Effects of Different β-Lactam Antibiotics on Indirect Tomato (Solanum lycopersicum L.) Shoot Organogenesis and Agrobacterium tumefaciens Growth Inhibition In Vitro
Abstract
1. Introduction
2. Results
2.1. The Effects of Different β-Lactam Antibiotics on In Vitro Tomato Morphogenesis
2.1.1. Callus Induction and Frequency of Its Formation
2.1.2. Efficiency of Tomato Shoot Organogenesis
2.2. The Effects of Different β-Lactam Antibiotics on A. tumefaciens Growth Inhibition In Vitro
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Explant Source, Antibiotics, and Culture Conditions
4.3. Histological Analysis of Callus Tissue
4.4. Agar Disk-Diffusion Method
4.5. Accounting Data and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gerszberg, A.; Hnatuszko-Konka, K.; Kowalczyk, T.; Kononowicz, A.K. Tomato (Solanum lycopersicum L.) in the service of biotechnology. Plant Cell Tiss. Organ Cult. 2015, 120, 881–902. [Google Scholar] [CrossRef]
- Hwang, H.H.; Yu, M.; Lai, E.M. Agrobacterium-mediated plant transformation: Biology and applications. Arab. Book 2017, 2017, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Ruma, D.; Dhaliwal, M.S.; Kaur, A.; Gosal, S.S. Transformation of tomato using biolistic gun for transient expression of the β-glucuronidase gene. Indian J. Biotechnol. 2009, 8, 363–369. [Google Scholar]
- Abu-El-Heba, G.A.; Hussein, G.M.; Abdalla, N.A. A rapid and efficient tomato regeneration and transformation system. Landbauforsch. Volkenrode 2008, 58, 103. [Google Scholar]
- Vinoth, S.; Gurusaravanan, P.; Jayabalan, N. Optimization of factors influencing microinjection method for Agrobacterium tumefaciens-mediated transformation of tomato. Appl. Biochem. Biotechnol. 2013, 169, 1173–1187. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Tanaka, H.; Yano, K.; Takagi, M. An effective transformation system for Lycopersicon peruvianum by electroporation. Jpn. J. Breed. 1992, 42, 487–495. [Google Scholar] [CrossRef]
- Ray, S.; Lahiri, S.; Halder, M.; Mondal, M.; Choudhuri, T.R.; Kundu, S. An efficient method of isolation and transformation of protoplasts from tomato leaf mesophyll tissue using the binary vector pCambia 1302. Int. Adv. Res. J. Sci. Eng. Technol. 2015, 2, 146–150. [Google Scholar]
- Nicolia, A.; Andersson, M.; Hofvander, P.; Festa, G.; Cardi, T. Tomato protoplasts as cell target for ribonucleoprotein (RNP)-mediated multiplexed genome editing. Plant Cell Tiss. Organ. Cult. 2021, 144, 463–467. [Google Scholar] [CrossRef]
- Rao, A.Q.; Bakhsh, A.; Kiani, S.; Shahzad, K.; Shahid, A.A.; Husnain, T.; Riazuddin, S. The myth of plant transformation. Biotechnol. Adv. 2009, 27, 753–763. [Google Scholar] [CrossRef]
- Khaliluev, M.R.; Kharchenko, P.N.; Dolgov, S.V. Development of regeneration system and study of the transformation potential of a commercial tomato variety. Russ. Agric. Sci. 2010, 36, 175–179. [Google Scholar] [CrossRef]
