In Silico and In Vitro Screening of Antipathogenic Properties of Melianthus comosus (Vahl) against Pseudomonas aeruginosa
Abstract
1. Introduction
2. Results
2.1. Crude Extract Yields and Gas Chromatography–Mass Spectrophotometry (GC-MS) Profiling of Extracts
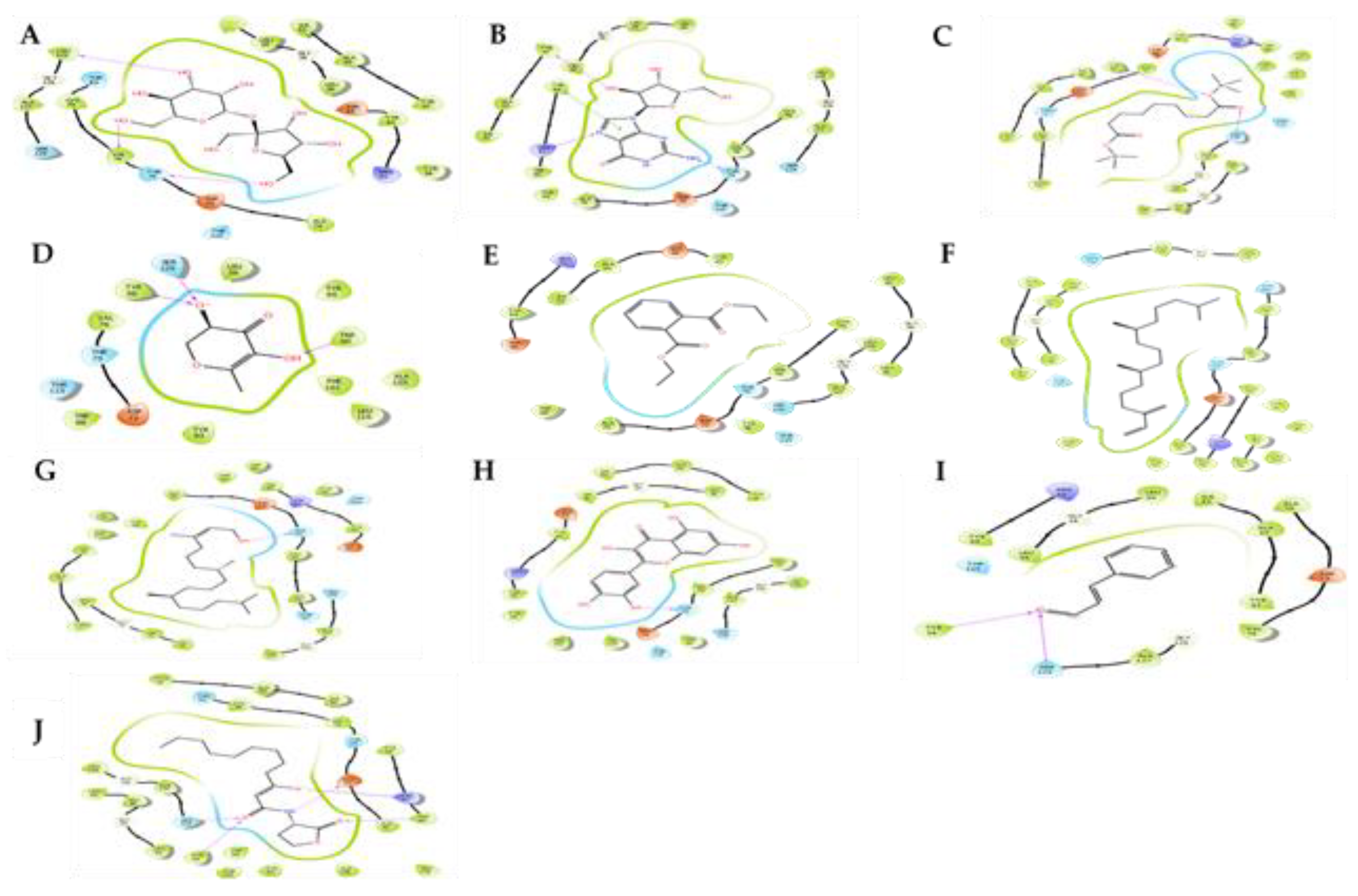
2.2. In Silico Modeling of Identified Compounds against QS Receptors CviR’
2.3. In Silico Modeling of Identified Compounds against LasR Protein
2.4. In Vitro Antibacterial Validation of M. comosus Crude Extracts against P. aeruginosa
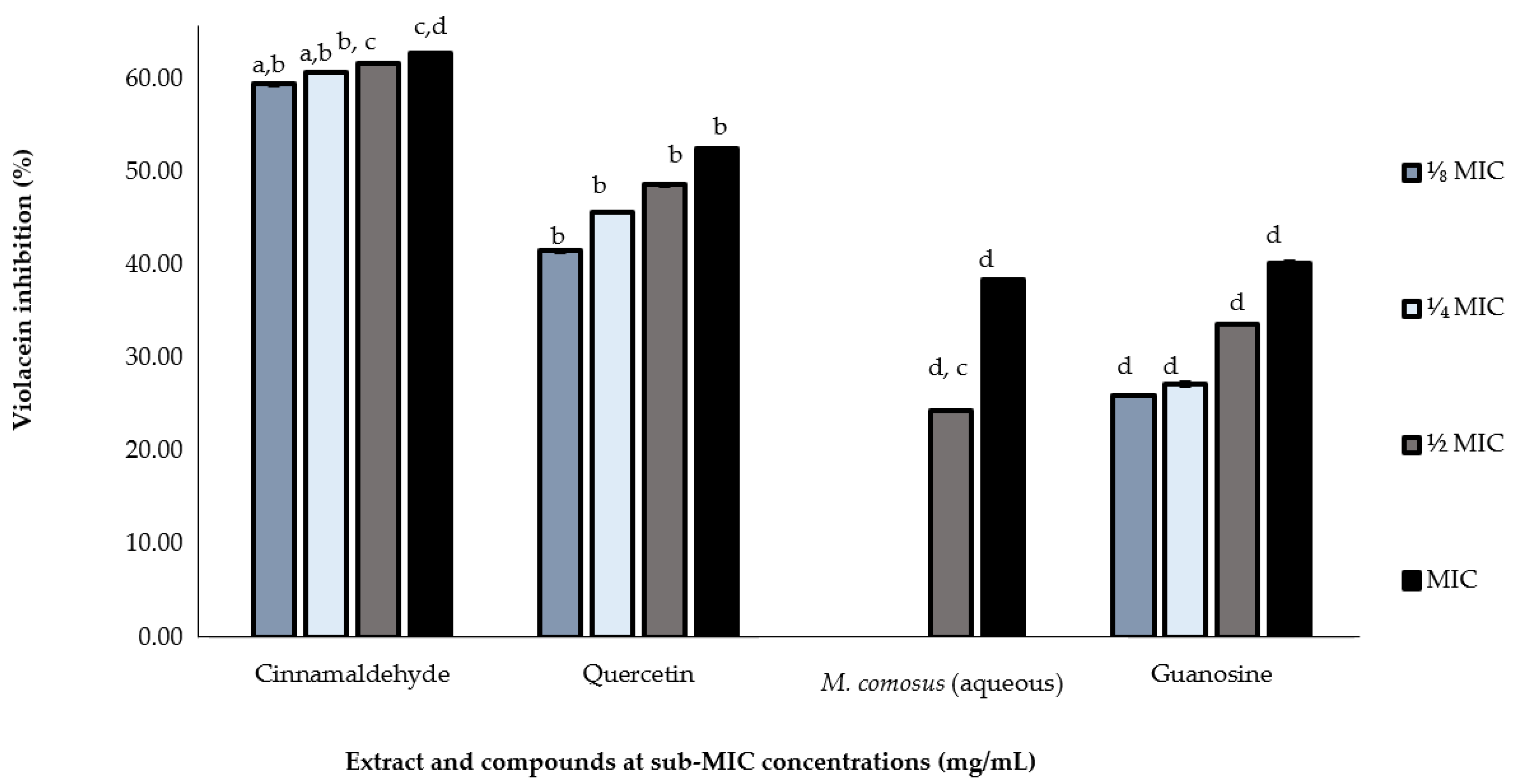
2.5. In Vitro Validation of Quorum Sensing-Dependent Violacein Inhibition (QSI)
2.6. P. aeruginosa Biofilm Formation Inhibition: Cell Attachment and Biofilm Development
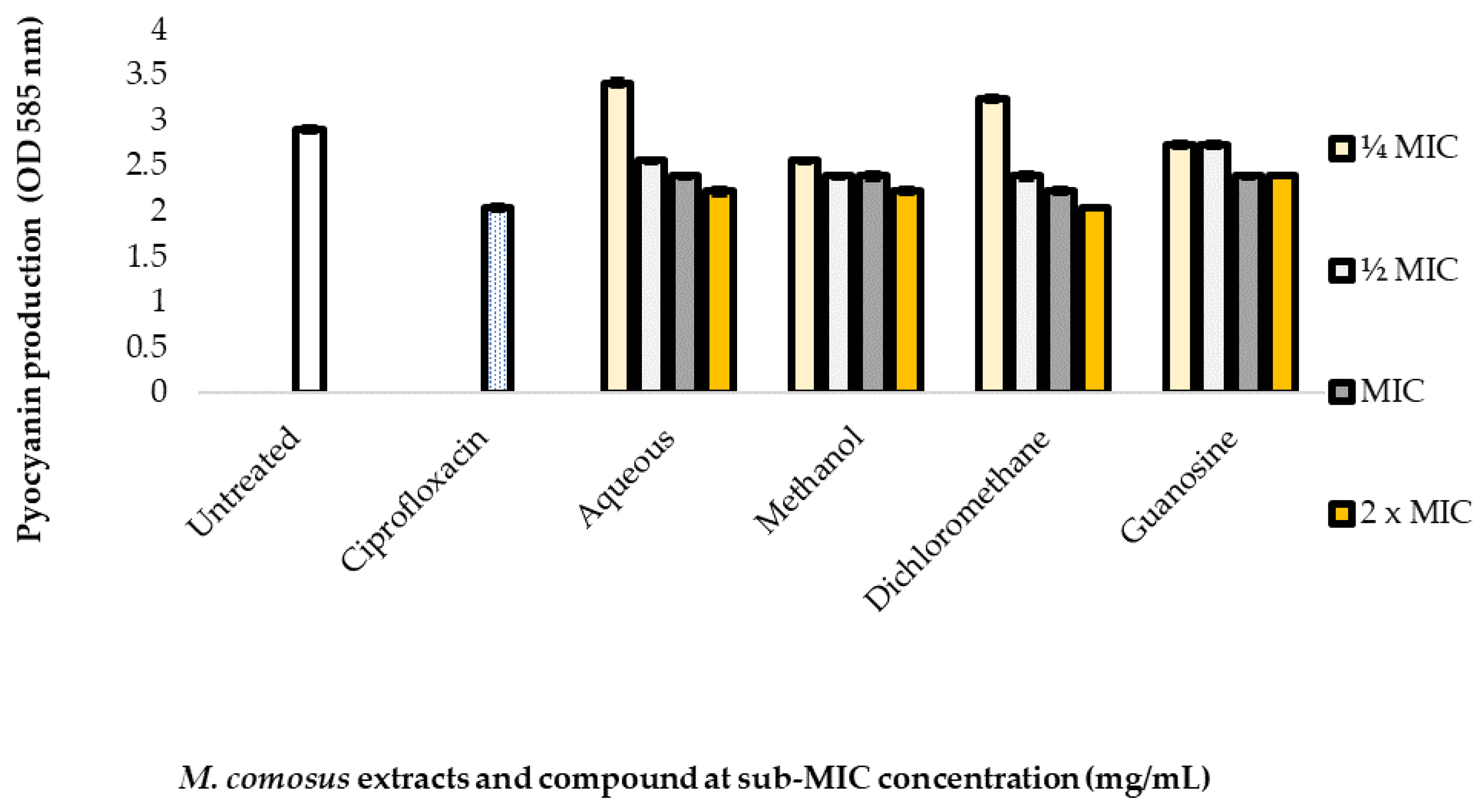
2.7. Inhibitory Effect of Plant Extracts and Compound on Pyocyanin Production
2.8. Effect of Plant Extracts and Compound on Swimming Motility
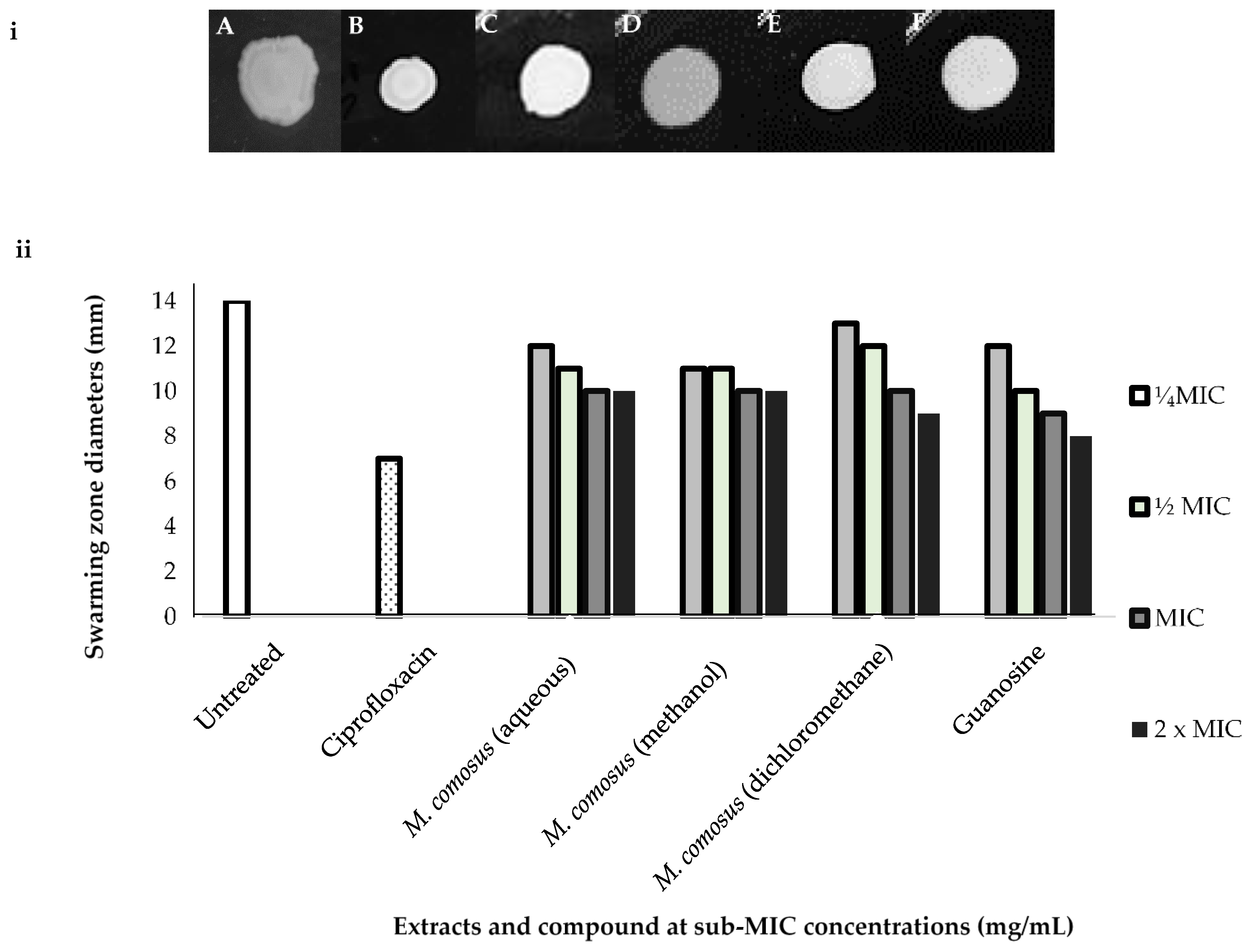
2.9. Effect of Plant Extracts and Compound on Swarming Motility
3. Discussion
4. Materials and Methods
4.1. Plant Collection, Preparation, and Extraction
4.2. Identification of Phytochemical Compounds Using Gas Chromatography–Mass Spectrophotometry (GC-MS)
4.3. Molecular Docking Studies
4.4. Bacterial Strain and Growth Conditions
4.5. Antibacterial Activity Using a Microdilution Assay
4.6. Evaluation of Plant Extracts for Anti-Quorum Sensing (AQS) Potential
4.6.1. Qualitative Anti-Quorum Sensing Assay
4.6.2. Quantitative Anti-Quorum Sensing Assay
4.7. Effect of Plant Extracts and Selected Compounds on Cell Attachment and Biofilm Development
4.8. Inhibition of Quorum Sensing Mediated Virulence Determinants—Pyocyanin Assay
4.9. Swimming and Swarming Motility Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malgaonkar, A.; Nair, M. Quorum sensing in Pseudomonas aeruginosa mediated by RhlR is regulated by a small RNA PhrD. Sci. Rep. 2019, 9, 432. [Google Scholar] [CrossRef]
- Singh, S.; Bhatia, S. In silico identification of albendazole as a quorum sensing inhibitor and its in vitro verification using CviR and LasB receptors based assay systems. Biompact 2018, 8, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Cosa, S.; Rakoma, J.R.; Yusuf, A.A.; Tshikalange, T.E. Calpurnia Aurea (Aiton) Benth extracts reduce quorum sensing controlled virulence factors in Pseudomonas aeruginosa. Molecules 2020, 25, 2283. [Google Scholar] [CrossRef]
