinal disorders | Powdered bark is taken with cold water or a pinch; is smoked, sometimes mixed with Cannabis sativa leaf to treat colds and a dry cough. Bark is used for chest complaints | Used for malaria, toothache, transmitted infections. Emetic is used for febrile complaints and for rheumatism. Powdered bark is mixed with fats and applied topically for inflammation | [13,14,39,40,42,43,71] |
| Ziziphus mucronata Willd. (Rhamnaceae) | OUTER BARK APPEARANCE Grey to brown or dark brown. Very rough, with very prominent vertical cracks, and some horizontal but less prominent cracks. Mature bark with irregular cracks. INNER BARK APPEARANCE Yellowish red (5 YR 5/8). Smooth with vertical lines. | The bark is used to treat boils, skin infections, and measles. Steam baths from the bark are used to purify skin texture | The bark is used to treat dysentery and other stomach ailments | Infusion is taken as emetics for respiratory ailments and chronic coughs | Bark is used to treat tubercular gland swelling. Decoction is used for rheumatism, gonorrhea, and chlamydia | [13,14,40,51,71] |
| Bark Samples | B. cereus ATCC 11175 | S. aureus ATCC 25923 | S. epidermidis ATCC 12228 | E. faecalis ATCC 29121 | P. aeruginosa ATCC 27853 | E. coli ATCC 8739 | S. typhimurium ATCC 14028 | S. sonnei ATCC 9290 | K. pneumoniae ATCC 13883 | M. catarrhalis ATCC 23246 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Solvent extracts | M * | DCM ** | M | DCM | M | DCM | M | DCM | M | DCM | M | DCM | M | DCM | M | DCM | M | DCM | M | D |
| A. adianthifolia | NT *** | NT | 4.00 | 3.33 | 4.00 | 1.33 | NT | NT | 2.66 | 2.66 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT |
| B. lucens | 0.33 | 0.50 | NT | NT | NT | NT | 0.50 | 0.50 | NT | NT | 2.00 | 1.00 | 0.66 | 1.33 | 1.33 | 2.00 | NT | NT | NT | NT |
| C. capense | NT | NT | 5.33 | 5.33 | 4.00 | 4.00 | NT | NT | 4.00 | 2.66 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT |
| C. camphora | NT | NT | 1.00 | 1.00 | NT | NT | NT | NT | 1.66 | 2.66 | NT | NT | NT | NT | NT | NT | 1.66 | 2.00 | 1.33 | 2.00 |
| C. sylvaticus | 0.75 | 0.50 | NT | NT | NT | NT | 2.00 | 1.00 | NT | NT | 4.00 | 1.00 | 2.00 | 1.66 | 8.00 | 3.00 | NT | NT | NT | NT |
| C. latifolia | NT | NT | 1.66 | 0.83 | NT | NT | NT | NT | 2.00 | 1.00 | NT | NT | NT | NT | NT | NT | 1.66 | 1.66 | 2.00 | 0.83 |
| D. rotundifolia | 1.33 | 0.50 | NT | NT | NT | NT | 0.75 | 0.66 | NT | NT | 1.50 | 1.66 | 1.00 | 1.00 | 2.00 | 2.66 | NT | NT | NT | NT |
| E. capensis | 0.25 | 0.50 | 2.00 | 5.33 | 2.00 | 4.00 | 2.00 | 2.00 | 1.00 | 1.00 | 1.00 | 8.00 | 2.00 | 2.00 | 1.00 | 2.66 | NT | NT | NT | NT |
| E. transvaalense | 0.41 | 0.20 | 1.66 | 0.33 | 1.66 | 0.26 | 0.50 | 0.26 | 0.83 | 0.37 | 0.66 | 0.41 | 1.33 | 1.00 | 1.00 | 0.75 | NT | NT | NT | NT |
| E. lysistemon | 0.20 | 0.12 | 0.20 | 0.20 | 0.12 | 0.004 | 0.16 | 0.10 | 0.50 | 0.50 | 1.00 | 0.41 | 1.00 | 1.00 | 0.16 | 0.16 | NT | NT | NT | NT |
| E. lasianthum | 1.33 | 0.83 | NT | NT | NT | NT | 1.66 | 1.33 | NT | NT | 1.00 | 2.00 | 0.83 | 3.00 | 1.66 | 8.00 | NT | NT | NT | NT |
| G. livingstonei | 0.06 | 0.12 | NT | NT | NT | NT | 0.18 | 0.75 | NT | NT | 0.41 | 2.00 | 0.25 | 2.00 | 0.83 | 0.50 | NT | NT | NT | NT |
| H. caffrum | NT | NT | 3.33 | 4.00 | 1.00 | 1.00 | NT | NT | 0.66 | 1.66 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT |
| K. africana | 1.66 | 0.16 | NT | NT | NT | NT | 2.66 | 0.83 | NT | NT | 1.50 | 1.33 | 2.00 | 2.66 | 4.00 | 1.66 | NT | NT | NT | NT |
| O. bullata | 0.66 | 0.66 | NT | NT | NT | NT | 1.33 | 1.50 | NT | NT | 1.66 | 2.66 | 1.50 | 8.00 | 2.66 | 4.00 | NT | NT | NT | NT |
| P. africanum | NT | NT | 2.00 | 0.83 | NT | NT | NT | NT | 1.66 | 1.66 | NT | NT | NT | NT | NT | NT | 1.00 | 0.66 | 2.00 | 2.00 |
| P. viridiflorum | NT | NT | 3.33 | 1.66 | 4.00 | 4.00 | NT | NT | 1.33 | 1.00 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT |
| P. africana | NT | NT | 1.33 | 0.83 | NT | NT | NT | NT | 0.83 | 0.50 | NT | NT | NT | NT | NT | NT | 1.66 | 0.50 | 1.33 | 1.00 |
| P. rostratus | NT | NT | 0.50 | 0.50 | NT | NT | NT | NT | 0.25 | 0.20 | NT | NT | NT | NT | NT | NT | 0.66 | 0.83 | 0.66 | 0.41 |
| R. melanophloeos | NT | NT | 0.41 | 0.41 | 0.25 | 0.66 | NT | NT | 0.50 | 0.50 | NT | NT | NT | NT | NT | NT | 0.50 | 0.50 | 0.50 | 0.41 |
