Prevalence and Risk Factors for Multidrug-Resistant Organisms Colonization in Long-Term Care Facilities Around the World: A Review
Abstract
1. Introduction
2. Results and Discussion
2.1. Studies Included
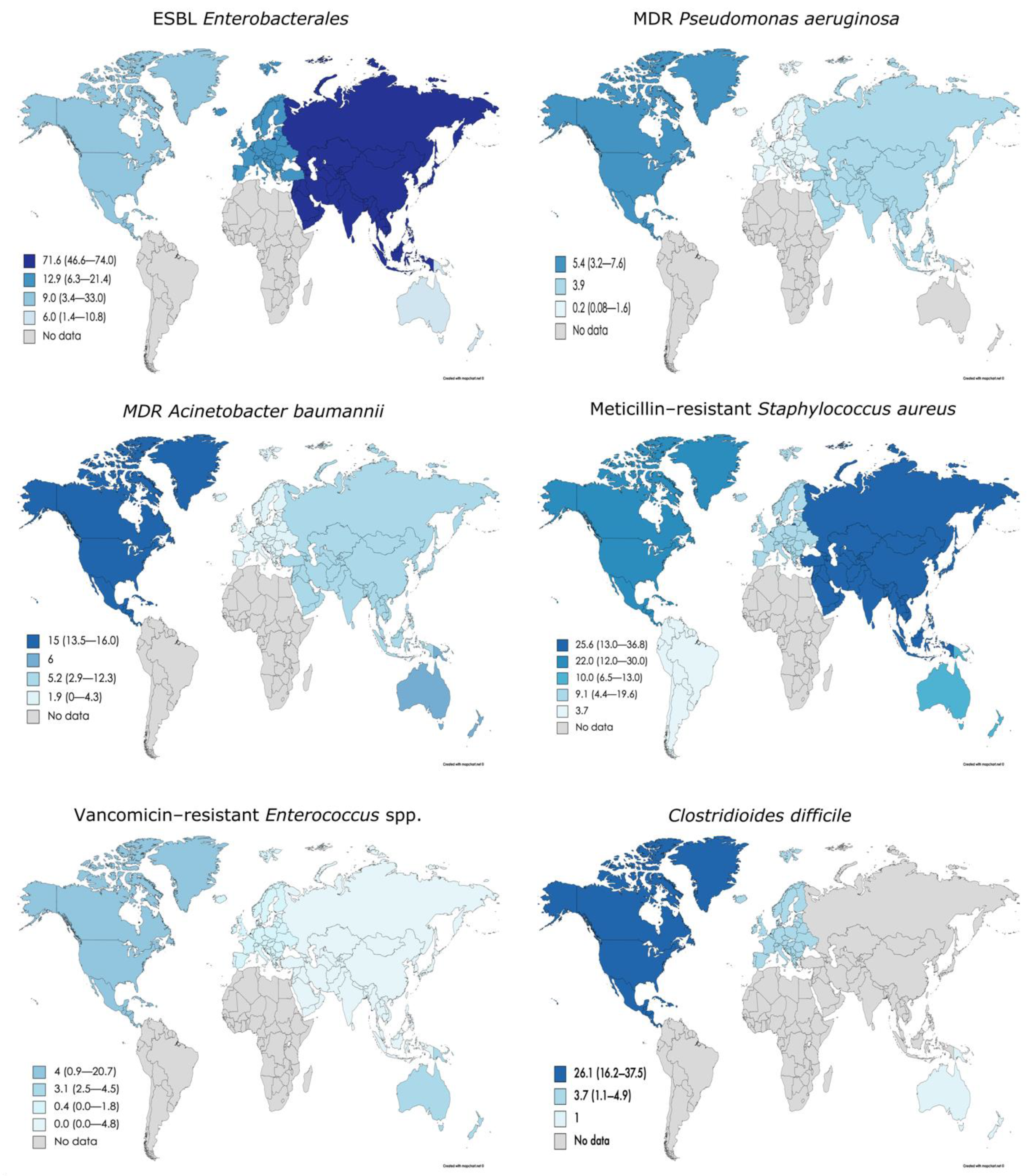
2.2. MDRO Prevalence
2.3. MDRO Co-Colonization
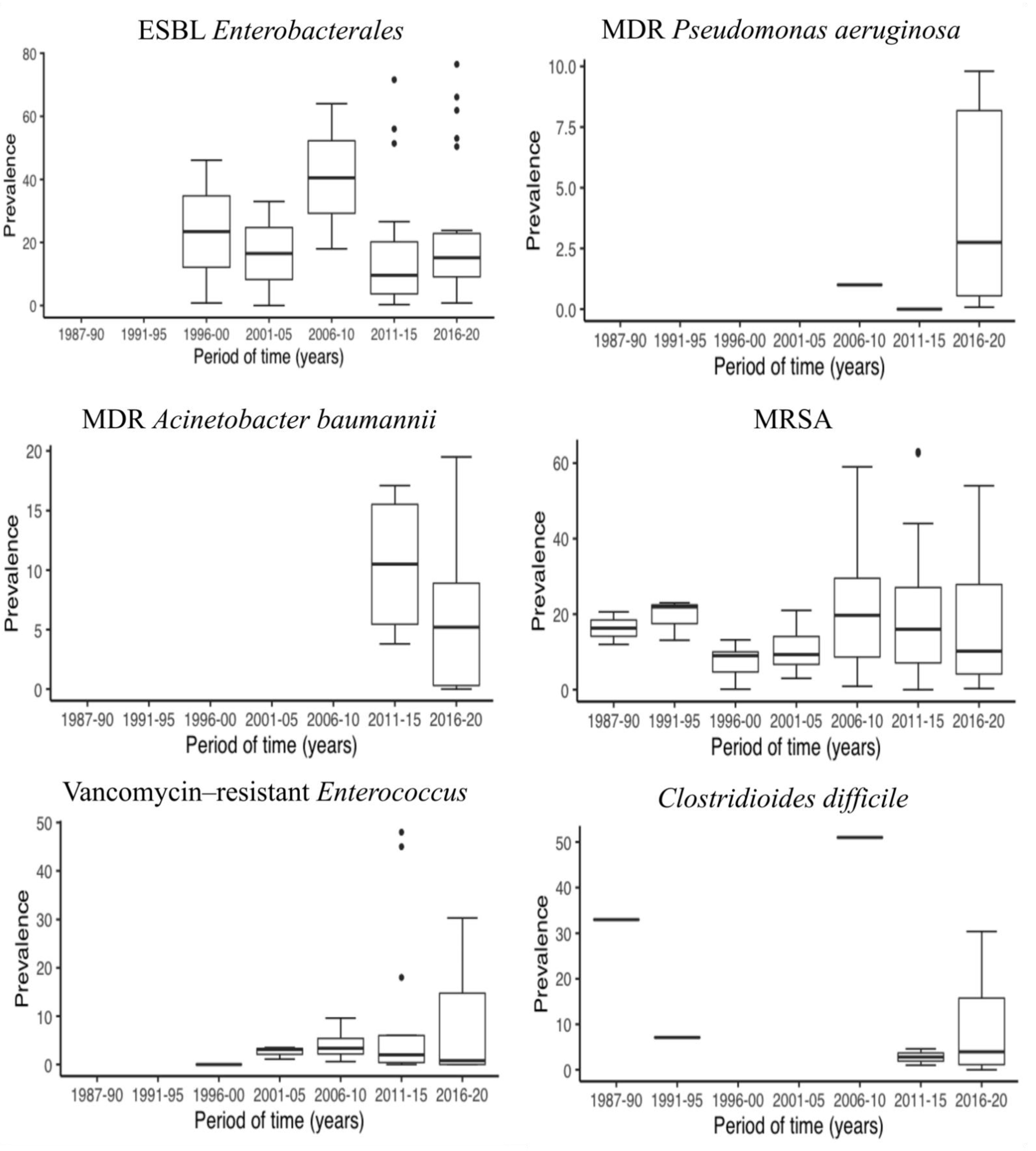
2.4. Changes in MDRO Prevalence Over Time
2.5. Risk Factors for Colonization
2.5.1. Reside in Nursing Homes
2.5.2. Age
2.5.3. Sex
2.5.4. Underlying Diseases: Dementia (Lower Cognitive Status), Diabetes, Cancer and Chronic Wound (Pressure/Decubitus Ulcer)
2.5.5. Dependence or Disability
2.5.6. Medical Devices: Indwelling or Invasive Devices, Urinary Catheters, and Gastrointestinal Tubes (Feeding or Percutaneous Enteral Gastrostomy Tubes)
2.5.7. Antibiotics Use in the Preceding Months
2.5.8. Hospital Admission in the Previous 12 Months, Any Department
2.5.9. Previous Colonization by MDRO
3. Materials and Methods
3.1. Search Strategy
3.2. Study Selection and Eligibility Criteria
3.3. Definitions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Stepan, D.; Ušaj, L.; Šter, M.P.; Galun, M.S.; Smole, H.; Beović, B. Antimicrobial prescribing in long-term care facilities: A nationwide point-prevalence study, Slovenia, 2016. Eurosurveillance 2018, 23, 1800100. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, D.; Briggs, R.; Holmerová, I.; Samuelsson, O.; Gordon, A.L.; Martin, F.C.; The Special Interest Group in Long Term Care of the European Geriatric Medicine Society. COVID-19 highlights the need for universal adoption of standards of medical care for physicians in nursing homes in Europe. Eur. Geriatr. Med. 2020, 11, 645–650. [Google Scholar] [CrossRef]
- Infectious Diseases Society of America; Spellberg, B.; Blaser, M.; Guidos, R.J.; Boucher, H.W.; Bradley, J.S.; Eisenstein, B.I.; Gerding, D.; Lynfield, R.; Reller, L.B.; et al. Combating Antimicrobial Resistance: Policy Recommendations to Save Lives. Clin. Infect. Dis. 2011, 52, S397–S428. [Google Scholar] [CrossRef]
- van Duin, D.; Paterson, D.L. Multidrug-Resistant Bacteria in the Community. Infect. Dis. Clin. N. Am. 2016, 30, 377–390. [Google Scholar] [CrossRef]
- Vehreschild, M.J.G.T.; Haverkamp, M.; Biehl, L.M.; Lemmen, S.; Fätkenheuer, G. Vancomycin-resistant enterococci (VRE): A reason to isolate? Infection 2019, 47, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, J.P.; Montero, M.; Oliver, A.; Sorlí, L.; Luque, S.; Gómez-Zorrilla, S.; Benito, N.; Grau, S. Epidemiology and Treatment of Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa Infections. Clin. Microbiol. Rev. 2019, 32, 00031-19. [Google Scholar] [CrossRef] [PubMed]
- Perez, F.; Hujer, A.M.; Hujer, K.M.; Decker, B.K.; Rather, P.N.; Bonomo, R.A. Global Challenge of Multidrug-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2007, 51, 3471–3484. [Google Scholar] [CrossRef]
- Barbut, F.; Jones, G.; Eckert, C. Epidemiology and control of Clostridium difficile infections in healthcare settings. Curr. Opin. Infect. Dis. 2011, 24, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Korsak, N.; Taminiau, B.; Avesani, V.; Van Broeck, J.; Delmée, M.; Daube, G. Clostridium difficile infection in elderly nursing home residents. Anaerobe 2014, 30, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Bell, B.G.; Schellevis, F.; Stobberingh, E.; Goossens, H.; Pringle, M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014, 14, 13. [Google Scholar] [CrossRef]
- Molina, J.; Peñalva, G.; Gil-Navarro, M.V.; Praena, J.; Lepe, J.A.; Pérez-Moreno, M.A.; Ferrándiz, C.; Aldabó, T.; Aguilar, M.; Olbrich, P.; et al. Long-Term Impact of an Educational Antimicrobial Stewardship Program on Hospital-Acquired Candidemia and Multidrug-Resistant Bloodstream Infections: A Quasi-Experimental Study of Interrupted Time-Series Analysis. Clin. Infect. Dis. 2017, 65, 1992–1999. [Google Scholar] [CrossRef] [PubMed]
- Batina, N.G.; Crnich, C.J.; Döpfer, D. Acquisition and persistence of strain-specific methicillin-resistant Staphylococcus aureus and their determinants in community nursing homes. BMC Infect. Dis. 2017, 17, 752. [Google Scholar] [CrossRef]
- Kohler, P.; Fulchini, R.; Albrich, W.C.; Egli, A.; Balmelli, C.; Harbarth, S.; Héquet, D.; Kahlert, C.R.; Kuster, S.P.; Petignat, C.; et al. Antibiotic resistance in Swiss nursing homes: Analysis of National Surveillance Data over an 11-year period between 2007 and 2017. Antimicrob. Resist. Infect. Control. 2018, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Mylotte, J.M.; Goodnough, S.; Tayara, A. Antibiotic-resistant organisms among long-term care facility residents on admission to an inpatient geriatrics unit: Retrospective and prospective surveillance. Am. J. Infect. Control. 2001, 29, 139–144. [Google Scholar] [CrossRef]
- Drayß, M.; Claus, H.; Hubert, K.; Thiel, K.; Berger, A.; Sing, A.; Van Der Linden, M.; Vogel, U.; Lâm, T.-T. Asymptomatic carriage of Neisseria meningitidis, Haemophilus influenzae, Streptococcus pneumoniae, Group A Streptococcus and Staphylococcus aureus among adults aged 65 years and older. PLoS ONE 2019, 14, e0212052. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, L.; Roukens, M.; De Greeff, S.; Meessen, N.; Natsch, S.; Stobberingh, E. Carriage of antimicrobial-resistant commensal bacteria in Dutch long-term-care facilities. J. Antimicrob. Chemother. 2016, 71, 2586–2592. [Google Scholar] [CrossRef]
- Brugnaro, P.; Fedeli, U.; Pellizzer, G.; Buonfrate, D.; Rassu, M.; Boldrin, C.; Parisi, S.G.; Grossato, A.; Palù, G.; Spolaore, P. Clustering and Risk Factors of Methicillin-Resistant Staphylococcus aureus Carriage in Two Italian Long-Term Care Facilities. Infection 2008, 37, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Ludden, C.; Cormican, M.; Vellinga, A.; Johnson, J.R.; Austin, B.; Morris, D. Colonisation with ESBL-producing and carbapenemase-producing Enterobacteriaceae, vancomycin-resistant enterococci, and meticillin-resistant Staphylococcus aureus in a long-term care facility over one year. BMC Infect. Dis. 2015, 15, 168. [Google Scholar] [CrossRef]
- Giufrè, M.; Ricchizzi, E.; Accogli, M.; Barbanti, F.; Monaco, M.; De Araujo, F.P.; Farina, C.; Fazii, P.; Mattei, R.; Sarti, M.; et al. Colonization by multidrug-resistant organisms in long-term care facilities in Italy: A point-prevalence study. Clin. Microbiol. Infect. 2017, 23, 961–967. [Google Scholar] [CrossRef][Green Version]