- Kostylev, M.; Kim, D.Y.; Smalley, N.E.; Salukhe, I.; Greenberg, E.P. Evolution of the Pseudomonas Aeruginosa Quorum- Sensing Hierarchy. Proc. Natl. Acad. Sci. USA 2019, 116, 7027–7032. [Google Scholar] [CrossRef]
- Ulrey, R.K.; Barksdale, S.M.; Zhou, W.; Van Hoek, M.L. Cranberry proanthocyanidins have anti-biofilm properties against Pseudomonas aeruginosa. BMC. Complementary Altern. Med. 2014, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, M.; Inaba, T.; Kiyokawa, T.; Obana, N.; Yawata, Y.; Nomura, N. Environmental factors that shape biofilm formation. Biosci. Biotechnol. Biochem. 2016, 80, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, S.; Ranganathan, S.; Rao, D.; Syed, A.; Ameen, F.; Busi, S. Attenuation of quorum sensing regulated virulence and biofilm development in Pseudomonas aeruginosa PAO1 by Diaporthe phaseolorum SSP12. Microb. Pthogenes 2018, 118, 177–189. [Google Scholar] [CrossRef]
- Pena, R.T.; Blasco, L.; Ambroa, A.; González-pedrajo, B.; Fernández-garcía, L.; López, M.; Bleriot, I.; Bou, G.; García-contreras, R.; Wood, T.K.; et al. Relationship between quorum sensing and secretion systems. Front. Microbiol. 2019, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Knight, S.L.M.C.; Iglewski, B.H. The Pseudomonas quinolone signal regulates Rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 2000, 182, 2702–2708. [Google Scholar]
- Lee, J.; Zhang, L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein. Cell 2015, 6, 26–41. [Google Scholar] [CrossRef]
- Ueda, A.; Wood, T. Connecting quorum sensing, c-Di-GMP, pel polysaccharide, and biofilm formation in Pseudomonas aeruginosa through tyrosine phosphatase TpbA. PLoS Pathogens. 2009, 5, 1–15. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Ortega-Ramirez, L.A.; Gutierrez-Pacheco, M.M.; Bernal-Mercado, A.T.; Rodriguez-Garcia, I.; Gonzalez-Aguilar, G.A.; Ponce, A.; Moreira, M.R.; Roura, S.I.; Ayala-Zavala, J.F. Oregano essential oil-pectin edible films as anti-quorum sensing and food antimicrobial agents. Front. Micribiol 2014, 5, 699. [Google Scholar] [CrossRef]
- Ahmad, A.; Viljoen, A.M.; Chenia, H.Y. The impact of plant volatiles on bacterial quorum sensing. Lett. Appl. Microbiol. 2014, 60, 8–19. [Google Scholar] [CrossRef]
- Patel, P.; Joshi, C.; Kothari, V. Antipathogenic potential of a polyherbal wound-care formulation (herboheal) against certain wound-infective Gram-negative bacteria. Adv. Pharmacol. Sci. 2019, 1–17. [Google Scholar] [CrossRef]