| R. caffra | NT | NT | 3.33 | 4.00 | 0.50 | 1.00 | NT | NT | 1.66 | 1.66 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT |
| S. brachypetala | 1.00 | 0.50 | NT | NT | NT | NT | 0.83 | 0.66 | NT | NT | 1.00 | 0.33 | 0.83 | 0.66 | 0.83 | 1.33 | NT | NT | NT | NT |
| S. birrea | 1.00 | 0.50 | NT | NT | NT | NT | 0.66 | 0.50 | NT | NT | 1.00 | 0.50 | 0.83 | 2.00 | 0.83 | 2.00 | NT | NT | NT | NT |
| S. longepeduncu-lata | NT | NT | 8.00 | 2.00 | 4.00 | 1.33 | NT | NT | 1.33 | 1.66 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT |
| S. henningsii | 2.66 | 1.00 | NT | NT | NT | NT | 1.50 | 8.00 | NT | NT | 8.00 | 2.66 | 8.00 | 2.00 | 8.00 | 8.00 | NT | NT | NT | NT |
| S. cordatum | NT | NT | 1.66 | 2.66 | NT | NT | NT | NT | 2.00 | 3.33 | NT | NT | NT | NT | NT | NT | 1.00 | 0.66 | 1.66 | 2.66 |
| T. emetica | 3.33 | 1.66 | NT | NT | NT | NT | 0.83 | 1.66 | NT | NT | 5.33 | 1.66 | 1.66 | 1.66 | 3.33 | 8.00 | NT | NT | NT | NT |
| W. salutaris | NT | NT | 1.66 | 0.50 | NT | NT | NT | NT | 1.33 | 0.25 | NT | NT | NT | NT | NT | NT | 1.00 | 1.33 | 2.00 | 0.41 |
| V. karroo | 2.00 | 0.66 | NT | NT | NT | NT | 2.00 | 0.66 | NT | NT | 8.00 | 1.66 | 4.00 | 1.00 | 8.00 | 4.00 | NT | NT | NT | NT |
| V. robusta | NT | NT | 1.66 | 2.66 | NT | NT | NT | NT | 0.83 | 1.66 | NT | NT | NT | NT | NT | NT | 1.00 | 1.66 | 1.00 | 1.00 |
| Z. mucronata | 1.66 | 0.66 | 0.50 | 0.83 | 0.50 | 1.33 | 1.00 | 1.00 | 0.83 | 0.83 | 0.66 | 1.66 | 0.25 | 1.00 | 0.50 | 1.33 | NT | NT | NT | NT |
| Ciprofloxacin positive control (µg/mL) | 0.07 | 0.05 | 0.02 | <0.02 | <0.02 | 0.66 | <0.02 | 0.07 | 0.03 | 0.05 | ||||||||||
| Acetone negative control | 8.00 | >8.00 | >8.00 | >8.00 | >8.00 | >8.00 | >8.00 | >8.00 | >8.00 | >8.00 | ||||||||||
| DMSO negative control | 8.00 | 8.00 | 8.00 | 4.00 | 4.00 | 6.66 | 6.66 | 6.66 | 4.00 | 4.00 | ||||||||||
2.2.3. Schotia brachypetala
2.2.4. Sclerocarya birrea
2.2.5. Pterocelastrus rostratus
2.2.6. Rapanea melanophloeos
2.2.7. Warburgia salutaris
2.3. Pharmacokinetics Perspective
2.4. Brine Shrimp Toxicity Assay
3. Materials and Methods
3.1. The Study Area: The Muthi Markets
3.1.1. The Faraday Muthi Market
3.1.2. The Kwa Mai-Mai Muthi Market
3.1.3. Trader Information and Data Recorded
3.2. Preparation of Plant Extracts
3.3. Test Pathogens
3.4. Antimicrobial Assay
3.5. Brine Shrimp Toxicity Screening
3.5.1. Sample Preparation
3.5.2. Cytotoxic Brine Shrimp Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angyalossy, V.; Pace, M.R.; Evert, R.F.; Marcati, C.R.; Oskolski, A.A.; Terrazas, T.; Kotina, E.; Lens, F.; Mazzoni-Viveiros, S.C.; Angeles, G.; et al. Iawa list of microscopic bark features. IAWA J. 2016, 37, 517–615. [Google Scholar] [CrossRef]
- Evert, R.F. Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development, 3rd ed.; National Library of Australia: Canberra, Australia, 2006. [Google Scholar]
- Jablonsky, M.; Nosalova, J.; Sladkova, A.; Haz, A.; Kreps, F.; Valka, J.; Miertus, S.; Frecer, V.; Ondrejovic, M.; Sima, J.; et al. Valorisation of softwood bark through extraction of utilizable chemicals: A review. Biotechnol. Adv. 2017, 35, 726–750. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Biswas, R.; Sharma, A.; Banerjee, S.; Biswas, S.; Katiyar, C.K. Validation of medicinal herbs for anti-tyrosinase potential. J. Herb. Med. 2018, 14, 1–16. [Google Scholar] [CrossRef]
- Pagliosa, C.M.; Vieira, M.A.; Podestá, R.; Maraschin, M.; Zeni, A.L.B.; Amante, E.R.; Amboni, R.D.M.C. Methylxanthines, phenolic composition, and antioxidant activity of bark from residues from mate tree harvesting (Ilex par-aguariensis a. St. Hil.). Food Chem. 2010, 122, 173–178. [Google Scholar] [CrossRef]
- Naik, R.; Harmalkar, D.S.; Xu, X.; Jang, K.; Lee, K. Bioactive benzofuran derivatives: Moracins a-z in medicinal chemistry. Eur. J. Med. Chem. 2015, 90, 379–393. [Google Scholar] [CrossRef]
- Sabandar, C.W.; Jalil, J.; Ahmat, N.; Aladdin, N.-A. Medicinal uses, chemistry and pharmacology of Dillenia species (dilleniaceae). Phytochemistry 2017, 134, 6–25. [Google Scholar] [CrossRef]
- Nganou, B.K.; Mbaveng, A.T.; Fobofou, S.A.T.; Fankam, A.G.; Bitchagno, G.T.M.; Mpetga, J.D.S.; Wessjohann, L.A.; Kuete, V.; Efferth, T.; Tanea, P. Furoquinolines and dihydrooxazole alkaloids with cytotoxic activity from the stem bark of Araliopsis soyauxii. Fitoterapia 2019, 133, 193–199. [Google Scholar] [CrossRef]