- Nucleo, E.; Caltagirone, M.; Marchetti, V.M.; D’Angelo, R.; Fogato, E.; Confalonieri, M.; Reboli, C.; March, A.; Sleghel, F.; Soelva, G.; et al. Colonization of long-term care facility residents in three Italian Provinces by multidrug-resistant bacteria. Antimicrob. Resist. Infect. Control. 2018, 7, 33. [Google Scholar] [CrossRef]
- March, A.; Aschbacher, R.; Sleghel, F.; Soelva, G.; Kaczor, M.; Migliavacca, R.; Piazza, A.; Mattioni Marchetti, V.; Pagani, L.; Scalzo, K.; et al. Colonization of residents and staff of an Italian long-term care facility and an adjacent acute care hospital geriatric unit by multidrug-resistant bacteria. New Microbiol. 2017, 40, 258–263. [Google Scholar] [PubMed]
- March, A.; Aschbacher, R.; Dhanji, H.; Livermore, D.M.; Böttcher, A.; Sleghel, F.; Maggi, S.; Noale, M.; Larcher, C.; Woodford, N. Colonization of residents and staff of a long-term-care facility and adjacent acute-care hospital geriatric unit by multiresistant bacteria. Clin. Microbiol. Infect. 2010, 16, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Trick, W.E.; Weinstein, R.A.; DeMarais, P.L.; Kuehnert, M.J.; Tomaska, W.; Nathan, C.; Rice, T.W.; McAllister, S.K.; Carson, L.A.; Jarvis, W.R. Colonization of Skilled-Care Facility Residents with Antimicrobial-Resistant Pathogens. J. Am. Geriatr. Soc. 2001, 49, 270–276. [Google Scholar] [CrossRef]
- Roghmann, M.-C.; Lydecker, A.D.; Hittle, L.; DeBoy, R.T.; Nowak, R.G.; Johnson, J.K.; Mongodin, E.F. Comparison of the Microbiota of Older Adults Living in Nursing Homes and the Community. mSphere 2017, 2, e00210-17. [Google Scholar] [CrossRef]
- Hogardt, M.; Proba, P.; Mischler, D.; Cuny, C.; Kempf, V.A.; Heudorf, U. Current prevalence of multidrug-resistant organisms in long-term care facilities in the Rhine-Main district, Germany, 2013. Eurosurveillance 2015, 20, 21171. [Google Scholar] [CrossRef] [PubMed]
- Tsao, F.-Y.; Kou, H.-W.; Huang, Y.-C. Dissemination of methicillin-resistant Staphylococcus aureus sequence type 45 among nursing home residents and staff in Taiwan. Clin. Microbiol. Infect. 2015, 21, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Hudson, L.O.; Reynolds, C.; Spratt, B.G.; Enright, M.C.; Quan, V.; Kim, D.; Hannah, P.; Mikhail, L.; Alexander, R.; Moore, D.F.; et al. Diversity of Methicillin-Resistant Staphylococcus aureus Strains Isolated from Residents of 26 Nursing Homes in Orange County, California. J. Clin. Microbiol. 2013, 51, 3788–3795. [Google Scholar] [CrossRef]
- Cheng, V.C.C.; Chen, J.H.K.; Ng, W.C.; Wong, J.Y.H.; Chow, D.M.K.; Law, T.C.; So, S.Y.C.; Wong, S.C.Y.; Chan, T.C.; Chan, F.H.W.; et al. Emergence of Carbapenem-Resistant Acinetobacter baumannii in Nursing Homes With High Background Rates of MRSA Colonization. Infect. Control Hosp. Epidemiol. 2016, 37, 983–986. [Google Scholar] [CrossRef]
- Mody, L.; Kauffman, C.A.; Donabedian, S.; Zervos, M.; Bradley, S.F. Epidemiology of Staphylococcus aureus Colonization in Nursing Home Residents. Clin. Infect. Dis. 2008, 46, 1368–1373. [Google Scholar] [CrossRef]
- Denis, O.; Jans, B.; Deplano, A.; Nonhoff, C.; De Ryck, R.; Suetens, C.; Struelens, M.J. Epidemiology of methicillin-resistant Staphylococcus aureus (MRSA) among residents of nursing homes in Belgium. J. Antimicrob. Chemother. 2009, 64, 1299–1306. [Google Scholar] [CrossRef]
- Jans, B.; Schoevaerdts, D.; Huang, T.-D.; Berhin, C.; Latour, K.; Bogaerts, P.; Nonhoff, C.; Denis, O.; Catry, B.; Glupczynski, Y. Epidemiology of Multidrug-Resistant Microorganisms among Nursing Home Residents in Belgium. PLoS ONE 2013, 8, e64908. [Google Scholar] [CrossRef]
- Kotilainen, P.; Routamaa, M.; Peltonen, R.; Evesti, P.; Eerola, E.; Salmenlinna, S.; Vuopio-Varkila, J.; Rossi, T. Eradication of methicillin-resistant Staphylococcus aureus from a health center ward and associated nursing home. Arch. Intern. Med. 2001, 161, 859–863. [Google Scholar] [CrossRef]
- Min, L.; Galecki, A.; Mody, L. Functional Disability and Nursing Resource Use Are Predictive of Antimicrobial Resistance in Nursing Homes. J. Am. Geriatr. Soc. 2015, 63, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Van Dulm, E.; Tholen, A.T.R.; Pettersson, A.; Van Rooijen, M.S.; Willemsen, I.; Molenaar, P.; Damen, M.; Gruteke, P.; Oostvogel, P.; Kuijper, E.J.; et al. High prevalence of multidrug resistant Enterobacteriaceae among residents of long term care facilities in Amsterdam, the Netherlands. PLoS ONE 2019, 14, e0222200. [Google Scholar] [CrossRef] [PubMed]
- Verrall, A.; Merchant, R.; Dillon, J.; Ying, D.; Fisher, D. Impact of nursing home residence on hospital epidemiology of meticillin-resistant Staphylococcus aureus: A perspective from Asia. J. Hosp. Infect. 2013, 83, 250–252. [Google Scholar] [CrossRef]
- Stone, N.D.; Lewis, D.R.; Lowery, H.K.; Darrow, L.A.; Kroll, C.M.; Gaynes, R.P.; Jernigan, J.A.; McGowan, J.E.; Tenover, F.C.; Richards, C.L. Importance of Bacterial Burden Among Methicillin-Resistant Staphylococcus aureus Carriers in a Long-Term Care Facility. Infect. Control Hosp. Epidemiol. 2008, 29, 143–148. [Google Scholar] [CrossRef]
- Hübner, N.; Dittmann, K.; Begunk, R.; Kramer, A. Infection control measures and prevalence of multidrug-resistant organisms in non-hospital care settings in northeastern Germany: Results from a one-day point prevalence study. J. Hosp. Infect. 2017, 97, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.L.; Shaffer, M.L.; Loeb, M.B.; Givens, J.L.; Habtemariam, D.; Kiely, D.K.; D’Agata, E. Infection Management and Multidrug-Resistant Organisms in Nursing Home Residents With Advanced Dementia. JAMA Intern. Med. 2014, 174, 1660–1667. [Google Scholar] [CrossRef]
- Ruscher, C.; Pfeifer, Y.; Layer, F.; Schaumann, R.; Levin, K.; Mielke, M. Inguinal skin colonization with multidrug-resistant bacteria among residents of elderly care facilities: Frequency, persistence, molecular analysis and clinical impact. Int. J. Med. Microbiol. 2014, 304, 1123–1134. [Google Scholar] [CrossRef]
- Greenland, K.; Rijnders, M.I.; Mulders, M.; Haenen, A.; Spalburg, E.; Van De Kassteele, J.; De Neeling, A.; Stobberingh, E. Low Prevalence of Methicillin-Resistant Staphylococcus Aureus in Dutch Nursing Homes. J. Am. Geriatr. Soc. 2011, 59, 768–769. [Google Scholar] [CrossRef] [PubMed]