- Khaliluev, M.R.; Bogoutdinova, L.R.; Baranova, G.B.; Baranova, E.N.; Kharchenko, P.N.; Dolgov, S.V. Influence of genotype, explant type, and component of culture medium on in vitro callus induction and shoot organogenesis of tomato (Solanum lycopersicum L.). Biol. Bull. 2014, 41, 512–521. [Google Scholar] [CrossRef]
- Kumar, S.; Jindal, S.K.; Sarao, N.K.; Dhaliwal, M.S. Development of an efficient In vitro regeneration protocol in tomato (Solanum lycopersicum L.). Agric. Res. J. 2017, 54, 475–479. [Google Scholar] [CrossRef]
- Rashid, R.; Bal, S.S. Effect of hormones on direct shoot regeneration in hypocotyl explants of tomato. Not. Sci. Biol. 2010, 2, 70–73. [Google Scholar] [CrossRef][Green Version]
- Devi, R.; Dhaliwal, M.S.; Gosal, S.S. In vitro direct plant regeneration protocol for tomato genotypes. Indian J. Horticult. 2013, 70, 369–372. [Google Scholar]
- Saeed, W.; Naseem, S.; Gohar, D.; Ali, Z. Efficient and reproducible somatic embryogenesis and micropropagation in tomato via novel structures-Rhizoid Tubers. PLoS ONE 2019, 14, e0215929. [Google Scholar] [CrossRef] [PubMed]
- Godishala, V.; Mangamoori, L.; Nanna, R. Plant regeneration via somatic embryogenesis in cultivated tomato (Solanum lycopersicum L.). J. Cell Tiss. Res. 2011, 11, 2521. [Google Scholar]
- Ashakiran, K.; Chidambareswaran, M.; Govindasamy, V.; Sivankalyani, V.; Girija, S. Somatic embryogenesis for Agrobacterium mediated transformation of tomato-Solanum lycopersicum. L. Int. J. Biotechnol. Appl. 2011, 3, 72–79. [Google Scholar]
- Trujillo-Moya, C.; Gisbert, C.; Vilanova, S.; Nuez, F. Localization of QTLs for in vitro plant regeneration in tomato. BMC Plant Biol. 2011, 11, 1–13. [Google Scholar] [CrossRef][Green Version]
- Basté, E.M.; Pratta, G.R.; Zorzoli, R. Genetic analysis of the in vitro culture response in tomato. Plant Cell Tiss. Organ Cult. 2007, 88, 233–239. [Google Scholar] [CrossRef]
- Sánchez-López, J.; Atarés, A.; Jáquez-Gutiérrez, M.; Ortiz-Atienza, A.; Capel, C.; Pineda, B.; García-Sogo, B.; Yuste-Lisbona, F.J.; Lozano, R.; Moreno, V. Approaching the genetic dissection of indirect adventitious organogenesis process in tomato explants. Plant Sci. 2021, 302, 110721. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, J.; Jie, E.Y.; Choi, S.H.; Jiang, L.; Ahn, W.S.; Kim, C.Y.; Kim, S.W. Temporal and spatial expression analysis of shoot-regeneration regulatory genes during the adventitious shoot formation in hypocotyl and cotyledon explants of tomato (cv. Micro-Tom). Int. J. Mol. Sci. 2020, 21, 5309. [Google Scholar] [CrossRef]
- Stavridou, E.; Τzioutziou, N.A.; Madesis, P.; Labrou, N.E.; Nianiou-Obeidat, I. Effect of different factors on regeneration and transformation efficiency of tomato (Lycopersicum esculentum) hybrids. Czech J. Gen. Plant Breed. 2019, 55, 120–127. [Google Scholar] [CrossRef]
- Chetty, V.J.; Ceballos, N.; Garcia, D.; Narváez-Vásquez, J.; Lopez, W.; Orozco-Cárdenas, M.L. Evaluation of four Agrobacterium tumefaciens strains for the genetic transformation of tomato (Solanum lycopersicum L.) cultivar Micro-Tom. Plant Cell Rep. 2013, 32, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Enikeev, A.G.; Kopytina, T.V.; Semenova, L.A.; Natyaganova, A.V.; Gamanetz, L.V.; Volkova, O.D. Agrobacterial transformation as complex biotical stressing factor. J. Stress Physiol. Biochem. 2008, 4, 11–19. [Google Scholar]