- Mangal, P.; Khare, P.; Jagtap, S.; Bishnoi, M.; Kondepudi, K.K.; Bhutani, K.K. Screening of six ayurvedic medicinal plants for anti-obesity potential: An investigation on bioactive constituents from Oroxylum indicum (l.) kurz bark. J. Ethnopharmacol. 2017, 197, 138–146. [Google Scholar] [CrossRef]
- Misra, H.; Mehta, B.K.; Jain, D.C. Optimization of extraction conditions and hptlc-uv method for determination of quinine in different extracts of Cinchona species bark. Rec. Nat. Prod. 2008, 2, 107–115. [Google Scholar]
- Donehower, R.C. The clinical development of paclitaxel: A successful collaboration of academia, industry and the national cancer institute. Stem Cells 1996, 14, 25–28. [Google Scholar] [CrossRef]
- Desborough, M.J.R. The aspirin story—From willow to wonder drug. Br. J. Haematol. 2017, 177, 674–683. [Google Scholar] [CrossRef]
- Grace, O.M.; Prendergast, H.D.V.; Jäger, A.K.; van Staden, J. Bark medicines used in traditional healthcare in kwazulu-natal, South Africa: An inventory. S. Afr. J. Bot. 2003, 69, 301–363. [Google Scholar] [CrossRef]
- Hutchings, A.; Scott, A.H.; Lewis, G.; Cunningham, A. Zulu Medicinal Plants: An Inventory; University of Natal Press: Pietermaritzburg, South Africa, 1996. [Google Scholar]
- Mander, M.; Ntuli, L.; Diederichs, N.; Mavundla, K. Economics of the traditional medicine trade in South Africa care delivery. S. Afr. Health Rev. 2007, 2007, 189–196. [Google Scholar]
- Mathabe, M.C.; Nikolova, R.V.; Lall, N.; Nyazema, N.Z. Antibacterial activities of medicinal plants used for the treatment of diarrhoea in Limpopo Province, South Africa. J. Ethnopharmacol. 2006, 105, 286–293. [Google Scholar] [CrossRef]
- Williams, V.L. Trade and socio-economic value of forest and woodland reserves within the medicinal plant market in Johannesburg. In Indigenous Forests and Woodlands in South Africa: Policy, People and Practice; Lawes, M.J., Eeley, H.A.C., Shackleton, C.M., Geach, B.G.S., Eds.; University of KwaZulu-Natal Press: Scottsville, South Africa, 2004. [Google Scholar]
- Mander, M. An overview of the medicinal plant market in South Africa. In Indigenous Forests and Woodlands in South Africa: Policy, People and Practice; Lawes, M.J., Eeley, H.A.C., Shackleton, C.M., Geach, B.G.S., Eds.; University of KwaZulu-Natal Press: Scottsville, South Africa, 2004. [Google Scholar]
- Williams, V.L.; Victor, J.E.; Crouch, N.R. Red listed medicinal plants of South Africa: Status, trends and assessment challenges. S. Afr. J. Bot. 2013, 86, 23–25. [Google Scholar] [CrossRef]
- Williams, V.L.; Wojtasik, E.M.; Witkowski, E.T.F. Ethno-ecological evidence for Hydnora abyssinica occurring in Johannesburg and Durban traditional medicine markets. S. Afr. J. Bot. 2011, 77, 268–279. [Google Scholar] [CrossRef][Green Version]
- Grace, O.M.; Prendergast, H.D.V.; van Staden, J.; Jäger, A.K. The status of bark in South African traditional health care. S. Afr. J. Bot. 2002, 68, 21–30. [Google Scholar] [CrossRef][Green Version]
- Mbendana, D.; Mamabolo, K.; Truter, M.; Kritzinger, Q.; Ndhlala, A.R. Practices at herbal (muthi) markets in Gauteng, South Africa and their impact on the health of the consumers: A case study of KwaMai-Mai and Marabastad muthi markets. S. Afr. J. Bot. 2019, 126, 30–39. [Google Scholar] [CrossRef]
- Khumalo, G.P. An Inventory of the Most Popular Medicinal Barks Sold on the Johannesburg Muthi Markets and the Antimicrobial Activity of Selected Extracts and Isolated Chemical Compounds. Master’s Thesis, University of Johannesburg, Johannesburg, South Africa, 2018. [Google Scholar]
- Cumes, D. South African indigenous healing: How it works. Explore 2013, 9, 58–65. [Google Scholar] [CrossRef]
- Fennel, C.W.; Lindsey, K.L.; McGaw, L.J.; Sparg, S.G.; Stafford, G.I.; Elgorashi, E.E.; Grace, O.M.; van Staden, J. Assessing African medicinal plants for efficacy and safety: Pharmacological screening and toxicology. J. Ethnopharmacol. 2004, 94, 205–217. [Google Scholar] [CrossRef]
- Verschaeve, L.; van Staden, J. Mutagenic and antimutagenic properties of extracts from South African traditional medicinal plants. J. Ethnopharmacol. 2008, 119, 575–587. [Google Scholar] [CrossRef]