- Khan, M.F.; Tang, H.; Lyles, J.T.; Pineau, R. Antibacterial properties of medicinal plants from Pakistan against multidrug-resistant ESKAPE. Front. Pharmacol. 2018, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Maroyi, A. A review of the ethnomedicinal uses, phytochemistry and pharmacological properties of Melianthus comosus Vahl. J. Pharmceu. Scien. Resear. 2019, 11, 3655–3660. [Google Scholar]
- Eloff, J.N.; Angeh, I.E.; Mcgaw, L.J. Solvent-solvent fractionation can increase the antifungal activity of a Melianthus comosus (Melianthaceae) acetone extract to yield a potentially useful commercial antifungal product. Industrial. Crops. Products 2018, 111, 69–77. [Google Scholar] [CrossRef]
- Almeida, R.E.D.; Molina, R.D.I.; Viola, C.M.; Luciardi, M.C.; Peñalver, C.N.; Bardón, A.; Arena, M.E. Comparison of seven structurally related coumarins on the inhibition of quorum sensing of Pseudomonas aeruginosa and Chromobacterium violaceum. Bioorg. Chem. 2017, 73, 37–42. [Google Scholar] [CrossRef]
- Paul, J.; Guzman, M.D.; Padilla, L.V.; Science, C. Anti-quorum sensing activity of Tetracera scandens and Aleurites moluccana leaf extracts against Chromobacterium violaceum. Microbiol. Res. J. Intern. 2017, 22, 1–10. [Google Scholar] [CrossRef]
- Andrade-Ochoa, S.; Correa-Basurto, J.; Rodríguez-Valdez, L.M.; Sánchez-Torres, L.E.; Nogueda-Torres, B.; Nevárez-Moorillón, G.V. In vitro and in silico studies of terpenes, terpenoids and related compounds with larvicidal and pupaecidal activity against Culex quinquefasciatus Say (Diptera: Culicidae). Chem. Cent. J. 2018, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Chenia, H.Y. Anti-quorum sensing potential of crude Kigelia africana fruit extracts. Sensors 2013, 2802–2817. [Google Scholar] [CrossRef]
- Pereira, A.S.P.; Haan, H.D.; Peña-garcía, J.; Moreno, M.M. Exploring African medicinal plants for potential anti-diabetic compounds with the DIA-DB inverse virtual screening web server. Molecules 2019, 24, 2002. [Google Scholar] [CrossRef]
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Molec. Aspec. Medici. 2006, 1–93. [Google Scholar] [CrossRef] [PubMed]
- Erhabor, C.R.; Erhabor, J.O.; Mcgaw, L.J. The potential of South African medicinal plants against microbial biofilm and quorum sensing of foodborne pathogens: A review. S. Afr. J. Bot. 2019. [Google Scholar] [CrossRef]