- Gibson, K.E.; McNamara, S.E.; Cassone, M.; Perri, M.B.; Zervos, M.; Mody, L.; on behalf of the Targeted Infection Prevention (TIP) Study Team, Ann Arbor, Michigan. Methicillin-Resistant Staphylococcus aureus:Site of Acquisition and Strain Variation in High-Risk Nursing Home Residents with Indwelling Devices. Infect. Control Hosp. Epidemiol. 2014, 35, 1458–1465. [Google Scholar] [CrossRef][Green Version]
- Reynolds, C.; Quan, V.; Kim, D.; Peterson, E.; Dunn, J.; Whealon, M.; Terpstra, L.; Meyers, H.; Cheung, M.; Lee, B.; et al. Methicillin-Resistant Staphylococcus aureus (MRSA) Carriage in 10 Nursing Homes in Orange County, California. Infect. Control Hosp. Epidemiol. 2011, 32, 91–93. [Google Scholar] [CrossRef]
- Murphy, S.; Denman, S.; Bennett, R.G.; Greenough, W.B.; Lindsay, J.; Zelesnick, L.B. Methicillin-ResistantStaphylococcus aureusColonization in a Long-Term-Care Facility. J. Am. Geriatr. Soc. 1992, 40, 213–217. [Google Scholar] [CrossRef]
- Storch, G.A.; Radcliff, J.L.; Meyer, P.L.; Hinrichs, J.H. Methicillin-Resistant Staphylococcus aureus in a Nursing Home. Infect. Control. 1987, 8, 24–29. [Google Scholar] [CrossRef][Green Version]
- Muder, R.R.; Brennen, C.; Wagener, M.M.; Vickers, R.M.; Rihs, J.D.; Hancock, G.A.; Yee, Y.C.; Miller, J.M.; Yu, V.L. Methicillin-Resistant Staphylococcal Colonization and Infection in a Long-Term Care Facility. Ann. Intern. Med. 1991, 114, 107–112. [Google Scholar] [CrossRef]
- Eveillard, M.; Charru, P.; Rufat, P.; Hippeaux, M.-C.; Lancien, E.; Benselama, F.; Branger, C. Methicillin-resistant Staphylococcus aureus carriage in a long-term care facility: Hypothesis about selection and transmission. Age Ageing 2008, 37, 294–299. [Google Scholar] [CrossRef][Green Version]
- Cox, R.; Bowie, P. Methicillin-resistant Staphylococcus aureus colonization in nursing home residents: A prevalence study in Northamptonshire. J. Hosp. Infect. 1999, 43, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Suetens, C.; Niclaes, L.; Jans, B.; Verhaegen, J.; Schuermans, A.; Van Eldere, J.; Buntinx, F. Methicillin-Resistant Staphylococcus aureus Colonization Is Associated with Higher Mortality in Nursing Home Residents with Impaired Cognitive Status. J. Am. Geriatr. Soc. 2006, 54, 1854–1860. [Google Scholar] [CrossRef] [PubMed]
- Nillius, D.; Von Müller, L.; Wagenpfeil, S.; Klein, R.; Herrmann, M. Methicillin-Resistant Staphylococcus aureus in Saarland, Germany: The Long-Term Care Facility Study. PLoS ONE 2016, 11, e0153030. [Google Scholar] [CrossRef][Green Version]
- Namnyak, S.; Adhami, Z.; Wilmore, M.; Keynes, H.; Hampton, K.; Mercieca, E.; Roker, K. Methicillin-resistant Staphylococcus aureus: A questionnaire and microbiological survey of nursing and residential homes in Barking, Havering and Brentwood. J. Infect. 1998, 36, 67–72. [Google Scholar] [CrossRef]
- Bradley, S.F.; Terpenning, M.S.; Ramsey, M.A.; Zarins, L.T.; Jorgensen, K.A.; Sottile, W.S.; Schaberg, D.R.; Kauffman, C.A. Methicillin-resistant Staphylococcus aureus: Colonization and Infection in a Long-term Care Facility. Ann. Intern. Med. 1991, 115, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Monaco, M.; Bombana, E.; Trezzi, L.; Regattin, L.; Brusaferro, S.; Pantosti, A.; Goglio, A. Meticillin-resistant Staphylococcus aureus colonising residents and staff members in a nursing home in Northern Italy. J. Hosp. Infect. 2009, 73, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Horner, C.; Parnell, P.; Hall, D.; Kearns, A.; Heritage, J.; Wilcox, M. Meticillin-resistant Staphylococcus aureus in elderly residents of care homes: Colonization rates and molecular epidemiology. J. Hosp. Infect. 2013, 83, 212–218. [Google Scholar] [CrossRef]
- Ho, P.-L.; Lai, E.L.; Chow, K.-H.; Chow, L.S.; Yuen, K.-Y.; Yung, R.W. Molecular epidemiology of methicillin-resistant Staphylococcus aureus in residential care homes for the elderly in Hong Kong. Diagn. Microbiol. Infect. Dis. 2008, 61, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Dulon, M.; Kleinmüller, O.; Nienhaus, A.; Schablon, A. MRSA Prevalence and Risk Factors among Health Personnel and Residents in Nursing Homes in Hamburg, Germany—A Cross-Sectional Study. PLoS ONE 2017, 12, e0169425. [Google Scholar] [CrossRef][Green Version]
- Friedewald, M.; de Wit, D. MRSA screening of nursing home, residents admitted to hospital on the NSW Central Coast. Aust. Infect. Control. 2001, 6, 119–121. [Google Scholar] [CrossRef]
- Gruber, I.; Heudorf, U.; Werner, G.; Pfeifer, Y.; Imirzalioglu, C.; Ackermann, H.; Brandt, C.; Besier, S.; Wichelhaus, T.A. Multidrug-resistant bacteria in geriatric clinics, nursing homes, and ambulant care—Prevalence and risk factors. Int. J. Med. Microbiol. 2013, 303, 405–409. [Google Scholar] [CrossRef] [PubMed]
- O’Fallon, E.; Schreiber, R.; Kandel, R.; D’Agata, E.M.C. Multidrug-Resistant Gram-Negative Bacteria at a Long-Term Care Facility: Assessment of Residents, Healthcare Workers, and Inanimate Surfaces. Infect. Control Hosp. Epidemiol. 2009, 30, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Pop-Vicas, A.; Mitchell, S.L.; Kandel, R.; Schreiber, R.; D’Agata, E.M.C. Multidrug-Resistant Gram-Negative Bacteria in a Long-Term Care Facility: Prevalence and Risk Factors. J. Am. Geriatr. Soc. 2008, 56, 1276–1280. [Google Scholar] [CrossRef]
- Chen, H.; Au, K.M.; Hsu, K.E.; Lai, C.K.; Myint, J.; Mak, Y.F.; Lee, S.Y.; Wong, T.Y.; Tsang, N.C. Multidrug-resistant organism carriage among residents from residential care homes for the elderly in Hong Kong: A prevalence survey with stratified cluster sampling. Hong Kong Med. J. 2018, 24, 350–360. [Google Scholar] [CrossRef]
- Da Silveira, M.; Cunha, M.D.L.R.D.S.D.; De Souza, C.S.M.; Correa, A.A.F.; Fortaleza, C.M.C.B. Nasal colonization with methicillin-resistant Staphylococcus aureus among elderly living in nursing homes in Brazil: Risk factors and molecular epidemiology. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kwetkat, A.; Pfister, W.; Pansow, D.; Pletz, M.W.; Sieber, C.C.; Hoyer, H. Naso- and oropharyngeal bacterial carriage in nursing home residents: Impact of multimorbidity and functional impairment. PLoS ONE 2018, 13, e0190716. [Google Scholar] [CrossRef] [PubMed]