- Cock, I.E.; Van Vuuren, S. South African food and medicinal plant extracts as potential antimicrobial food agents. J. Food Sci. Technol. 2015, 52, 6879–6899. [Google Scholar] [CrossRef]
- Ndhlala, A.R.; Ncube, B.; Okem, A.; Mulaudzi, R.B.; van Staden, J. Toxicology of some important medicinal plants in southern Africa. Food Chem. Toxicol. 2013, 62, 609–621. [Google Scholar] [CrossRef]
- Coe, F.G.; Parikh, D.M.; Johnson, C.A. Alkaloid presence and brine shrimp (Artemia salina) bioassay of medicinal species of Eastern Nicaragua. Pharm. Biol. 2010, 48, 439–445. [Google Scholar] [CrossRef]
- McGaw, L.J.; Elgorashi, E.E.; Eloff, J.N. Toxicity of African medicinal plants against normal animal and human cells. In Toxicological Survey of African Medicinal Plants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 181–233. [Google Scholar]
- Seleteng-Kose, L.; Moteetee, A.; van Vuuren, S. Medicinal plants used for the treatment of sexually transmitted infections in the Maseru District, Lesotho: Antimicrobial validation, phytochemical and cytotoxicity studies. S. Afr. J. Bot. 2019, 122, 457–466. [Google Scholar] [CrossRef]
- Ndhlala, A.R.; Stafford, G.I.; van Staden, J. Commercial herbal preparations in Kwazulu-Natal, South Africa: The urban face of traditional medicine. S. Afr. J. Bot. 2011, 77, 830–843. [Google Scholar] [CrossRef]
- Van Vuuren, S.; Williams, V.L.; Sooka, A.; Burger, A.; Van der Haar, L. Microbial contamination of traditional medicinal plants sold at the Faraday muthi market, Johannesburg, South Africa. S. Afr. J. Bot. 2014, 9, 98–100. [Google Scholar] [CrossRef]
- Van Wyk, B.-E.; Wink, M. Medicinal Plants of the World; CABI and Briza Publications: Pretoria, South Africa, 2017. [Google Scholar]
- White, R.M.; Cocks, M.; Herbert, D.G.; Hamer, M.L. Traditional medicines from forest animals. In Indigenous Forests and Woodlands in South Africa: Policy, People and Practice; Lawes, M.J., Eeley, H.A.C., Shackleton, C.M., Geach, B.G.S., Eds.; University of KwaZulu-Natal Press: Scottsville, South Africa, 2004. [Google Scholar]
- Mander, M. Marketing of Medicinal Plants in South Africa: A CASE study in Kwazulu-Natal; Food and Agriculture Organization of the United Nations: Rome, Italy, 1998. [Google Scholar]
- Ndawonde, B.G.; Zobolo, A.M.; Dlamini, E.T.; Siebert, S.J. A survey of plants sold by traders at Zululand muthi markets, with a view to selecting popular plant species for propagation in communal gardens. Afr. J. Range Forage Sci. 2007, 24, 103–107. [Google Scholar] [CrossRef]
- De Wet, H.; Nicki, S.; van Vuuren, S. Medicinal plants used for the treatment of various skin disorders by a rural community in Northern Maputaland, South Africa. J. Ethnobiol. Ethnomed. 2013, 9, 51–60. [Google Scholar] [CrossRef]
- Van Wyk, B.; Van Wyk, P.; Van Wyk, B.-E. Photographic Guide to Trees of Southern Africa; Briza Publications: Pretoria, South Africa, 2011. [Google Scholar]
- Van Wyk, B.-E.; Oudtshoorn, B.; Gericke, N. Medicinal Plants of South Africa, 2nd ed.; Briza Publications: Pretoria, South Africa, 2009. [Google Scholar]
- Watt, J.M.; Breyer-Brandwijk, M.G. The Medicinal and Poisonous Plants of Southern and East Africa, 2nd ed.; Livingstone: Edinburgh, UK, 1962. [Google Scholar]
- Mabogo, D.E.N. Ethnobotany of the Vhavenda. Masters Thesis, University of Pretoria, Pretoria, South Africa, 1990. [Google Scholar]
- Pujol, J. Naturafrica: The Herbalist Handbook: African Flora Medicinal Plants. Natural Healers Foundation; Thorolds Contemporary Africana, Law & General Booksellers (Frank R. Thorold Pty Ltd.): Sandton, South Africa, 1990. [Google Scholar]
- Prat, C.; Lacoma, A. Bacteria in the respiratory tract–how to treat? Or do not treat? Int. J. Infect. Dis. 2016, 51, 113–122. [Google Scholar] [CrossRef]
- Van Vuuren, S.F.; Holl, D. Antimicrobial natural product research: A review from a South African perspective for the years 2009–2016. J. Ethnopharmacol. 2017, 208, 236–252. [Google Scholar] [CrossRef]
- McGaw, L.J.; Lall, N.; Meyer, J.J.M.; Eloff, J.N. The potential of South African plants against Mycobacterium infections. J. Ethnopharmacol. 2008, 119, 482–500. [Google Scholar] [CrossRef] [PubMed]
- Statssa. Mortality and Causes of Death in South Africa, 1997–2003: Findings from Death Notification. Available online: http://www.statssa.gov.za/ (accessed on 21 April 2021).