- Mabona, U.; Viljoen, A.; Shikanga, E.; Marston, A.; Van Vuuren, S. Antimicrobial activity of southern African medicinal plants with dermatological relevance: From an ethnopharmacological screening approach, to combination studies and the isolation of a bioactive compound. J. Ethnopharmacol. 2013, 148, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Adonizio, A.L. Anti-quorum sensing agents from South Florida medicinal plants and their attenuation of Pseudomonas aeruginosa pathogenicity. PhD. Thesis, Florida International University, Miami, FL, USA, 2008. [Google Scholar]
- Aliyu, A.B.; Koorbanally, N.A.; Moodley, B.; Singh, P.; Chenia, H.Y. Quorum sensing inhibitory potential and molecular docking studies of sesquiterpene lactones from Vernonia blumeoides. Phytochemistry 2016, 126, 23–33. [Google Scholar] [CrossRef]
- Quecan, B.X.V.; Santos, J.T.C.; Rivera, M.L.C.; Hassimotto, N.M.A.; Almeida, F.A.; Otero, A.M. Effect of quercetin rich onion extracts on bacterial quorum sensing. Front. Microbiol. 2019, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Bajaj, A.V. Extra precision glide docking, free energy calculation and molecular dynamics studies of 1, 2-diarylethane derivatives as potent urease inhibitors. J. Mol. Modeling 2018, 24, 1–27. [Google Scholar] [CrossRef]
- Kumar, L.; Chhibber, S.; Kumar, R.; Kumar, M.; Harjai, K. Zingerone silences quorum sensing and attenuates virulence of Pseudomonas aeruginosa. Fitoterapia 2015, 102, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Avila, J.L.; Rojas, T.; Avila, A.; Polegre, M.A.; Robins, R.K. Biological activity of analogs of guanine and guanosine against American trypanosoma and leishmania spp. Antimicrob. Agents Chemother. 1987, 31, 447–451. [Google Scholar] [CrossRef]
- Van Vuuren, S.; Muhlarhi, T. Do South African medicinal plants used traditionally to treat infections respond differently to resistant microbial strains? S. Afr. J. Bot. 2017, 112, 186–192. [Google Scholar] [CrossRef]
- Kelmanson, J.E.; Ja, A.K.; Staden, J. Van. Zulu medicinal plants with antibacterial activity. J. Ethnopharmacol. 2000, 69, 241–246. [Google Scholar] [CrossRef]
- Setzer, W. The Phytochemistry of cherokee aromatic medicinal plants. Medicines. 2018, 5, 121. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The minimum inhibitory concentration of antibiotics: Methods, interpretation, clinical relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef]