- Manzur, A.; Domínguez, M.A.; de Gopegui, E.R.; Mariscal, D.; Gavalda, L.; Segura, F.; Perez, J.; Pujol, M. Natural history of meticillin-resistant Staphylococcus aureus colonisation among residents in community long term care facilities in Spain. J. Hosp. Infect. 2010, 76, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Foxman, B.; Mody, L.; Snitkin, E.S. Network of microbial and antibiotic interactions drive colonization and infection with multidrug-resistant organisms. Proc. Natl. Acad. Sci. USA 2017, 114, 10467–10472. [Google Scholar] [CrossRef] [PubMed]
- Fisch, J.; Lansing, B.; Wang, L.; Symons, K.; Cherian, K.; McNamara, S.; Mody, L. New Acquisition of Antibiotic-Resistant Organisms in Skilled Nursing Facilities. J. Clin. Microbiol. 2012, 50, 1698–1703. [Google Scholar] [CrossRef] [PubMed]
- Heinze, K.; Kabeto, M.; Martin, E.T.; Cassone, M.; Hicks, L.; Mody, L. Predictors of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci co-colonization among nursing facility patients. Am. J. Infect. Control. 2019, 47, 415–420. [Google Scholar] [CrossRef]
- Lee, C.-M.; Lai, C.-C.; Chiang, H.-T.; Lu, M.-C.; Wang, L.-F.; Tsai, T.-L.; Kang, M.-Y.; Jan, Y.-N.; Lo, Y.-T.; Ko, W.-C.; et al. Presence of multidrug-resistant organisms in the residents and environments of long-term care facilities in Taiwan. J. Microbiol. Immunol. Infect. 2017, 50, 133–144. [Google Scholar] [CrossRef]
- Budimir, A.; Pal, M.P.; Bošnjak, Z.; Mareković, I.; Vuković, D.; Križan, I.R.; Milas, J.; Plečko, V.; Kalenić, S. Prevalence and molecular characteristics of methicillin-resistant Staphylococcus aureus strains isolated in a multicenter study of nursing home residents in Croatia. Am. J. Infect. Control. 2014, 42, 1197–1202. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, F.-F.; Zhao, S.-Y.; Xiao, S.-Z.; Wang, Y.-C.; Guo, X.-K.; Ni, Y.-X.; Han, L.-Z. Prevalence and Molecular Epidemiology of Staphylococcus aureus among Residents of Seven Nursing Homes in Shanghai. PLoS ONE 2015, 10, e0137593. [Google Scholar] [CrossRef]
- Moschou, A.; Maraki, S.; Giormezis, N.; Moraitaki, H.; Stafylaki, D.; Militsopoulou, M.; Spiliopoulou, I.; Papadakis, J.; Samonis, G.; Kofteridis, D. Prevalence and molecular epidemiology of Staphylococcus aureus nasal colonization in four nursing home residents in Crete, Greece. J. Infect. Chemother. 2020, 26, 199–204. [Google Scholar] [CrossRef]
- Cretnik, T.Z.; Vovko, P.; Retelj, M.; Juteršek, B.; Harlander, T.; Kolman, J.; Gubina, M. Prevalence and Nosocomial Spread of Methicillin-ResistantStaphylococcus aureusin a Long-Term-Care Facility in Slovenia. Infect. Control Hosp. Epidemiol. 2005, 26, 184–190. [Google Scholar] [CrossRef]
- Barrufet, M.P.; Vendrell, E.; Force, L.; Sauca, G.; Rodríguez, S.; Martínez, E.; Palomera, E.; Serra-Prat, M.; Capdevila, J.A.; Cornudella, J.; et al. Prevalence and risk factors for meticillin-resistant Staphylococcus aureus in an acute care hospital and long-term care facilities located in the same geographic area. Rev. Esp. Quimioter. 2014, 27, 190–195. [Google Scholar] [PubMed]
- McKinnell, J.A.; Singh, R.D.; Miller, L.G.; Kleinman, K.; Gussin, G.; He, J.; Saavedra, R.; Dutciuc, T.D.; Estevez, M.; Chang, J.; et al. The SHIELD Orange County Project: Multidrug-resistant Organism Prevalence in 21 Nursing Homes and Long-term Acute Care Facilities in Southern California. Clin. Infect. Dis. 2019, 69, 1566–1573. [Google Scholar] [CrossRef]
- Mody, L.; Gibson, K.E.; Horcher, A.; Prenovost, K.; McNamara, S.E.; Foxman, B.; Kaye, K.S.; Bradley, S. Prevalence of and Risk Factors for Multidrug-Resistant Acinetobacter baumannii Colonization Among High-Risk Nursing Home Residents. Infect. Control Hosp. Epidemiol. 2015, 36, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Hoogendoorn, M.; Smalbrugge, M.; Stobberingh, E.E.; van Rossum, S.V.; Vlaminckx, B.J.; Thijsen, S.F. Prevalence of Antibiotic Resistance of the Commensal Flora in Dutch Nursing Homes. J. Am. Med. Dir. Assoc. 2013, 14, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Andersson, H.; Lindholm, C.; Iversen, A.; Giske, C.G.; Örtqvist, Å.; Kalin, M.; Fossum, B. Prevalence of antibiotic-resistant bacteria in residents of nursing homes in a Swedish municipality: Healthcare staff knowledge of and adherence to principles of basic infection prevention. Scand. J. Infect. Dis. 2012, 44, 641–649. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barr, B.; Wilcox, M.H.; Brady, A.; Parnell, P.; Darby, B.; Tompkins, D. Prevalence of Methicillin-ResistantStaphylococcus aureusColonization Among Older Residents of Care Homes in the United Kingdom. Infect. Control Hosp. Epidemiol. 2007, 28, 853–859. [Google Scholar] [CrossRef]
- Furuno, J.P.; Hebden, J.N.; Standiford, H.C.; Perencevich, E.N.; Miller, R.R.; Moore, A.C.; Strauss, S.M.; Harris, A.D. Prevalence of methicillin-resistant Staphylococcus aureus and Acinetobacter baumannii in a long-term acute care facility. Am. J. Infect. Control. 2008, 36, 468–471. [Google Scholar] [CrossRef]
- Manzur, A.; Gavalda, L.; de Gopegui, E.R.; Mariscal, D.; Domínguez, M.A.; Perez, J.; Segura, F.; Pujol, M. Prevalence of methicillin-resistant Staphylococcus aureus and factors associated with colonization among residents in community long-term-care facilities in Spain. Clin. Microbiol. Infect. 2008, 14, 867–872. [Google Scholar] [CrossRef]
- Baldwin, N.S.; Gilpin, D.; Hughes, C.M.; Kearney, M.P.; Gardiner, D.A.; Cardwell, C.; Tunney, M.M. Prevalence of Methicillin-Resistant Staphylococcus aureus Colonization in Residents and Staff in Nursing Homes in Northern Ireland. J. Am. Geriatr. Soc. 2009, 57, 620–626. [Google Scholar] [CrossRef]
- Flint, J.A.; Ryan, P.; Gordon, D.L. Prevalence of MRSA in South Australian nursing homes. Med. J. Aust. 1998, 169, 559–560. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.J.; Cheng, A.; Kennon, J.; Spelman, D.; Hale, D.; Melican, G.; Sidjabat, H.E.; Paterson, D.L.; Kong, D.; Peleg, A.Y. Prevalence of multidrug-resistant organisms and risk factors for carriage in long-term care facilities: A nested case-control study. J. Antimicrob. Chemother. 2014, 69, 1972–1980. [Google Scholar] [CrossRef]
- Latour, K.; Huang, T.-D.; Jans, B.; Berhin, C.; Bogaerts, P.; Noel, A.; Nonhoff, C.; Dodémont, M.; Denis, O.; Ieven, M.; et al. Prevalence of multidrug-resistant organisms in nursing homes in Belgium in 2015. PLoS ONE 2019, 14, e0214327. [Google Scholar] [CrossRef] [PubMed]