- York, T.; De Wet, H.; van Vuuren, S. Plants used for treating respiratory infection in rural Maputaland, Kwa Zulu-Natal, South Africa. J. Ethnopharmacol. 2011, 135, 696–710. [Google Scholar] [CrossRef]
- York, T.; van Vuuren, S.; De Wet, H. An antimicrobial evaluation of plants used for the treatment of respiratory infections in rural Maputaland, Kwa Zulu-Natal, South Africa. J. Ethnopharmacol. 2012, 144, 118–127. [Google Scholar] [CrossRef]
- De Wet, H.; Nkwanyana, M.N.; van Vuuren, S. Medicinal plants used for the treatment of diarrhoea in northern Maputaland, Kwazulu-Natal Province, South Africa. J. Ethnopharmacol. 2010, 130, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Samie, A.; Obi, C.L.; Bessong, P.O.; Namrita, L. Activity profiles of fourteen selected medicinal plants from Rural Venda communities in South Africa against fifteen clinical bacterial species. Afr. J. Biotechnol. 2005, 4, 1443–1451. [Google Scholar]
- Basson, W. Diarrhoea now the third biggest killer in South Africa. CSIR Sci. Scope 2009, 4, 31–33. [Google Scholar]
- Palombo, E.A. Phytochemicals from traditional medicinal plants used in the treatment of diarrhoea: Modes of action and effects on intestinal function. Phytother. Res. 2006, 20, 717–724. [Google Scholar] [CrossRef]
- Van Wyk, B.-E.; Wink, M. Phytomedicines, Herbal Drugs and Plant Poisons; Kew Publishers and Briza Publications: London, UK, 2015. [Google Scholar]
- Hutchings, A. A survey and analysis of traditional medicinal plants as used by the Zulu, Xhosa and Sotho. Bothalia 1989, 19, 111–123. [Google Scholar] [CrossRef]
- Ashbolt, N.J. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology 2004, 198, 229–238. [Google Scholar] [CrossRef]
- Pandey, P.K.; Kass, P.H.; Soupir, M.L.; Biswas, S.; Singh, V.P. Contamination of water resources by pathogenic bacteria. AMB Express 2014, 4, 51–67. [Google Scholar] [CrossRef]
- Madikizela, B.; Ndhlala, A.R.; Finnie, J.F.; van Staden, J. Ethnopharmacological study of plants from Pondoland used against diarrhoea. J. Ethnopharmacol. 2012, 141, 61–71. [Google Scholar] [CrossRef]
- Kokoska, L.; Kloucek, P.; Leuner, O.; Novy, P. Plant-derived products as antibacterial and antifungal agents in human health care. Curr. Med. Chem. 2019, 26, 5501–5541. [Google Scholar] [CrossRef]
- Chabán, M.F.; Karagianni, C.; Joray, M.B.; Toumpa, D.; Solá, C.; Crespo, M.I.; Palacios, S.M.; Athanassopoulos, C.M.; Carpinella, M.C. Antibacterial effects of extracts obtained from plants of argentina: Bioguided isolation of compounds from the anti-infectious medicinal plant lepechinia meyenii. J. Ethnopharmacol. 2019, 239, 111930. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- Joray, M.B.; Trucco, L.D.; González, M.L.; Napal, G.N.D.; Palacios, S.M.; Bocco, J.L.; Carpinella, M.C. Antibacterial and cytotoxic activity of compounds isolated from Flourensia oolepis. Evid. Based Complementary Altern. Med. 2015, 2015, 912484. [Google Scholar] [CrossRef] [PubMed]
- Mabona, U.; Viljoen, A.M.; Shikanga, E.; Marston, A.; van Vuuren, S. Antimicrobial activity of southern African medicinal plants with dermatological relevance: From an ethnopharmacological screening approach, to combination studies and the isolation of a bioactive compound. J. Ethnopharmacol. 2013, 148, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Rabe, T.; van Staden, J. Antibacterial activity of South African plants used for medicinal purposes. J. Ethnopharmacol. 1997, 56, 81–87. [Google Scholar] [CrossRef]
- Mukandiwa, L.; Naidoo, V.; Eloff, J.N. In vitro antibacterial activity of seven plants used traditionally to treat wound myiasis in animals in southern Africa. J. Med. Plants Res. 2012, 6, 4379–4388. [Google Scholar]
- Sadgrove, N.J.; Oliveira, T.B.; Khumalo, G.P.; van Vuuren, S.; van Wyk, B.-E. Antimicrobial isoflavones and derivatives from Erythrina (Fabaceae): Structure activity perspective (SAR & QSAR) on experimental and mined values against Staphylococcus aureus. Antibiotics 2020, 9, 223. [Google Scholar]
- Van Vuuren, S.; Nkwanyana, M.N.; De Wet, H. Antimicrobial evaluation of plants used for the treatment of diarrhoea in a rural community in northern Maputaland, Kwazulu-Natal, South Africa. BMC Complementary Altern. Med. 2015, 15, 254. [Google Scholar] [CrossRef] [PubMed]