- Gibbons, S. Anti-Staphylococcal plant natural products. Nat. Prod. Rep. 2004, 21, 263–277. [Google Scholar] [CrossRef]
- Mamabolo, M.P.; Muganza, F.M.; Tabize Olivier, M.; Olaokun, O.O.; Nemutavhanani, L.D. Evaluation of antigonorrhea activity and cytotoxicity of Helichrysum caespititium (DC) Harv. whole plant extracts. Biol. Med. 2018, 10, 1–4. [Google Scholar] [CrossRef]
- Baloyi, I.T.; Cosa, S.; Combrinck, S.; Leonard, C.M.; Viljoen, A.M. Anti-quorum sensing and antimicrobial activities of South African medicinal plants against uropathogens. S. Afr. J. Bot. 2019, 122, 484–491. [Google Scholar] [CrossRef]
- Koh, K.H.; Tham, F.Y. Screening of traditional Chinese medicinal plants for quorum-sensing inhibitors activity. J. Microbiol. Immunol. Infect. 2011, 44, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Cosa, S.; Chaudhary, S.K.; Chen, W.; Combrinck, S. Exploring common culinary herbs and spices as potential anti-quorum sensing agents. Nutirents 2019, 14, 739. [Google Scholar] [CrossRef] [PubMed]
- Vasavi, H.C.K.; Arun, A.B.; Rekha, P.D. Inhibition of quorum sensing in Chromobacterium violaceum by Syzygium cumini L. and Pimenta dioica L. Asian Pacific J. Trop. Biomed. 2013, 3, 954–959. [Google Scholar] [CrossRef]
- Ganesh, S.; Rai, R. Attenuation of quorum-sensing-dependent virulence factors and biofilm formation by medicinal plants against antibiotic resistant Pseudomonas aeruginosa. J. Tradit. Complement. Med. 2018, 8, 170–177. [Google Scholar] [CrossRef]
- Bettio, L.E.B.; Gil-Mohapel, J.; Rodrigues, A.L.S. Guanosine and its role in neuropathologies. Purinergic Signal. 2016, 12, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rui, X.; Wang, L.; Guan, Y.; Sun, X.; Dong, M. Polyphenolic extract from Rosa rugosa tea inhibits bacterial quorum sensing and biofilm formation. Food Control 2014, 42, 125–131. [Google Scholar] [CrossRef]
- Sarkar, R.; Chaudhary, S.K.; Sharma, A.; Yadav, K.K.; Nema, N.K.; Sekhoacha, M.; Karmakar, S.; Braga, F.C.; Gilbert, M.; Mukherjee, P.K.; et al. Anti-biofilm activity of Marula–a study with the standardized bark extract. J. Ethnopharmacol. 2014, 154, 170–175. [Google Scholar] [CrossRef]
- Da Júnior, S.D.C.; De Santos, J.V.O.; De Campos, L.A.A.; Pereira, M.A.; Magalhães, N.S.S.; Cavalcanti, I.M.F. Antibacterial and antibiofilm activities of quercetin against clinical isolates of Staphyloccocus aureus and Staphylococcus saprophyticus with resistance profile. Int. J. Environ. Agric. Biotechnol. 2018, 3, 1948–1958. [Google Scholar] [CrossRef]