- Mossong, J.; Gelhausen, E.; Decruyenaere, F.; Devaux, A.; Perrin, M.; Even, J.; Heisbourg, E. Prevalence, risk factors and molecular epidemiology of methicillin-resistantStaphylococcus aureus(MRSA) colonization in residents of long-term care facilities in Luxembourg, 2010. Epidemiol. Infect. 2012, 141, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Del Rosario-Quintana, C.; Tosco-Núñez, T.; Lorenzo, L.; Martín-Sánchez, A.M.; Molina-Cabrillana, J. Prevalencia y factores asociados a la colonización de microorganismos multirresistentes en centros de larga estancia de Gran Canaria. Revista Española de Geriatría y Gerontología 2015, 50, 232–236. [Google Scholar] [CrossRef]
- Vovko, P.; Retelj, M.; Cretnik, T.Z.; Jutersek, B.; Harlander, T.; Kolman, J.; Gubina, M. Risk Factors for Colonization With Methicillin-ResistantStaphylococcus aureusin a Long-Term-Care Facility in Slovenia. Infect. Control Hosp. Epidemiol. 2005, 26, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.-F.; Zhang, J.; Zhao, S.-Y.; Yang, Z.-R.; Zhang, Y.-L.; Xiao, S.-Z.; Wang, S.; Guo, X.-K.; Qu, J.-M.; Ni, Y.-X.; et al. Risk factors for methicillin-resistant Staphylococcus aureus carriage among residents in 7 nursing homes in Shanghai, China. Am. J. Infect. Control. 2016, 44, 805–808. [Google Scholar] [CrossRef]
- Becker, J.; Diel, R. Screening for Methicillin-resistant Staphylococcus aureus in a residence home for elderly in Germany. J. Occup. Med. Toxicol. 2017, 12, 3. [Google Scholar] [CrossRef][Green Version]
- El Emam, K.; Arbuckle, L.; Essex, A.; Samet, S.; Eze, B.; Middleton, G.; Buckeridge, D.; Jonker, E.; Moher, E.; Earle, C. Secure Surveillance of Antimicrobial Resistant Organism Colonization or Infection in Ontario Long Term Care Homes. PLoS ONE 2014, 9, e93285. [Google Scholar] [CrossRef]
- Cederna, J.E.; Terpenning, M.S.; Ensberg, M.; Bradley, S.F.; Kauffman, C.A. StaphylococcusaureusNasal Colonization in a Nursing Home: Eradication With Mupirocin. Infect. Control Hosp. Epidemiol. 1990, 11, 13–16. [Google Scholar] [CrossRef]
- Lasseter, G.; Charlett, A.; Lewis, D.L.; Donald, I.P.; Howelljones, R.; McNulty, C.A.M. Staphylococcus aureus carriage in care homes: Identification of risk factors, including the role of dementia. Epidemiol. Infect. 2010, 138, 686–696. [Google Scholar] [CrossRef]
- Galán-Sánchez, F.; Pérez-Eslava, M.; Machuca, J.; Trujillo-Soto, T.; Arca-Suárez, J.; Rodríguez-Iglesias, M. Staphylococcus aureus carriage in older populations in community residential care homes: Prevalence and molecular characterization of MRSA isolates. Enfermedades Infecciosas y Microbiología Clínica 2019, 37, 172–175. [Google Scholar] [CrossRef]
- Kerttula, A.-M.; Lyytikäinen, O.; Virolainen, A.; Finne-Soveri, H.; Agthe, N.; Vuopio-Varkila, J. Staphylococcus aureus colonization among nursing home residents in a large Finnish nursing home. Scand. J. Infect. Dis. 2007, 39, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-L.; Cesario, T.; Gupta, G.; Flionis, L.; Tran, C.; Decker, M.; Thrupp, L. Surveillance of colonization and infection with Staphylococcus aureus susceptible or resistant to methicillin in a community skilled-nursing facility. Am. J. Infect. Control. 1997, 25, 312–321. [Google Scholar] [CrossRef]
- Chan, T.C.; Cheng, V.C.C.; Hung, I.F.N.; Chan, F.H.W.; Ng, W.C.; Yuen, K.Y. The Association between Methicillin Resistant Staphylococcus aureus Colonization and Mortality in Chinese Nursing Home Older Adults: A 2-Year Prospective Cohort. J. Am. Med. Dir. Assoc. 2015, 16, 796–797. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.C.; Tai, J.W.; Wong, Z.S.; Chen, J.H.; Pan, K.B.; Hai, Y.; Ng, W.-C.; Chow, D.M.; Yau, M.C.; Chan, J.F.; et al. Transmission of methicillin-resistant staphylococcus aureus in the long term care facilities in Hong Kong. BMC Infect. Dis. 2013, 13, 1–205. [Google Scholar] [CrossRef]
- McKinnell, J.A.; Miller, L.G.; Singh, R.; Kleinman, K.; Peterson, E.M.; Evans, K.D.; Dutciuc, T.D.; Heim, L.; Gombosev, A.; Estevez, M.; et al. Prevalence of and Factors Associated With Multidrug Resistant Organism (MDRO) Colonization in 3 Nursing Homes. Infect. Control Hosp. Epidemiol. 2016, 37, 1485–1488. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, N.; Keane, C. Risk factors for colonization with methicillin-resistant Staphylococcus aureus among nursing home residents. J. Hosp. Infect. 2000, 45, 206–210. [Google Scholar] [CrossRef]
- Eveillard, M.; LaFargue, S.; Guet, L.; Mangeol, A.; Piquet, J.; Quenon, J.-L.; Fauvelle, F. Association between Institutionalization and Carriage of Multiresistant Bacteria in the Elderly at the Time of Admission to a General Hospital. Eur. J. Clin. Microbiol. Infect. Dis. 1999, 18, 133–136. [Google Scholar] [CrossRef]
- Dandachi, I.; Sokhn, E.S.; Najem, E.; Azar, E.; Daoud, Z. Carriage of beta-lactamase-producing Enterobacteriaceae among nursing home residents in north Lebanon. Int. J. Infect. Dis. 2016, 45, 24–31. [Google Scholar] [CrossRef][Green Version]
- Van Der Donk, C.; Schols, J.; Driessen, C.; Hagenouw, R.; Meulendijks, A.; Stobberingh, E. Prevalence and Spread of Multidrug Resistant Escherichia coli Isolates Among Nursing Home Residents in the Southern Part of the Netherlands. J. Am. Med. Dir. Assoc. 2013, 14, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Wiener, J.; Quinn, J.P.; Bradford, P.A.; Goering, R.; Nathan, C.; Bush, K.; Weinstein, R.A. Multiple Antibiotic–Resistant Klebsiella and Escherichia coli in Nursing Homes. JAMA 1999, 281, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Birgand, G.; Hayatgheib, N.; Bémer, P.; Guilloteau, V.; Legeay, C.; Perron, S.; Chapelet, G.; Corvec, S.; Bourigault, C.; Batard, E.; et al. Multi-drug-resistant Enterobacteriacae carriage in highly exposed nursing homes: Prevalence in western France. J. Hosp. Infect. 2017, 97, 258–259. [Google Scholar] [CrossRef] [PubMed]
- Cochard, H.; Aubier, B.; Quentin, R.; Van Der Mee-Marquet, N.; Du Centre, R.D.H. Extended-Spectrumβ-Lactamase–Producing Enterobacteriaceae in French Nursing Homes: An Association between High Carriage Rate among Residents, Environmental Contamination, Poor Conformity with Good Hygiene Practice, and Putative Resident-to-Resident Transmission. Infect. Control Hosp. Epidemiol. 2014, 35, 384–389. [Google Scholar] [CrossRef]