- Şen, A. Oxidative stress studies in plant tissue culture. In Antioxidant Enzyme; El-Missiry, M.A., Ed.; InTech: Rijeka, Croatia, 2012; pp. 59–88. [Google Scholar]
- Dolgikh, Y.I.; Stepanova, A.Y.; Trusova, S.V.; Chichkova, N.V.; Vartapetian, A.B. Mitochondria-targeted antioxidant provides for enhanced morphogenetic potential in plant tissue cultures. Russ. J. Plant Physiol. 2013, 60, 706–712. [Google Scholar] [CrossRef]
- Ahsan, N.; Lee, S.-H.; Lee, D.-G.; Anisuzzaman, M.; Alam, M.F.; Yoon, H.-S.; Choi, M.S.; Yang, J.-K.; Lee, B.H. The effects of wounding type, preculture, infection method and cocultivation temperature on the Agrobacterium-mediated gene transfer in tomatoes. Ann. Appl. Biol. 2007, 151, 363–372. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, Y.L.; Meng, Z.J.; Jiang, J. Optimization of factors affecting Agrobacterium-mediated transformation of Micro-Tom tomatoes. Genet. Mol. Res. 2012, 11, 661–671. [Google Scholar] [CrossRef]
- Dan, Y.; Zhang, S.; Zhong, H.; Yi, H.; Sainz, M.B. Novel compounds that enhance Agrobacterium-mediated plant transformation by mitigating oxidative stress. Plant. Cell Rep. 2015, 34, 291–309. [Google Scholar] [CrossRef]
- Pandey, N.; Cascella, M. Beta lactam antibiotics. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Zango, U.U.; Ibrahim, M.; Shawai, S.A.A. A review on β-lactam antibiotic drug resistance. MOJ Drug. Des. Dev. Ther. 2019, 3, 52–58. [Google Scholar]
- Saudagar, P.S.; Survase, S.A.; Singhal, R.S. Clavulanic acid: A review. Biotechnol. Adv. 2008, 26, 335–351. [Google Scholar] [CrossRef]
- Cheng, Z.M.; Schnurr, J.A.; Kapaun, J.A. Timentin as an alternative antibiotic for suppression of Agrobacterium tumefaciens in genetic transformation. Plant Cell Rep. 1998, 17, 646–649. [Google Scholar] [CrossRef]
- Dunaeva, S.E.; Osledkin, Y.S. Bacterial microorganisms associated with the plant tissue culture: Identification and possible role. Sel’skokhozyaistvennaya Biol. 2015, 50, 3–15. [Google Scholar] [CrossRef]
- Cheong, E.J.; Na, M.; Jeong, U. The effect of endophytic bacteria on in vitro shoot growth of Prunus yedoensis and its identification and elimination. Vitro Cell. Dev. Biol. Plant. 2019, 56, 200–206. [Google Scholar] [CrossRef]
- Ogawa, Y.; Mii, M. Screening for highly active β-lactam antibiotics against Agrobacterium tumefaciens. Arch. Microbiol. 2004, 181, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.M.; Pandian, S.K.; Manikandan, R. The effect of different antibiotics on the elimination of Agrobacterium and high frequency Agrobacterium-mediated transformation of indica rice (Oryza sativa L.). Czech J. Genet. Plant Breed. 2012, 48, 120–130. [Google Scholar] [CrossRef]
- Farzaneh, A.; Adel, Y.; Ali, N.; Younes, G. Determine effective concentrations of β-lactam antibiotics against three strains of Agrobacterium tumefaciens and phytotoxicity on Tomato and Tobacco. Int. J. Agron. Plant. Prod. 2013, 11, 2919–2925. [Google Scholar]