- Chong, Y.M.; How, K.Y.; Yin, W.F.; Chan, K.G. The effects of Chinese herbal medicines on the quorum sensing-regulated virulence in Pseudomonas aeruginosa PAO1. Molecules 2018, 23, 972. [Google Scholar] [CrossRef] [PubMed]
- Lau, G.W.; Hassett, D.J.; Ran, H.; Kong, F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol. Med. 2004, 10, 599–606. [Google Scholar] [CrossRef]
- Al-haidari, R.A.; Shaaban, M.I.; Ibrahim, S.R.M.; Mohamed, G.A. Anti-quorum sensing activity of some medicinal plants. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 67–71. [Google Scholar] [CrossRef]
- Murray, T.S.; Ledizet, M.; Kazmierczak, B.I. Swarming motility, secretion of type 3 effectors and biofilm formation phenotypes exhibited within a large cohort of Pseudomonas aeruginosa clinical isolates. J. Med. Microbiol. 2010, 59, 511–520. [Google Scholar] [CrossRef]
- Lakshmanan, D.; Nanda, J.; Jeevaratnam, K. Inhibition of swarming motility of Pseudomonas aeruginosa by methanol extracts of Alpinia officinarum Hance and Cinnamomum tamala T. Nees and Eberm. Nat. Prod. Res. 2018, 32, 1307–1311. [Google Scholar] [CrossRef]
- Bouyahya, A.; Dakka, N.; Et-Touys, A.; Abrini, J.; Bakri, Y. Medicinal plant products targeting quorum sensing for combating bacterial infections. Asian Pac. J. Trop. Med. 2017, 10, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.M.; Sattenapally, N.; Parikh, H.I.; Li, W.; Rumbaugh, K.P.; German, N.A. Design, synthesis, and evaluation of compounds capable of reducing Pseudomonas aeruginosa virulence. Eur. J. Med. Chem. 2020, 185, 111800. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Pauw, E.; Eloff, J.N. Which tree orders in southern Africa have the highest antimicrobial activity and selectivity against bacterial and fungal pathogens of animals? BMC Complement. Altern. Med. 2014, 14, 1–12. [Google Scholar] [CrossRef]
- Famuyide, I.M.; Aro, A.O.; Fasina, F.O.; Eloff, J.N.; McGaw, L.J. Antibacterial and antibiofilm activity of acetone leaf extracts of nine under-investigated South African Eugenia and Syzygium (Myrtaceae) species and their selectivity indices. BMC Complement. Altern. Med. 2019, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Paul Bhattacharya, S.; Mitra, A.; Bhattacharya, A.; Sen, A. Quorum quenching activity of pentacyclic triterpenoids leads to inhibition of biofilm formation by Acinetobacter Baumannii. Biofouling 2020, 36, 922–937. [Google Scholar] [CrossRef] [PubMed]