- Leitner, E.; Zechner, E.; Ullrich, E.; Zarfel, G.; Luxner, J.; Pux, C.; Pichler, G.; Schippinger, W.; Krause, R.; Zollner-Schwetz, I. Low prevalence of colonization with multidrug-resistant gram-negative bacteria in long-term care facilities in Graz, Austria. Am. J. Infect. Control. 2018, 46, 76–80. [Google Scholar] [CrossRef]
- Stuart, R.L.; Kotsanas, D.; Webb, B.; Vandergraaf, S.; Gillespie, E.E.; Hogg, G.G.; Korman, T. Prevalence of antimicrobial-resistant organisms in residential aged care facilities. Med. J. Aust. 2011, 195, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, S.; Caillon, J.; Marquet, A.; Grandjean, G.; Potel, G.; Ballereau, F.; Microbiology Laboratories of the MedQual Network. Epidemiology of third-generation cephalosporin-resistant community-acquired Enterobacteria isolated from elderly patients. Méd. Mal. Infect. 2014, 44, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Machado, E.; Fernandes, S.; Peixe, L.; Novais, Â. Different Escherichia coli B2-ST131 clades (B and C) producing extended-spectrum β-lactamases (ESBL) colonizing residents of Portuguese nursing homes. Epidemiol. Infect. 2017, 145, 3303–3306. [Google Scholar] [CrossRef]
- Terveer, E.; Fallon, M.; Kraakman, M.; Ormond, A.; Fitzpatrick, M.; Caljouw, M.; Martin, A.; Van Dorp, S.; Wong, M.; Kuijper, E. Corrigendum to “Spread of ESBL-producing Escherichia coli in nursing home residents in Ireland and the Netherlands may reflect infrastructural differences” [J Hosp Infect 103 (2019) 160–164]. J. Hosp. Infect. 2020, 103, 160–164. [Google Scholar] [CrossRef]
- Valenza, G.; Nickel, S.; Pfeifer, Y.; Pietsch, M.; Voigtländer, E.; Lehner-Reindl, V.; Höller, C. Prevalence and genetic diversity of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli in nursing homes in Bavaria, Germany. Veter- Microbiol. 2017, 200, 138–141. [Google Scholar] [CrossRef]
- Jallad, M.A.; Naoufal, R.; Irani, J.; Azar, E. Extended Spectrum Beta-Lactamase Carriage State among Elderly Nursing Home Residents in Beirut. Sci. World J. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Luvsansharav, U.-O.; Hirai, I.; Niki, M.; Nakata, A.; Yoshinaga, A.; Matsuura, N.; Kawakami, F. Fecal carriage of CTX-M β-lactamase-producing Enterobacteriaceae in nursing homes in the Kinki region of Japan. Infect. Drug Resist. 2013, 6, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Gorrie, C.L.; Mirceta, M.; Wick, R.R.; Judd, L.M.; Wyres, K.L.; Thomson, N.R.; Strugnell, R.A.; Pratt, N.F.; Garlick, J.S.; Watson, K.M.; et al. Antimicrobial-Resistant Klebsiella pneumoniae Carriage and Infection in Specialized Geriatric Care Wards Linked to Acquisition in the Referring Hospital. Clin. Infect. Dis. 2018, 67, 161–170. [Google Scholar] [CrossRef]
- Pulcini, C.; Clerc-Urmes, I.; Attinsounon, C.A.; Fougnot, S.; Thilly, N. Antibiotic resistance of Enterobacteriaceae causing urinary tract infections in elderly patients living in the community and in the nursing home: A retrospective observational study. J. Antimicrob. Chemother. 2018, 74, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Hagel, S.; Makarewicz, O.; Hartung, A.; Weiß, D.; Stein, C.; Brandt, C.; Schumacher, U.; Ehricht, R.; Patchev, V.; Pletz, M.W. ESBL colonization and acquisition in a hospital population: The molecular epidemiology and transmission of resistance genes. PLoS ONE 2019, 14, e0208505. [Google Scholar] [CrossRef]
- Rooney, P.J.; O’Leary, M.C.; Loughrey, A.C.; McCalmont, M.; Smyth, B.; Donaghy, P.; Badri, M.; Woodford, N.; Karisik, E.; Livermore, D.M. Nursing homes as a reservoir of extended-spectrum -lactamase (ESBL)-producing ciprofloxacin-resistant Escherichia coli. J. Antimicrob. Chemother. 2009, 64, 635–641. [Google Scholar] [CrossRef]
- Blom, A.; Ahl, J.; Månsson, F.; Resman, F.; Tham, J. The prevalence of ESBL-producing Enterobacteriaceae in a nursing home setting compared with elderly living at home: A cross-sectional comparison. BMC Infect. Dis. 2016, 16, 111. [Google Scholar] [CrossRef] [PubMed]
- Broussier, M.; Gbaguidi-Haoré, H.; Rachidi-Berjamy, F.; Bertrand, X.; Slekovec, C. Prevalence, genetic diversity of and factors associated with ESBL-producing Enterobacterales carriage in residents of French nursing homes. J. Hosp. Infect. 2020, 104, 469–475. [Google Scholar] [CrossRef]
- Willemsen, I.; Nelson, J.; Hendriks, Y.; Mulders, A.; Verhoeff, S.; Mulder, P.; Roosendaal, R.; Van Der Zwaluw, K.; Verhulst, C.; Bergh, M.K.-V.D.; et al. Extensive Dissemination of Extended Spectrum β-Lactamase–Producing Enterobacteriaceae in a Dutch Nursing Home. Infect. Control Hosp. Epidemiol. 2015, 36, 394–400. [Google Scholar] [CrossRef]
- Overdevest, I.; Haverkate, M.; Veenemans, J.; Hendriks, Y.; Verhulst, C.; Mulders, A.; Couprie, W.; Bootsma, M.; Johnson, J.; Kluytmans, J. Prolonged colonisation with Escherichia coli O25:ST131 versus other extended-spectrum beta-lactamase-producing E. coli in a long-term care facility with high endemic level of rectal colonisation, the Netherlands, 2013 to 2014. Eurosurveillance 2016, 21, 30376. [Google Scholar] [CrossRef]
- Arvand, M.; Moser, V.; Pfeifer, Y. Prevalence of extended-spectrum-β-lactamase-producing Escherichia coli and spread of the epidemic clonal lineage ST131 in nursing homes in Hesse, Germany. J. Antimicrob. Chemother. 2013, 68, 2686–2688. [Google Scholar] [CrossRef]
- Lautenbach, E.; Han, J.; Santana, E.; Tolomeo, P.; Bilker, W.B.; Maslow, J. Colonization with Extended-Spectrum β-Lactamase-ProducingEscherichia coliandKlebsiellaSpecies in Long-Term Care Facility Residents. Infect. Control Hosp. Epidemiol. 2012, 33, 302–304. [Google Scholar] [CrossRef]
- Van Der Mee-Marquet, N.; Savoyen, P.; Domelier-Valentin, A.-S.; Mourens, C.; Quentin, R.; Réseau des Hygiénistes du Centre Study Group. CTX-M–Type Fluoroquinolone-Resistant Escherichia coli: Analysis of the Colonization of Residents and Inanimate Surfaces 1 Year after a First Case of Urinary Tract Infection at a Nursing Home in France. Infect. Control Hosp. Epidemiol. 2010, 31, 968–970. [Google Scholar] [CrossRef]
- Bertrand, X.; Amara, M.; Sauget, M.; Clément, M.-C.; Talon, D.; Domelier-Valentin, A.-S.; Quentin, R.; van der Mee-Marquet, N. Extended-spectrum beta-lactamase-producing Enterobacteriacae: Unexpected low prevalence of carriage in elderly French residents. Age Ageing 2012, 41, 233–237. [Google Scholar] [CrossRef][Green Version]