- Kaikabo, A.A.; Eloff, J.N. Antibacterial activity of two bioflavonoids from Garcinia livingstonei leaves against Mycobacterium smegmatis. J. Ethnopharmacol. 2011, 138, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Kaikabo, A.A.; Samuel, B.B.; Eloff, J.N. Isolation and activity of two antibacterial biflavonoids from Garcinia livingstonei leaf extract. Nat. Prod. Commun. 2009, 4, 1–4. [Google Scholar] [CrossRef]
- Boakye, P.A.; Brierley, S.M.; Pasilis, S.P.; Balemba, O.B. Garcinia buchananii bark extract is an effective anti-diarrheal remedy for lactose-induced diarrhea. J. Ethnopharmacol. 2012, 142, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, B.-E.; Oudtshoorn, B.V.; Gericke, N. Medicinal Plants of South Africa; Briza: Pretoria, South Africa, 1997. [Google Scholar]
- Corrigan, B.M.; van Wyk, A.E.; Geldenhuys, C.J.; Jardine, J.M. Ethnobotanical plant uses in the Kwanibela Peninsula, St Lucia, South Africa. S. Afr. J. Bot. 2011, 77, 346–359. [Google Scholar] [CrossRef]
- Sagbo, I.J.; Mbeng, W.O. Plants used for cosmetics in the eastern cape province of South Africa: A case study of skin care. Pharmacogn. Rev. 2015, 12, 139–156. [Google Scholar] [CrossRef]
- Tamokou, J.D.; Mpetga, D.J.S.; Lunga, P.K.; Tene, M.; Tane, P.; Kuiate, J.R. Antioxidant and antimicrobial activities of ethyl acetate extract, fractions and compounds from stem bark of Albizia adianthifolia. BMC Complementary Altern. Med. 2012, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Philander, L.A. An ethnobotany of Western Cape Rasta bush medicine. J. Ethnopharmacol. 2011, 138, 578–594. [Google Scholar] [CrossRef]
- Stafford, G.I.; Pendersen, M.E.; van Staden, J.; Jäger, A.K. Review on plants with cns-effects used in traditional South African medicine against mental diseases. J. Ethnopharmacol. 2008, 119, 513–537. [Google Scholar] [CrossRef]
- Kishore, N.; Lall, N. Are plants used for skin care in South Africa fully explored? J. Ethnopharmacol. 2014, 153, 61–84. [Google Scholar]
- Van Wyk, B.-E.; Gericke, N. People’s Plants: A Guide to Useful Plants of Southern Africa, 2nd ed.; Briza Publications: Pretoria, South Africa, 2018. [Google Scholar]
- Maroyi, A. Traditional usage, phytochemistry and pharmacology of Croton sylvaticus Hochst. ex c. Krauss. Asian Pac. J. Trop. Med. 2017, 10, 423–429. [Google Scholar] [CrossRef]
- Lall, N.; Meyer, J.J.M. In vitro inhibition of drug resistant and drug sensitive strains of Mycobacterium tuberculosis by ethnobotanically selected South African plants. J. Ethnopharmacol. 1999, 66, 347–354. [Google Scholar] [CrossRef]
- Reid, K.A.; Jäger, A.K.; van Staden, J. Pharmacological and phytochemical properties of Dombeya rotundifolia. S. Afr. J. Bot. 2001, 67, 349–353. [Google Scholar] [CrossRef]
- Vambe, M.; Aremu, A.O.; Chukwujekwu, J.C.; Finnie, J.F.; van Staden, J. Antibacterial screening, synergy studies and phenolic content of seven South African medicinal plants against drug-sensitive and -resistant microbial strains. S. Afr. J. Bot. 2018, 114, 250–259. [Google Scholar] [CrossRef]
- Mthethwa, N.S.; Oyedeji, B.A.O.; Obi, L.C.; Aiyegoro, A.O. Anti-staphylococcal, anti-hiv and cytotoxicity studies of four South African medicinal plants and isolation of bioactive compounds from Cassine transvaalensis (burtt. Davy) codd. BMC Complementary Altern. Med. 2014, 14, 512. [Google Scholar] [CrossRef] [PubMed]
- Khumalo, G.P.; Sadgrove, N.J.; van Vuuren, S.; Van Wyk, B.-E. Antimicrobial lupenol triterpenes and a polyphenol from Elaeodendron transvaalense, a popular southern African medicinal bark. S. Afr. J. Bot. 2019, 122, 518–521. [Google Scholar] [CrossRef]
- Masevhe, N.A.; McGaw, L.J.; Eloff, J.N. The traditional use of plants to manage candidiasis and related infections in Venda, South Africa. J. Ethnopharmacol. 2015, 168, 364–372. [Google Scholar] [CrossRef]
- Pillay, C.C.; Jäger, A.K.; Mulholland, D.A.; Van Staden, J. Cyclooxygenase inhibiting and anti-bacterial activities of South African Erythrina species. J. Ethnopharmacol. 2001, 74, 231–237. [Google Scholar] [CrossRef]
- Mulholland, D.A.; Mwangi, E.M.; Dlova, N.C.; Plant, N.; Crouch, N.R.; Coombes, P.H. Non-toxic melanin production inhibitors from Garcinia livingstonei (clusiaceae). J. Ethnopharmacol. 2013, 149, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Chinsembu, K.C.; Hijarunguru, A.; Mbangu, A. Ethnomedicinal plants used by traditional healers in the management of hiv/aids opportunistic diseases in Rundu, Kavango East Region, Namibia. S. Afr. J. Bot. 2015, 100, 33–42. [Google Scholar] [CrossRef]