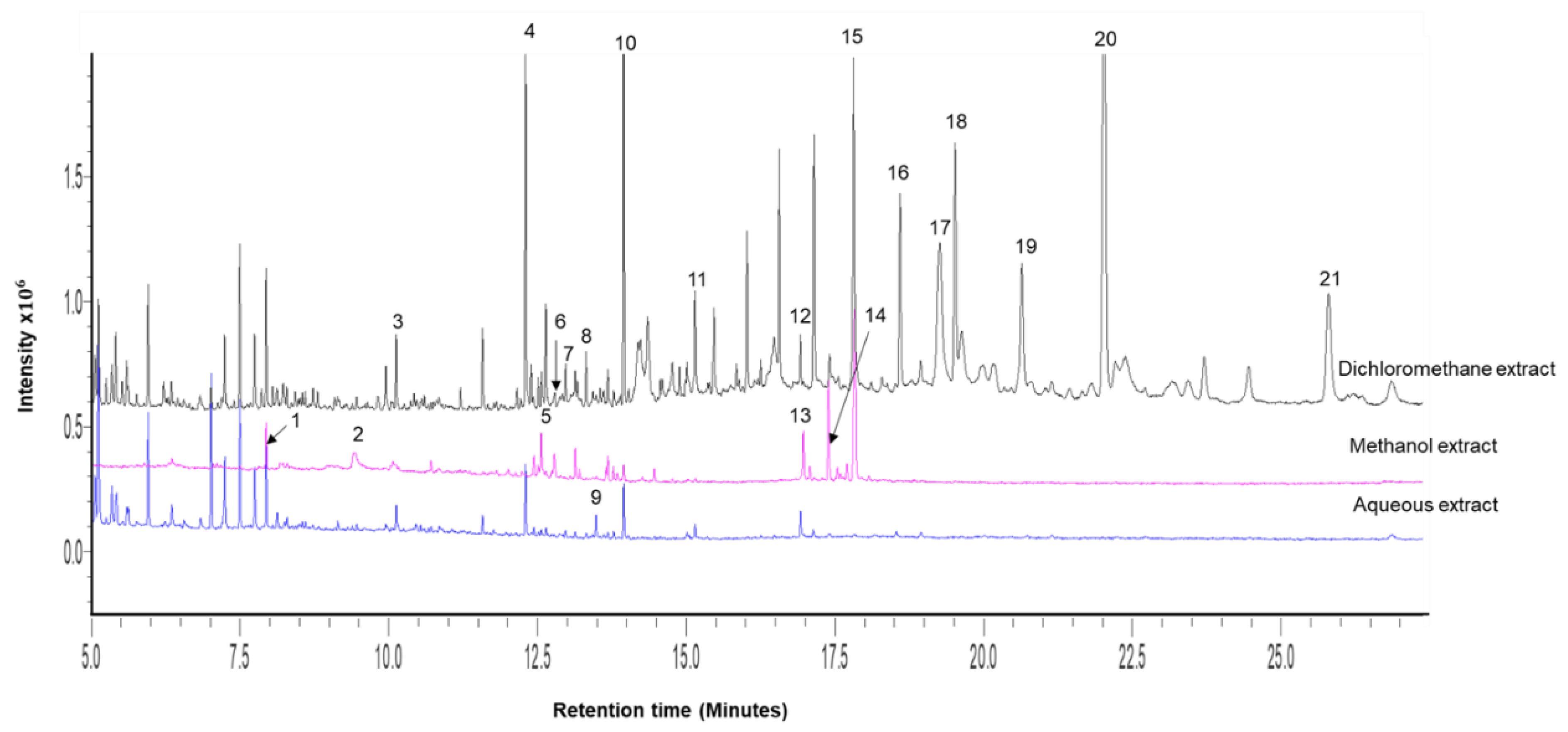


| Peak # | Ret. Time (min) | Name | Molecular Weight | Molecular Formula | M. comosus Extracts | ||
|---|---|---|---|---|---|---|---|
| AQ | ME | DCM | |||||
| 1 | 4.453 | Decane | 142 | C10H22 | 14.1% | 11.3% | |
| 2 | 6.479 | 2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one | 144 | C6H8O4 | 5.0% | ||
| 3 | 7.815 | Cyclooctane * | 112 | C8H16 | 1.2% | ||
| 4 | 9.389 | Guanosine | 283 | C10H13N4O | 9.8% | 22.3% | |
| 5 | 10.07 | Propanoic acid * | 174 | C8H18O2Si | 1.4% | ||
| 6 | 10.837 | 1,2-Benzenedicarboxylic acid, diethyl ester | 236 | C12H14NO6 | 64.1% | 52.8% | 2.0% |
| 7 | 11.576 | Octanedioic acid * | 318 | C14H30O4Si2 | 1.2% | 1.2% | |
| 8 | 12.267 | (-)-Loliolide | 196 | C11H16O3 | 0.2% | ||
| 9 | 12.298 | Azelaic acid * | 332 | C15H32O4Si2 | 4.8% | 5.6% | |
| 10 | 12.44 | D-Galactose, 2,3,4,5,6-pentakis-O * | 540 | C21H52O6Si5 | 0.4% | 2.5% | |
| 11 | 12.512 | D- (-)-Fructofuranose, pentakis ether * | 541 | C21H52O6Si5 | 2.0% | 0.5% | |
| 12 | 12.597 | Neophytadiene | 278 | C20H38 | 2.4% | 5.4% | 0.7% |
| 13 | 12.638 | Tetradecanoic acid * | 300 | C17H36O2Si | 1.4% | ||
| 14 | 12.894 | Cyclopentadecanol | 226 | C15H30O | 2.2% | 0.4% | |
| 15 | 12.945 | Decanedioic acid * | 346 | C16H34O4Si2 | 0.45% | ||
| 16 | 13.483 | Benzoic acid 3,4,5-tris(trimethylsiloxy) * | 458 | C19H38O5Si4 | 1.5% | ||
| 18 | 13.949 | Hexadecanoic acid * | 328 | C19H40O2Si | 3.4% | 1.4% | 5.4% |
| 19 | 14.407 | Tetramethyl hexadecenol (Trans phytol) | 296 | C20H40O | 4.8% | 0.9% | |
| 20 | 14.89 | Docosane | 310 | C22H46 | 0.4% | ||
| 21 | 15.125 | Octadecanoic acid * | 356 | C21H44O2Si | 1.4% | ||
| 22 | 15.47 | Tricosane | 324 | C23H48 | 1.2% | ||
| 23 | 16.025 | Tetracosane | 338 | C24H50 | 2.5% | ||
| 24 | 16.562 | Pentacosane | 352 | C25H52 | 4.2% | ||
| 25 | 16.919 | 1,2-Benzenedicarboxylic acid, mono(2-ethylhexyl) ester * | 390 | C24H38O4 | 1.9% | 0.7% | |
| 26 | 16.969 | Sucrose * | 342 | C12H22O11 | 4.8% | ||
| 27 | 17.146 | Hexacosane | 366 | C26H54 | 5.7% | ||
| 28 | 17.7 | Thymol-. beta. -d-glucopyranoside, tetrakis * | 600 | C28H56O6Si4 | 2.2% | ||
| 29 | 17.808 | Heptacosane | 380 | C27H56 | 7.0% | ||
| 30 | 17.814 | Tetratetracontane * | 618 | C44H90 | 6.9% | ||
| 31 | 18.584 | Octacosane | 394 | C28H58 | 6.4% | ||
| 32 | 19.505 | Nonacosane | 408 | C29H60 | 9.6% | ||