- Etherton-Beer, C.D.; Inglis, T.; Waterer, G. Prevalence of oropharyngeal antibiotic-resistant flora among residents of aged care facilities: A pilot study. Respirology 2015, 20, 1139–1141. [Google Scholar] [CrossRef] [PubMed]
- Benenson, S.; Cohen, M.J.; Block, C.; Stern, S.; Weiss, Y.; Moses, A.E.; JIRMI Group. Vancomycin-Resistant Enterococci in Long-Term Care Facilities. Infect. Control Hosp. Epidemiol. 2009, 30, 786–789. [Google Scholar] [CrossRef] [PubMed]
- Elizaga, M.L.; Weinstein, R.A.; Hayden, M.K. Patients in Long-Term Care Facilities: A Reservoir for Vancomycin-Resistant Enterococci. Clin. Infect. Dis. 2002, 34, 441–446. [Google Scholar] [CrossRef]
- Padiglione, A.A.; Grabsch, E.; Wolfe, R.; Gibson, K.; Grayson, M.L. The Prevalence of Fecal Colonization With VRE Among Residents of Long-Term—Care Facilities in Melbourne, Australia. Infect. Control Hosp. Epidemiol. 2001, 22, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Silverblatt, F.J.; Tibert, C.; Mikolich, D.; Blazek-D’Arezzo, J.; Alves, J.; Tack, M.; Agatiello, P. Preventing the Spread of Vancomycin-Resistant Enterococci in a Long-Term Care Facility. J. Am. Geriatr. Soc. 2000, 48, 1211–1215. [Google Scholar] [CrossRef]
- Cunha, C.; Kassakian, S.Z.; Chan, R.; Tenover, F.C.; Ziakas, P.; Chapin, K.C.; Mermel, L.A. Screening of nursing home residents for colonization with carbapenem-resistant Enterobacteriaceae admitted to acute care hospitals: Incidence and risk factors. Am. J. Infect. Control. 2016, 44, 126–130. [Google Scholar] [CrossRef]
- Prasad, N.; Labaze, G.; Kopacz, J.; Chwa, S.; Platis, D.; Pan, C.X.; Russo, D.; LaBombardi, V.J.; Osorio, G.; Pollack, S.; et al. Asymptomatic rectal colonization with carbapenem-resistant Enterobacteriaceae and Clostridium difficile among residents of a long-term care facility in New York City. Am. J. Infect. Control. 2016, 44, 525–532. [Google Scholar] [CrossRef]
- Ben-David, D.; Masarwa, S.; Navon-Venezia, S.; Mishali, H.; Fridental, I.; Rubinovitch, B.; Smollan, G.; Carmeli, Y.; Schwaber, M.J.; Israel PACF CRKP (Post-Acute-Care Facility Carbapenem-Resistant Klebsiella pneumoniae) Working Group. Carbapenem-Resistant Klebsiella pneumoniaein Post-Acute-Care Facilities in Israel. Infect. Control Hosp. Epidemiol. 2011, 32, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Prabaker, K.; Lin, M.Y.; McNally, M.; Cherabuddi, K.; Ahmed, S.; Norris, A.; Lolans, K.; Odeh, R.; Chundi, V.; Weinstein, R.A.; et al. Transfer from High-Acuity Long-Term Care Facilities Is Associated with Carriage ofKlebsiella pneumoniaeCarbapenemase–ProducingEnterobacteriaceae: A Multihospital Study. Infect. Control Hosp. Epidemiol. 2012, 33, 1193–1199. [Google Scholar] [CrossRef]
- Reuben, J.; Donegan, N.; Wortmann, G.; DeBiasi, R.; Song, X.; Kumar, P.; McFadden, M.; Clagon, S.; Mirdamadi, J.; White, D.; et al. Healthcare Antibiotic Resistance Prevalence—DC (HARP-DC): A Regional Prevalence Assessment of Carbapenem-Resistant Enterobacteriaceae (CRE) in Healthcare Facilities in Washington, District of Columbia. Infect. Control Hosp. Epidemiol. 2017, 38, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Leitner, E.; Schreiner, E.; Neuhold, M.; Bozic, M.; Pux, C.; Pichler, G.; Schippinger, W.; Steinmetz, I.; Krause, R.; Zollner-Schwetz, I. Low prevalence of Clostridium difficile colonization in patients in long-term care facilities in Graz, Austria: A point-prevalence study. Am. J. Infect. Control. 2020, 48, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Taminiau, B.; Korsak, N.; Avesani, V.; Van Broeck, J.; Brach, P.; Delmée, M.; Daube, G. Longitudinal survey of Clostridium difficile presence and gut microbiota composition in a Belgian nursing home. BMC Microbiol. 2016, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.J.; Gilliland, S.S.; Vance-Bryan, K.; Moody, J.A.; Larsson, A.J.; Rotschafer, J.C.; Guay, D.R.P. Clostridium difficileColonization in Residents of Long-Term Care Facilities: Prevalence and Risk Factors. J. Am. Geriatr. Soc. 1993, 41, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.R.; Bennett, R.G.; Laughon, B.E.; Iii, W.B.G.; Bartlett, J.G. Postantibiotic Colonization withClostridium difficilein Nursing Home Patients. J. Am. Geriatr. Soc. 1990, 38, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Riggs, M.M.; Sethi, A.K.; Zabarsky, T.F.; Eckstein, E.C.; Jump, R.L.P.; Donskey, C.J. Asymptomatic Carriers Are a Potential Source for Transmission of Epidemic and Nonepidemic Clostridium difficile Strains among Long-Term Care Facility Residents. Clin. Infect. Dis. 2007, 45, 992–998. [Google Scholar] [CrossRef]
- Arvand, M.; Moser, V.; Schwehn, C.; Bettge-Weller, G.; Hensgens, M.P.; Kuijper, E.J. High Prevalence of Clostridium difficile Colonization among Nursing Home Residents in Hesse, Germany. PLoS ONE 2012, 7, e30183. [Google Scholar] [CrossRef]
- Mortensen, E.; Trivedi, K.K.; Rosenberg, J.; Cody, S.H.; Long, J.; Jensen, B.J.; Vugia, D.J. Multidrug-Resistant Acinetobacter baumannii Infection, Colonization, and Transmission Related to a Long-Term Care Facility Providing Subacute Care. Infect. Control Hosp. Epidemiol. 2014, 35, 406–411. [Google Scholar] [CrossRef] [PubMed]
- O’Fallon, E.; Kandell, R.; Schreiber, R.; D’Agata, E.M.C. Acquisition of Multidrug-Resistant Gram-Negative Bacteria: Incidence and Risk Factors within a Long-Term Care Population. Infect. Control Hosp. Epidemiol. 2010, 31, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Kahvecioglu, D.; Ramiah, K.; Mcmaughan, D.; Garfinkel, S.; McSorley, V.; Nguyen, Q.N.; Yang, M.; Pugliese, C.; Mehr, D.; Phillips, C.D. Multidrug-Resistant Organism Infections in US Nursing Homes: A National Study of Prevalence, Onset, and Transmission across Care Settings, October 1, 2010-December 31, 2011. Infect. Control Hosp. Epidemiol. 2014, 35, S48–S55. [Google Scholar] [CrossRef] [PubMed]
- D’Agata, E.M.C.; Habtemariam, D.; Mitchell, S. Multidrug-Resistant Gram-Negative Bacteria: Inter- and Intradissemination Among Nursing Homes of Residents With Advanced Dementia. Infect. Control Hosp. Epidemiol. 2015, 36, 930–935. [Google Scholar] [CrossRef]