- Ogawa, Y.; Mii, M. Meropenem and moxalactam: Novel β-lactam antibiotics for efficient Agrobacterium-mediated transformation. Plant Sci. 2007, 172, 564–572. [Google Scholar] [CrossRef]
- Hu, W.; Phillips, G.C. A combination of overgrowth-control antibiotics improves Agrobacterium tumefaciens-mediated transformation efficiency for cultivated tomato (L. esculentum). Vitro Cell. Dev. Biol. Plant 2001, 37, 12–18. [Google Scholar] [CrossRef]
- Ling, H.Q.; Kriseleit, D.; Ganal, M.W. Effect of ticarcillin/potassium clavulanate on callus growth and shoot regeneration in Agrobacterium-mediated transformation of tomato (Lycopersicon esculentum Mill.). Plant Cell Rep. 1998, 17, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Ieamkhang, S.; Chatchawankanphanich, O. Augmentin® as an alternative antibiotic for growth suppression of Agrobacterium for tomato (Lycopersicon esculentum) transformation. Plant Cell Tiss. Organ Cult. 2005, 82, 213–220. [Google Scholar] [CrossRef]
- Mamidala, P.; Nanna, R.S. Influence of antibiotics on regeneration efficiency in tomato. Plant Omics J. 2009, 2, 135–140. [Google Scholar]
- Costa, M.G.C.; Nogueira, F.T.S.; Figueira, M.L.; Otoni, W.C.; Brommonschenkel, S.H.; Cecon, P.R. Influence of the antibiotic timentin on plant regeneration of tomato (Lycopersicon esculentum Mill.) cultivars. Plant Cell Rep. 2000, 19, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Gerszberg, A.; Grzegorczyk-Karolak, I. Influence of selected antibiotics on the tomato regeneration in in vitro cultures. Not. Bot. Hort. Agrobot. 2019, 47, 558–564. [Google Scholar] [CrossRef]
- Horsch, R.B.; Frye, J.E.; Hoffman, N.F.; Eichoholtz, D.; Rogers, S.G.; Fraley, R.T. A simple and general method for transferring genes into plants. Science 1985, 227, 1229–1231. [Google Scholar]
- McCormick, S.; Niedermeyer, J.; Fry, J.; Barnason, A.; Horsch, R.; Fraley, R. Leaf disc transformation of cultivated tomato (L. esculentum) using Agrobacterium tumefaciens. Plant Cell Rep. 1986, 5, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Perveen, S.; Khan, T.A.; Shaheen, H.; Naz, R.; Hyder, M.Z.; Ijaz, B.; Saqlan Naqvi, S.M.; Yasmin, T. Morpho-physiological investigations in transgenic tomato (Solanum lycopersicum L.) over expressing OsRGLP1 gene. Vitro Cell. Dev. Biol. Plant 2021, 1–16. [Google Scholar] [CrossRef]
- Kazemi, E.M.; Jonoubi, P.; Majd, A.; Pazhouhandeh, M. Reduction of negative effects of cefotaxime in tomato transformation by using FeEDDHA. Int. J. Farm. Alli. Sci. 2014, 3, 538–542. [Google Scholar]
- Gulevich, A.A.; Kurenina, L.V.; Baranova, E.N. Application of a system for targeting Fe-dependent superoxide dismutase and choline oxidase enzymes to chloroplast as a strategy for effective plant resistance to abiotic stresses. Russ. Agric. Sci. 2018, 44, 118–123. [Google Scholar] [CrossRef]