| 33 | 19.262 | 1-Hentetracontanol * | 592 | C41H84O | 7.2% | ||
| 34 | 20.625 | Triacontane | 422 | C30H62 | 5.0% | ||
| 35 | 22.00 | Dotriacontane | 450 | C32H66 | 4.6% | 33.9% | |
| 36 | 22.03 | Hexatraicontane * | 506 | C36H74 | 21.6% | ||
| 37 | 22.86 | alpha-Tocopherol (Vitamin E) | 430 | C29H50O2 | 1.6% | ||
| 38 | 23.66 | Tetratricontane | 478 | C34H70 | 3.0% | ||
| 39 | 26.7 | Stigmast-5-en-3-ol, (3. beta.) | 414 | C29H50O | 2.7% | 4.6% | |
| Melianthus comosus Extracts | MIC (mg/mL) |
|---|---|
| Aqueous | 0.78 |
| Methanol | 0.78 |
| Acetone | 1.56 |
| Ethyl acetate | 6.25 |
| Dichloromethane | 0.78 |
| Compounds | |
| Guanosine | 0.031 |
| 1% DMSO | ≥6.25 |
| Ciprofloxacin | 0.001 |
| Quercetin | 0.008 |
| Plant Species | Half Y | IC50 (mg/mL) |
|---|---|---|
| Melianthus comosus (aqueous) | 37.66 | 1.52 |
| Compounds | ||
| Cinnamaldehyde | 56.35 | 0.087 |
| Quercetin | 33.44 | 0.043 |
| Guanosine | 21.05 | 0.064 |
| M. comosus Extracts | Cell Attachment (%) | Biofilm Development (%) |
|---|---|---|
| Aqueous | −47.91 ± 0.51 a | −57.21 ± 0.34 b |
| Methanol | −10.17 ± 0.12 b | −48.54 ± 0.45 a,b |
| Dichloromethane | −14.25 ± 0.46 b | −128.36 ± 0.59 a |
| Compounds | ||
| Guanosine | 78.88 ± 0.11 d | 34.85 ± 1.08 c |
| Ciprofloxacin | 52.88 ± 0.18 c | 39.43 ± 0.30 c |
| Quercetin | 55.14 ± 0.03 c | 44.35 ± 0.05 c,d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baloyi, I.T.; Adeosun, I.J.; Yusuf, A.A.; Cosa, S. In Silico and In Vitro Screening of Antipathogenic Properties of Melianthus comosus (Vahl) against Pseudomonas aeruginosa. Antibiotics 2021, 10, 679. https://doi.org/10.3390/antibiotics10060679
Baloyi IT, Adeosun IJ, Yusuf AA, Cosa S. In Silico and In Vitro Screening of Antipathogenic Properties of Melianthus comosus (Vahl) against Pseudomonas aeruginosa. Antibiotics. 2021; 10(6):679. https://doi.org/10.3390/antibiotics10060679
Chicago/Turabian StyleBaloyi, Itumeleng T., Idowu J. Adeosun, Abdullahi A. Yusuf, and Sekelwa Cosa. 2021. "In Silico and In Vitro Screening of Antipathogenic Properties of Melianthus comosus (Vahl) against Pseudomonas aeruginosa" Antibiotics 10, no. 6: 679. https://doi.org/10.3390/antibiotics10060679
APA StyleBaloyi, I. T., Adeosun, I. J., Yusuf, A. A., & Cosa, S. (2021). In Silico and In Vitro Screening of Antipathogenic Properties of Melianthus comosus (Vahl) against Pseudomonas aeruginosa. Antibiotics, 10(6), 679. https://doi.org/10.3390/antibiotics10060679