- Mody, L.; Krein, S.L.; Saint, S.K.; Min, L.C.; Montoya, A.; Lansing, B.; McNamara, S.E.; Symons, K.; Fisch, J.; Koo, E.; et al. A Targeted Infection Prevention Intervention in Nursing Home Residents With Indwelling Devices. JAMA Intern. Med. 2015, 175, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Ziakas, P.D.; Zacharioudakis, I.M.; Zervou, F.N.; Grigoras, C.; Pliakos, E.E.; Mylonakis, E. Asymptomatic Carriers of Toxigenic C. difficile in Long-Term Care Facilities: A Meta-Analysis of Prevalence and Risk Factors. PLoS ONE 2015, 10, e0117195. [Google Scholar] [CrossRef]
- Donskey, C.J.; Kundrapu, S.; Deshpande, A. Colonization Versus Carriage of Clostridium difficile. Infect. Dis. Clin. N. Am. 2015, 29, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Simor, A.E.; Bradley, S.F.; Strausbaugh, L.J.; Crossley, K.; Nicolle, L.E. SHEA Long-Term–Care Committee Clostridium difficilein Long-Term–Care Facilities for the Elderly. Infect. Control Hosp. Epidemiol. 2002, 23, 696–703. [Google Scholar] [CrossRef]
- Thompson, G.; Shindruk, C.L.; Adekoya, A.A.; Demczuk, L.; McClement, S. Meanings of ‘centredness’ in long-term care facilities: A scoping review protocol. BMJ Open 2018, 8, e022498. [Google Scholar] [CrossRef]
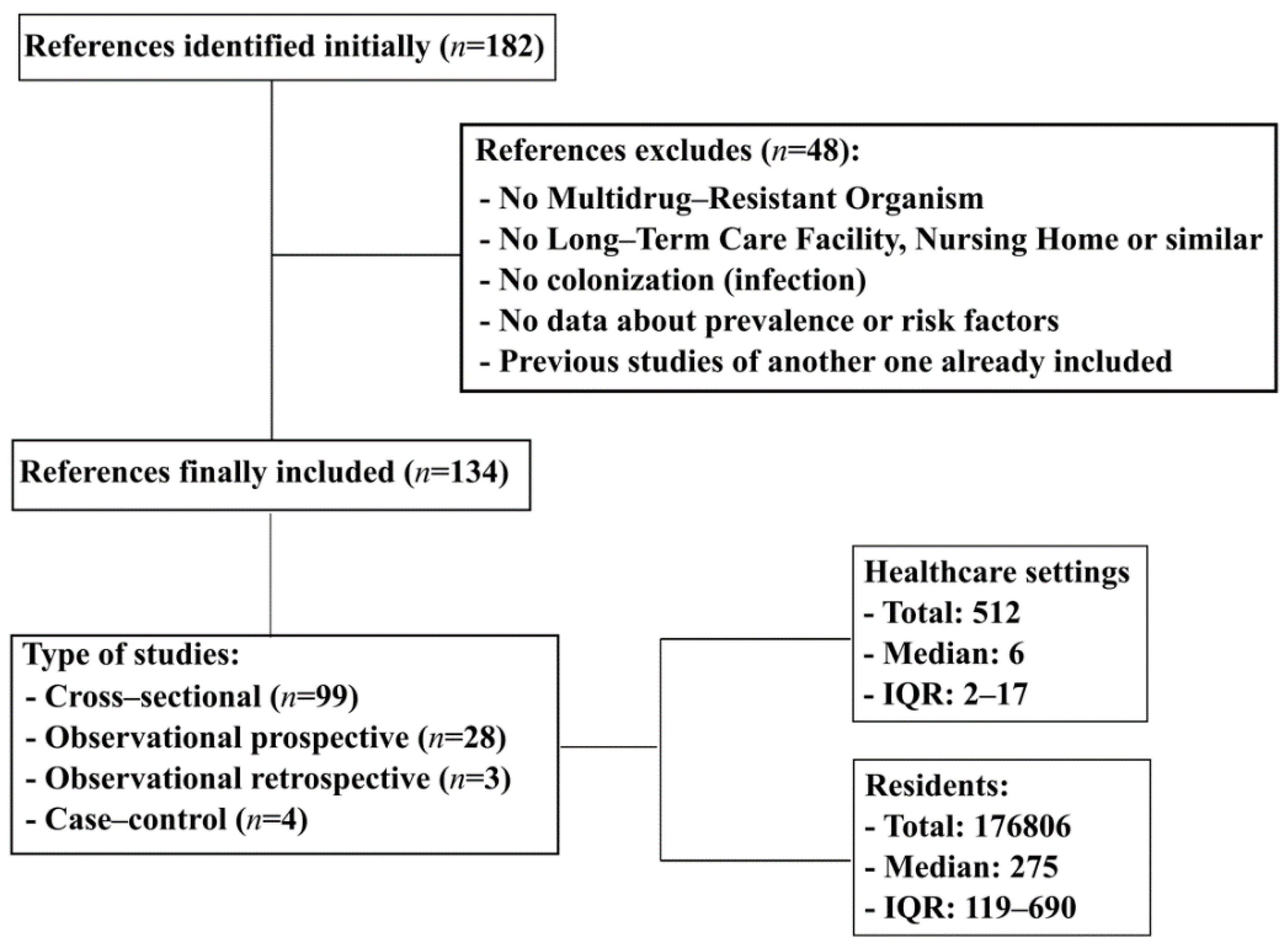
| Multidrug-Resistant Organism | No. of Articles n = 134 | Percentage of Articles n = 134 (%) | Microorganism Global Prevalence n = 134 (Median, IQR) | Prevalence in Europe n = 70 (Median %, IQR) | Prevalence in North America n = 41 (Median %, IQR) | Prevalence in South America n = 1 (Median %, IQR) | Prevalence in Asia n = 15 (median %, IQR) | Prevalence in Oceania n = 7 (Median %, IQR) | Prevalence in Africa n = 0 (Median %, IQR) |
|---|---|---|---|---|---|---|---|---|---|
| ESBL Enterobacterales | 51 | 38.0 | 11.6 (5.5–24.5) | 12.9 (6.3–21.4) | 9 (3.4–33) | - | 71.6 (46.6–74) | 6.0 (1.4–10.8) | - |
| ESBL Escherichia coli | 33 | 24.6 | 15.0 (7.7–41.4) | 15.3 (7.8–41.2) | 15 (2.9–30.3) | - | 82.7 (50.4–86.1) | 10.4 (5.6–11.2) | - |
| ESBL Klebsiella pneumoniae | 22 | 16.4 | 2.9 (0.4–7.1) | 4.2 (0.6–6.5) | 0.2 (0.0–4.8) | - | 9.1 (8.8–9.4) | 1.7 | - |
| Carbapenem resistant Enterobacterales | 27 | 20.1 | 0.8 (0.0–4.2) | 0.2 (0.0–0.9) | 5.0 (2.0–7.9) | - | 6.9 (1.4–14.6) | 0.4 (0.3–0.5) | - |
| MDR Pseudomonas aeruginosa | 8 | 5.9 | 1.3 (0.2–5.3) | 0.2 (0.08–1.6) | 5.4 (3.2–7.6) | - | 3.9 | - | - |
| MDR Acinetobacter baumannii | 11 | 8.2 | 5.8 (2.2–13.5) | 1.9 (0–4.3) | 15.0 (13.5–16.0) | - | 5.2 (2.9–12.3) | 6.0 | - |
| Meticillin-resistant Staphylococcus aureus | 88 | 65.6 | 13.2 (6.6–25) | 9.1 (4.4–19.6) | 22.0 (12.0–30.0) | 3.7 | 25.6 (13–36.8) | 10 (6.5–13) | - |
| Vancomycin-resistant Enterococcus spp. | 36 | 26.8 | 1.5 (0.06–6.9) | 0.4 (0.0–1.8) | 4.0 (0.9–20.7) | - | 0.0 (0.0–4.8) | 3.1 (2.5–4.5) | - |
| Clostridioides difficile | 11 | 8.2 | 5.1 (1.9–24.8) | 3.7 (1.1–4.9) | 26.1 (16.2–37.5) | - | - | 1.0 | - |
| Multidrug-Resistant Organism | No. of Articles (2015 or Before) n = 90 | No. of Articles (After 2015) n = 44 | Prevalence of MDR (2015 or Before) Median (IQR) | Prevalence of MDR (After 2015) Median (IQR) | Difference (%) |
|---|---|---|---|---|---|
| ESBL Enterobacterales | 30 | 22 | 10.5 (3.5–31.4) | 15.1 (9.1–19.9) | 4.6 |
| ESBL Escherichia coli | 19 | 14 | 18.0 (5.5–40.9) | 14.4 (8.1–41.4) | −3.6 |
| ESBL Klebsiella pneumoniae | 10 | 12 | 0.7 (0.2–9.2) | 4.2 (0.7–6.3) | 3.5 |
| Carbapenem resistant Enterobacterales | 6 | 21 | 2.9 (0.1–7.5) | 0.8 (0–1.9) | −2.1 |
| MDR Pseudomonas aeruginosa | 2 | 6 | 0.5 (0.25–0.75) | 2.75 (0.5–8.2) | 2.25 |
| MDR Acinetobacter baumannii | 4 | 7 | 10.5 (5.4–15.5) | 5.2 (0.3–8.9) | −5.3 |
| Meticillin-resistant Staphylococcus aureus | 61 | 26 | 16.0 (7.8–23.3) | 9.6 (3.9–25.5) | −6.4 |
| Vancomycin-resistant Enterococcus spp. | 22 | 14 | 2.8 (0.5–5.5) | 0.6 (0.001–13.0) | −2.2 |
| Clostridioides difficile | 5 | 6 | 7.1 (4.6–33.0) | 3.9 (1.1–15.7) | −3.2 |
| Risk Factors for MDRO Colonization | Limitations and Common Characteristics |
|---|---|
| Age | An increase entails higher risk. There is not a cut-off established for colonization by MDROs. |
| Male sex | Confirmed in many studies by multivariate analysis |
| Dementia | An increase entails higher risk. There is not a cut-off established for colonization by MDROs. |
| Diabetes | Controversial results. Differences by MDRO type. |
| Cancer | Controversial results. Differences by MDRO type. |
| Chronic wound | Confirmed in many studies by multivariate analysis |
| Dependence | An increase entails higher risk. There is not a cut-off established for colonization by MDROs. |
| Medical devices | Confirmed in many studies by multivariate analysis |
| Previous antibiotic use | Confirmed in many studies by multivariate analysis |
| Previous hospitalization | Whether the risk could be increased by days of hospitalization is unknown. |
| Previous MDRO colonization | Controversial results. Differences by MDRO type. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Villodres, Á.; Martín-Gandul, C.; Peñalva, G.; Guisado-Gil, A.B.; Crespo-Rivas, J.C.; Pachón-Ibáñez, M.E.; Lepe, J.A.; Cisneros, J.M. Prevalence and Risk Factors for Multidrug-Resistant Organisms Colonization in Long-Term Care Facilities Around the World: A Review. Antibiotics 2021, 10, 680. https://doi.org/10.3390/antibiotics10060680
Rodríguez-Villodres Á, Martín-Gandul C, Peñalva G, Guisado-Gil AB, Crespo-Rivas JC, Pachón-Ibáñez ME, Lepe JA, Cisneros JM. Prevalence and Risk Factors for Multidrug-Resistant Organisms Colonization in Long-Term Care Facilities Around the World: A Review. Antibiotics. 2021; 10(6):680. https://doi.org/10.3390/antibiotics10060680
Chicago/Turabian StyleRodríguez-Villodres, Ángel, Cecilia Martín-Gandul, Germán Peñalva, Ana Belén Guisado-Gil, Juan Carlos Crespo-Rivas, María Eugenia Pachón-Ibáñez, José Antonio Lepe, and José Miguel Cisneros. 2021. "Prevalence and Risk Factors for Multidrug-Resistant Organisms Colonization in Long-Term Care Facilities Around the World: A Review" Antibiotics 10, no. 6: 680. https://doi.org/10.3390/antibiotics10060680
APA StyleRodríguez-Villodres, Á., Martín-Gandul, C., Peñalva, G., Guisado-Gil, A. B., Crespo-Rivas, J. C., Pachón-Ibáñez, M. E., Lepe, J. A., & Cisneros, J. M. (2021). Prevalence and Risk Factors for Multidrug-Resistant Organisms Colonization in Long-Term Care Facilities Around the World: A Review. Antibiotics, 10(6), 680. https://doi.org/10.3390/antibiotics10060680