- Chinsembu, K.C. Study of medicinal flora utilised by traditional healers in the management of sexually transmitted infections in Sesheke District, Western Province, Zambia. Rev. Bras. Farmacogn. 2016, 26, 268–274. [Google Scholar] [CrossRef]
- Madikizela, B.; McGaw, L.J. Pittosporum viridiflorum Sims (Pittosporaceae): A review on a useful medicinal plant native to South Africa and tropical Africa. J. Ethnopharmacol. 2017, 205, 217–230. [Google Scholar] [CrossRef]
- Bodeker, G.; Van’t Klooster, C.; Weisbord, E. Prunus africana (hook.F.) kalkman: The overexploitation of a medicinal plant species and its legal context. J. Altern. Complementary Med. 2014, 20, 810–822. [Google Scholar] [CrossRef]
- Otang, W.M.; Grierson, D.S.; Ndip, R.N. Phytochemical studies and antioxidant activity of two South African medicinal plants traditionally used for the management of opportunistic fungal infections in HIV/AIDS patients. BMC Complementary Altern. Med. 2012, 12, 43. [Google Scholar] [CrossRef]
- Maroyi, A. Traditional use of medicinal plants in south-central Zimbabwe: Review and perspectives. J. Ethnobiol. Ethnomed. 2013, 9, 31–49. [Google Scholar] [CrossRef] [PubMed]
- McGaw, L.J.; Jäger, A.K.; van Staden, J. Variation in antibacterial activity of Schotia species. S. Afr. J. Bot. 2002, 68, 41–46. [Google Scholar] [CrossRef]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef]
- Muthee, J.K.; Gakuya, D.W.; Mbaria, J.M.; Mulei, C.M. Phytochemical screening and toxicity of selected plants used as anthelmintics in Loitoktok Sub-County, Kenya. J. Phytopharm. 2016, 5, 15–19. [Google Scholar]
- Njume, C.; Afolayan, A.J.; Green, E.; Ndip, R.N. Volatile compounds in the stem bark of Sclerocarya birrea (anacardaceae) possess antimicrobial activity against drug- resistant strain of Helicobacter pylori. Int. J. Antimicrob. Agents 2011, 38, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Moyo, M.; Finnie, J.F.; van Staden, J. Antimicrobial and cyclooxygenase enzyme inhibitory activities of Sclerocarya birrea and Harpephyllum caffrum (anacardiaceae) plant extracts. J. Ethnopharmacol. 2011, 105, 286–293. [Google Scholar] [CrossRef][Green Version]
- Amenya, H.Z.; Gathumbi, P.K.; Mbaria, J.M.; Thaiyah, A.G.; Thoithi, G.N. Sub-acute toxicity of the chloroformic extract of Rapanea melanophloeos (l.) mez in rats. J. Ethnopharmacol. 2014, 154, 593–599. [Google Scholar] [CrossRef]
- Khumalo, G.P.; Sadgrove, N.J.; Van Vuuren, S.F.; Van Wyk, B.-E. Antimicrobial activity of volatile and non-volatile isolated compounds and extracts from the bark and leaves of Warburgia salutaris (canellaceae) against skin and respiratory pathogens. S. Afr. J. Bot. 2019, 122, 547–550. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Jones, G.L. From petri dish to patient: Bioavailability estimation and mechanism of action for antimicrobial and immunomodulatory natural products. Front. Microbiol. 2019, 10, 2470. [Google Scholar] [CrossRef] [PubMed]
- Bussmann, R.W.; Malca, G.; Glenn, A.; Sharon, D.; Nilsen, B.; Parris, B.; Dubose, D.; Ruiz, D.; Saleda, J.; Martinez, M.; et al. Toxicity of medicinal plants used in traditional medicine in northern Peru. J. Ethnopharmacol. 2011, 137, 121–140. [Google Scholar] [CrossRef]
- Oryema, C.; Ziraba, R.B.; Odyek, O.; Omagor, N.; Opio, A. Phytochemical properties and toxicity to brine shrimp of medicinal plants in Erute County, Lira District, Uganda. J. Med. Plants Res. 2011, 5, 5450–5457. [Google Scholar]
- Komane, B.M.; Olivier, E.I.; Viljoen, A.M. Trichilia emetica (meliaceae)—A review of traditional uses, biological activities and phytochemistry. Phytochem. Lett. 2011, 4, 1–9. [Google Scholar] [CrossRef]
- Prozesky, E.A.M.; Meyer, J.J.; Louw, A.I. In vitro antiplasmodial activity and cytotoxicity of ethnobotanically selected South African plants. J. Ethnopharmacol. 2001, 76, 239–245. [Google Scholar] [CrossRef]
- Germano, M.P.; D’Angelo, V.; Sanogo, R.; Catania, S.; Alma, R.; De Pascual, R.; Bisignano, G. Hepatoprotective and antibacterial effects of extracts from Trichilia emetica vahl. J. Ethnopharmacol. 2005, 96, 227–232. [Google Scholar] [CrossRef]
- Gerstner, J. Some factors affecting the perpetuation of our indigenous silva. J. S. Afr. For. Assoc. 1946, 13, 4–11. [Google Scholar] [CrossRef]
- Lewis, G.E. Testing the toxicity of extracts of southern African plants using brine shrimp (Artemia salina). S. Afr. J. Sci. 1995, 9, 382–384. [Google Scholar]
- Williams, V.L.; Whiting, M.J. A picture of health? Animal use and the faraday traditional medicine market, South Africa. J. Ethnopharmacol. 2016, 179, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; McLaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Sadgrove, N.J.; Simmonds, M.S.J. Topical and nutricosmetic products for healthy hair and dermal anti-aging using ‘dual-acting’ (2 for 1) plant-based peptides, hormones, and cannabinoids. FASEB BioAdvances 2021. [Google Scholar] [CrossRef]
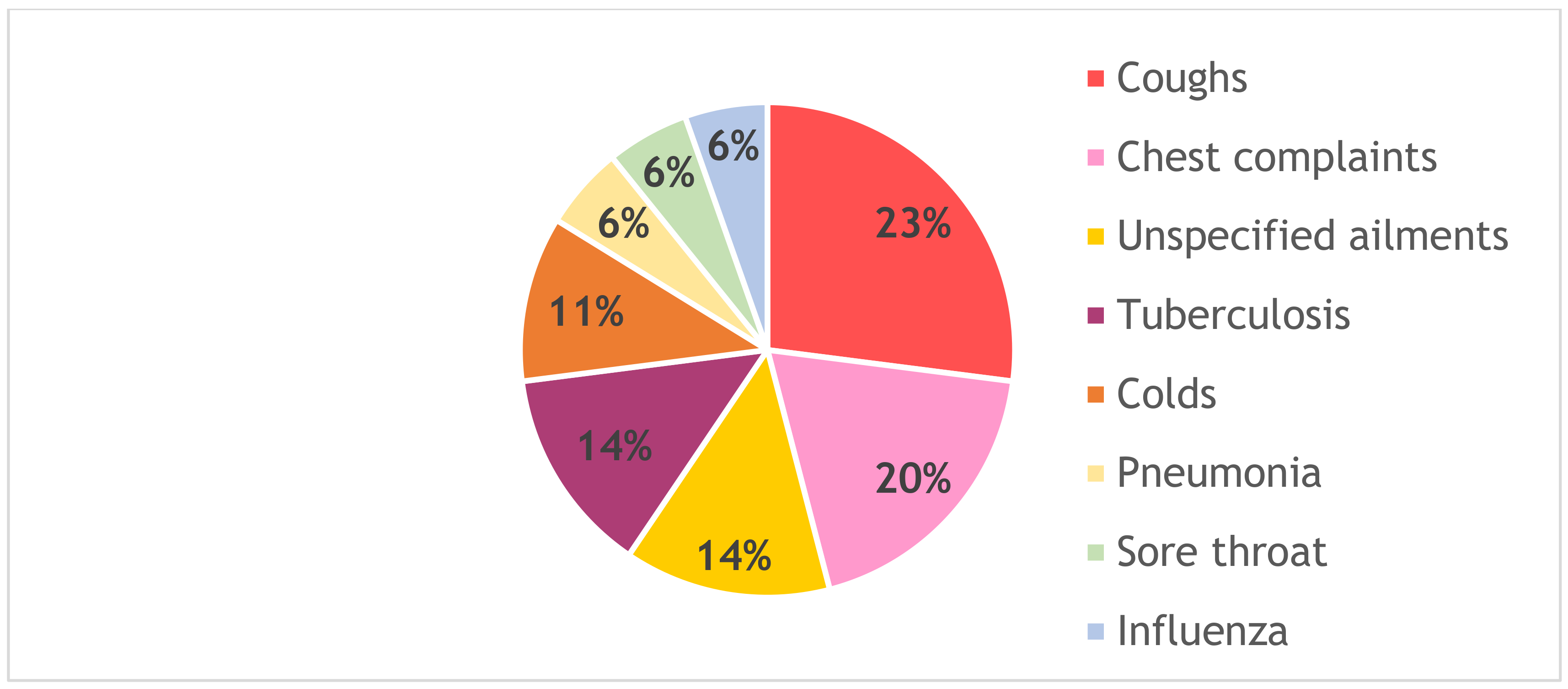
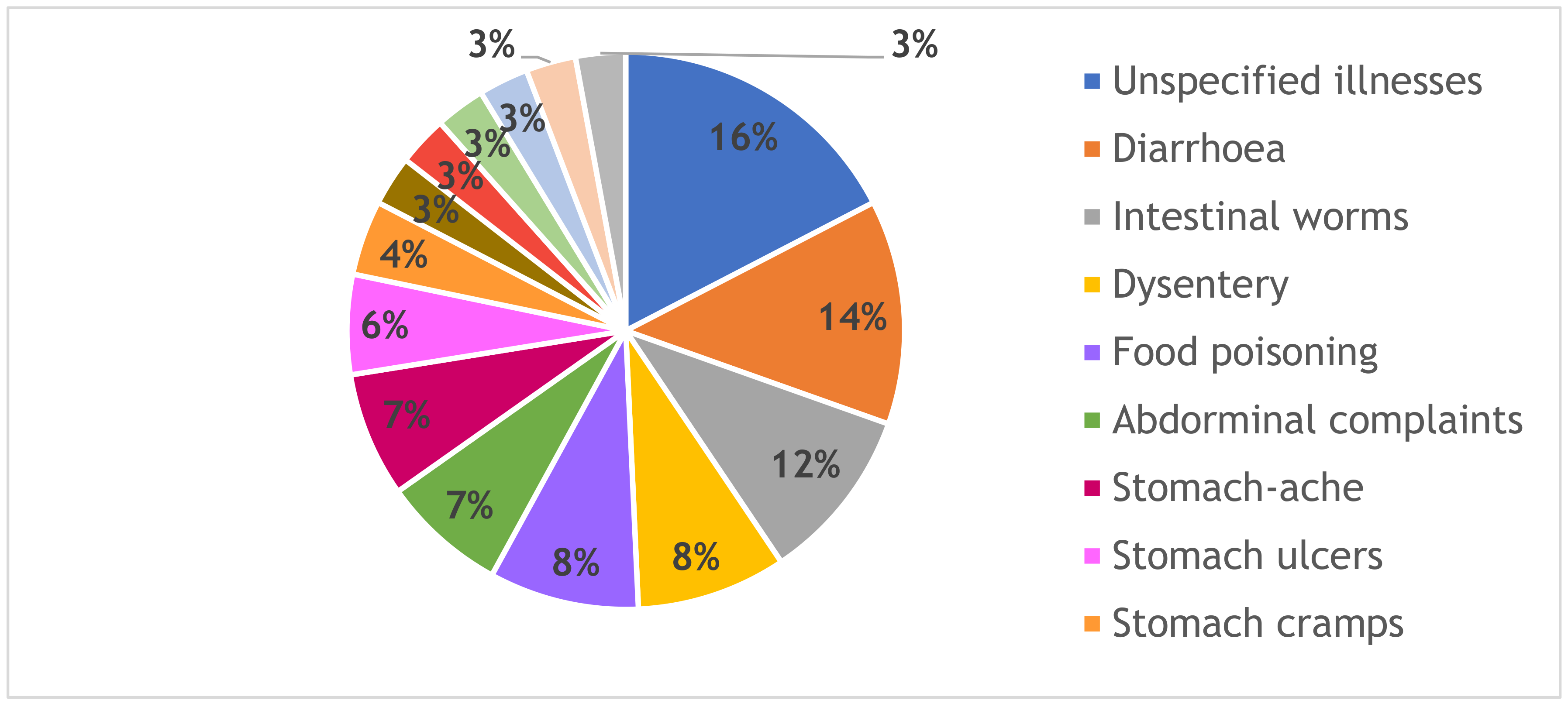
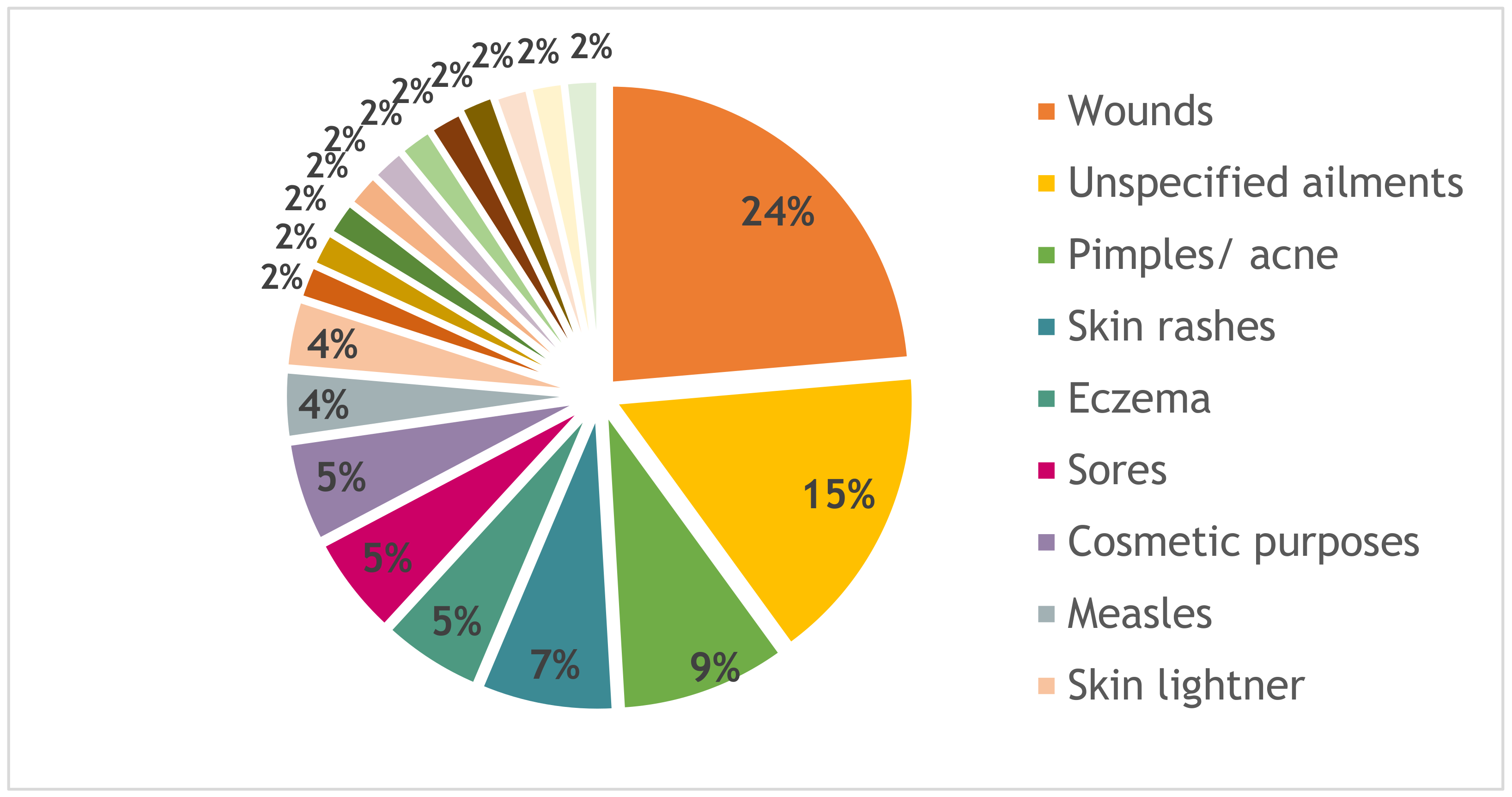



| Plant Species | Solvent Extract | Percentage Mortality @ 24 h | Percentage Mortality @ 48 h |
|---|---|---|---|
| Albizia adianthifolia | Methanol | 23.40 | 48.75 |
| Albizia adianthifolia | DCM | 4.00 | 6.87 |
| Bersama lucens | Methanol | 15.62 | 29.68 |
| Bersama lucens | DCM | 6.50 | 10.00 |
| Calodendrum capense | Methanol | 9.92 | 34.04 |
| Cinnamomum camphora | Methanol | 1.63 | 9.83 |
| Croton sylvaticus | Methanol | 13.64 | 32.71 |
| Cryptocarya latifolia | DCM | 18.17 | 30.88 |
| Dombeya rotundifolia | DCM | 7.84 | 7.84 |
| Ekebergia capensis | Methanol | 8.97 | 19.17 |
| Elaeodendron transvaalense | DCM | 22.37 | 48.00 |
| Erythrina lysistemon | Methanol | 26.51 | 37.05 |
| Erythrina lysistemon | DCM | 10.21 | 16.68 |
| Erythrophleum lasianthum | Methanol | 11.11 | 20.74 |
| Erythrophleum lasianthum | DCM | 11.34 | 18.55 |
| Garcinia livingstonei | Methanol | 19.12 | 46.85 |
| Harpephyllum caffrum | Methanol | 9.87 | 13.61 |
| Kigelia africana | Methanol | 3.25 | 6.00 |
| Ocotea bullata | Methanol | 8.85 | 12.19 |
| Peltophorum africana | Methanol | 20.40 | 25.85 |
| Pittosporum viridiflorum | DCM | 12.57 | 17.94 |
| Prunus africana | DCM | 22.00 | 32.00 |
| Pterocelastratus rostratus | DCM | 12.99 | 26.33 |
| Rapanea melanophloeos | Methanol | 14.42 | 23.09 |
| Rapanea melanophloeos | DCM | 11.84 | 17.33 |
| Rauvolfia caffra | Methanol | 3.25 | 4.01 |
| Schotia brachypetala | DCM | 24.00 | 36.10 |
| Sclerocarya birrea | Methanol | 6.38 | 12.46 |
| Securidaca longependunculata | Methanol | 18.33 | 30.89 |
| Strychnos henningsii | Methanol | 4.08 | 5.51 |
| Syzygium cordatum | Methanol | 8.22 | 20.00 |
| Trichilia emetica | Methanol | 40.95 | 69.52 |
| Trichilia emetica | DCM | 7.84 | 19.60 |
| Vachellia karroo | Methanol | 7.99 | 18.21 |
| Vachelia robusta | Methanol | 11.97 | 18.30 |
| Warburgia salutaris | DCM | 13.61 | 22.33 |
| Ziziphus mucronata | DCM | 8.33 | 15.18 |
| CONTROLS | |||
| Negative (salt water 32 mg/mL) | 0 | 0 | |
| Negative (2% DMSO) | 2.11 | 5.34 | |
| Positive control (1.6 mg/mL potassium dichromate) | 94% | 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khumalo, G.P.; Sadgrove, N.J.; Van Vuuren, S.F.; Van Wyk, B.-E. South Africa’s Best BARK Medicines Prescribed at the Johannesburg Muthi Markets for Skin, Gut, and Lung Infections: MIC’s and Brine Shrimp Lethality. Antibiotics 2021, 10, 681. https://doi.org/10.3390/antibiotics10060681
Khumalo GP, Sadgrove NJ, Van Vuuren SF, Van Wyk B-E. South Africa’s Best BARK Medicines Prescribed at the Johannesburg Muthi Markets for Skin, Gut, and Lung Infections: MIC’s and Brine Shrimp Lethality. Antibiotics. 2021; 10(6):681. https://doi.org/10.3390/antibiotics10060681
Chicago/Turabian StyleKhumalo, Gugulethu P., Nicholas J. Sadgrove, Sandy F. Van Vuuren, and Ben-Erik Van Wyk. 2021. "South Africa’s Best BARK Medicines Prescribed at the Johannesburg Muthi Markets for Skin, Gut, and Lung Infections: MIC’s and Brine Shrimp Lethality" Antibiotics 10, no. 6: 681. https://doi.org/10.3390/antibiotics10060681
APA StyleKhumalo, G. P., Sadgrove, N. J., Van Vuuren, S. F., & Van Wyk, B.-E. (2021). South Africa’s Best BARK Medicines Prescribed at the Johannesburg Muthi Markets for Skin, Gut, and Lung Infections: MIC’s and Brine Shrimp Lethality. Antibiotics, 10(6), 681. https://doi.org/10.3390/antibiotics10060681