- Shpakovski, G.V.; Spivak, S.G.; Berdichevets, I.N.; Babak, O.G.; Kubrak, S.V.; Kilchevsky, A.V.; Aralov, A.V.; Slovokhotov, I.Y.; Shpakovski, D.G.; Baranova, E.N.; et al. A key enzyme of animal steroidogenesis can function in plants enhancing their immunity and accelerating the processes of growth and development. BMC Plant Biol. 2017, 17, 189. [Google Scholar] [CrossRef]
- Mineykina, A.; Shumilina, D.; Bondareva, L.; Soldatenko, A.; Domblides, E. Effect of beta-lactam antibiotics on microspore embryogenesis in Brassica species. Plants 2020, 9, 489. [Google Scholar] [CrossRef]
- Danilova, S.A.; Dolgikh, Y.I. The stimulatory effect of the antibiotic cefotaxime on plant regeneration in maize tissue culture. Russ. J. Plant Physiol. 2004, 51, 559–562. [Google Scholar] [CrossRef]
- Gambhir, G.; Kumar, P.; Srivastava, D.K. Effect of antibiotic sensitivity on different cultured tissues and its significance in genetic transformation of cabbage Brassica oleracea. Biosci. Biotechnol. Res. Comm. 2017, 10, 652–661. [Google Scholar] [CrossRef]
- Stanišić, M.; Ninković, S.; Savić, J.; Ćosić, T.; Banjac, N. The effects of β-lactam antibiotics and hygromycin B on de novo shoot organogenesis in apple cv. Golden Delicious. Arch. Biol. Sci. 2018, 70, 179–190. [Google Scholar] [CrossRef]
- Grzebelus, E.; Skop, L. Effect of β-lactam antibiotics on plant regeneration in carrot protoplast cultures. Vitro Cell. Dev. Biol. Plant 2014, 50, 568–575. [Google Scholar] [CrossRef]
- Holford, P.; Newbury, H.J. The effects of antibiotics and their breakdown products on the in vitro growth of Antirrhinum majus. Plant Cell Rep. 1992, 11, 93–96. [Google Scholar] [CrossRef]
- Brethauer, S.; Held, M.; Panke, S. Clavulanic acid decomposition is catalyzed by the compound itself and by its decomposition products. J. Pharm. Sci. 2008, 97, 3451–3455. [Google Scholar] [CrossRef]
- Gómez-Ríos, D.; Ramírez-Malule, H.; Neubauer, P.; Junne, S.; Ríos-Estepa, R. Degradation kinetics of clavulanic acid in fermentation broths at low temperatures. Antibiotics 2019, 8, 6. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Schumacher, A.; Vranken, T.; Malhotra, A.; Arts, J.J.C.; Habibovic, P. In vitro antimicrobial susceptibility testing methods: Agar dilution to 3D tissue-engineered models. Eur. J. Clin. Microbiol. Infect Dis. 2018, 37, 187–208. [Google Scholar] [CrossRef]
- Chiu, C.-T.; Lai, C.-H.; Huang, Y.-H.; Yang, C.-H.; Lin, J.-N. Comparative analysis of gradient diffusion and disk diffusion with agar dilution for susceptibility testing of Elizabethkingia anophelis. Antibiotics 2021, 10, 450. [Google Scholar] [CrossRef]
- Böhm, M.E.; Razavi, M.; Flach, C.F.; Larsson, D.G. A Novel, Integron-regulated, class C β-lactamase. Antibiotics 2020, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F.A. Revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Bertani, G. Studies on lysogenesis I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacterial. 1951, 62, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Dospekhov, B.A. Methods of Field Experience; Agropromizdat: Moscow, Russia, 1985; p. 350. [Google Scholar]
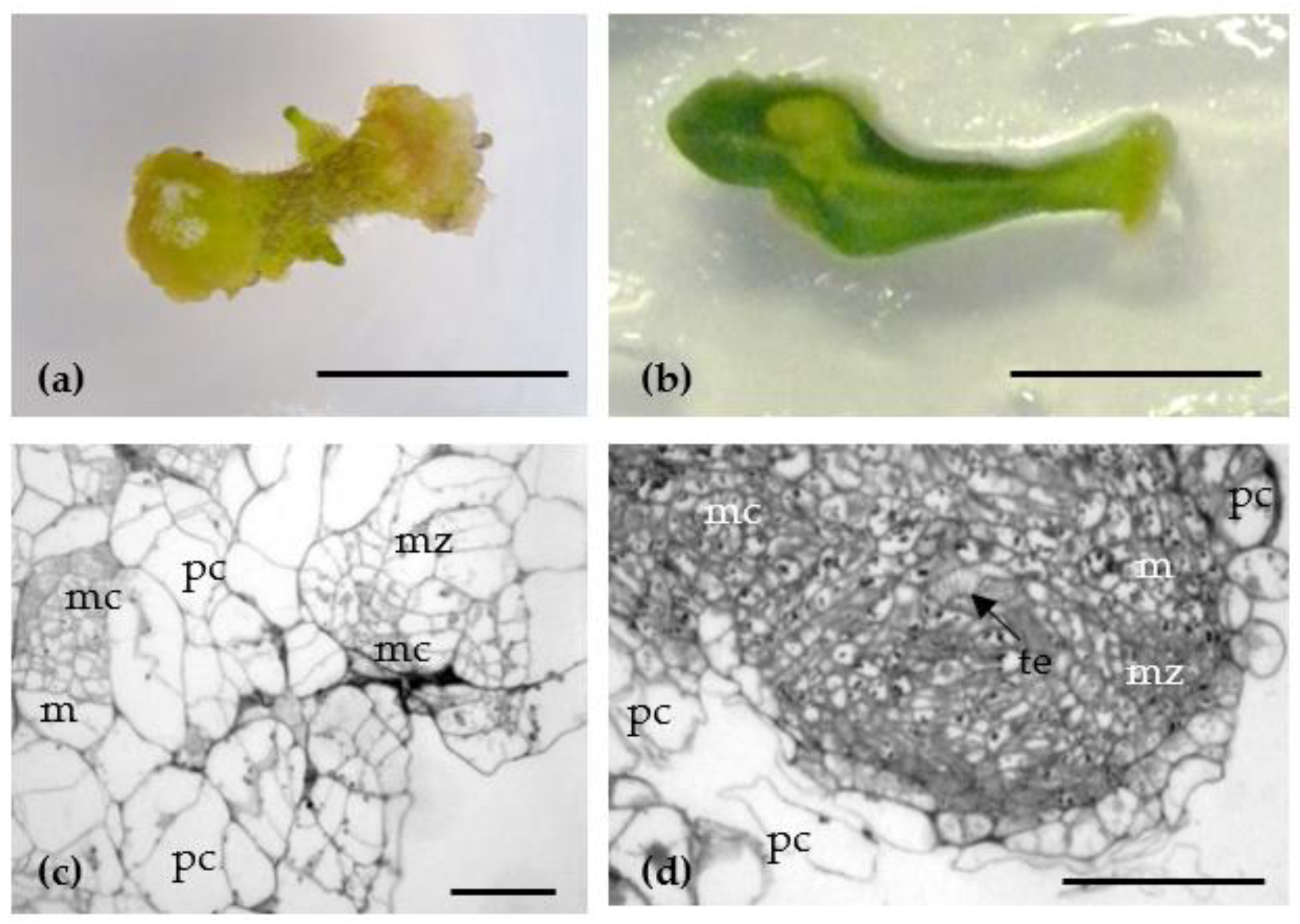
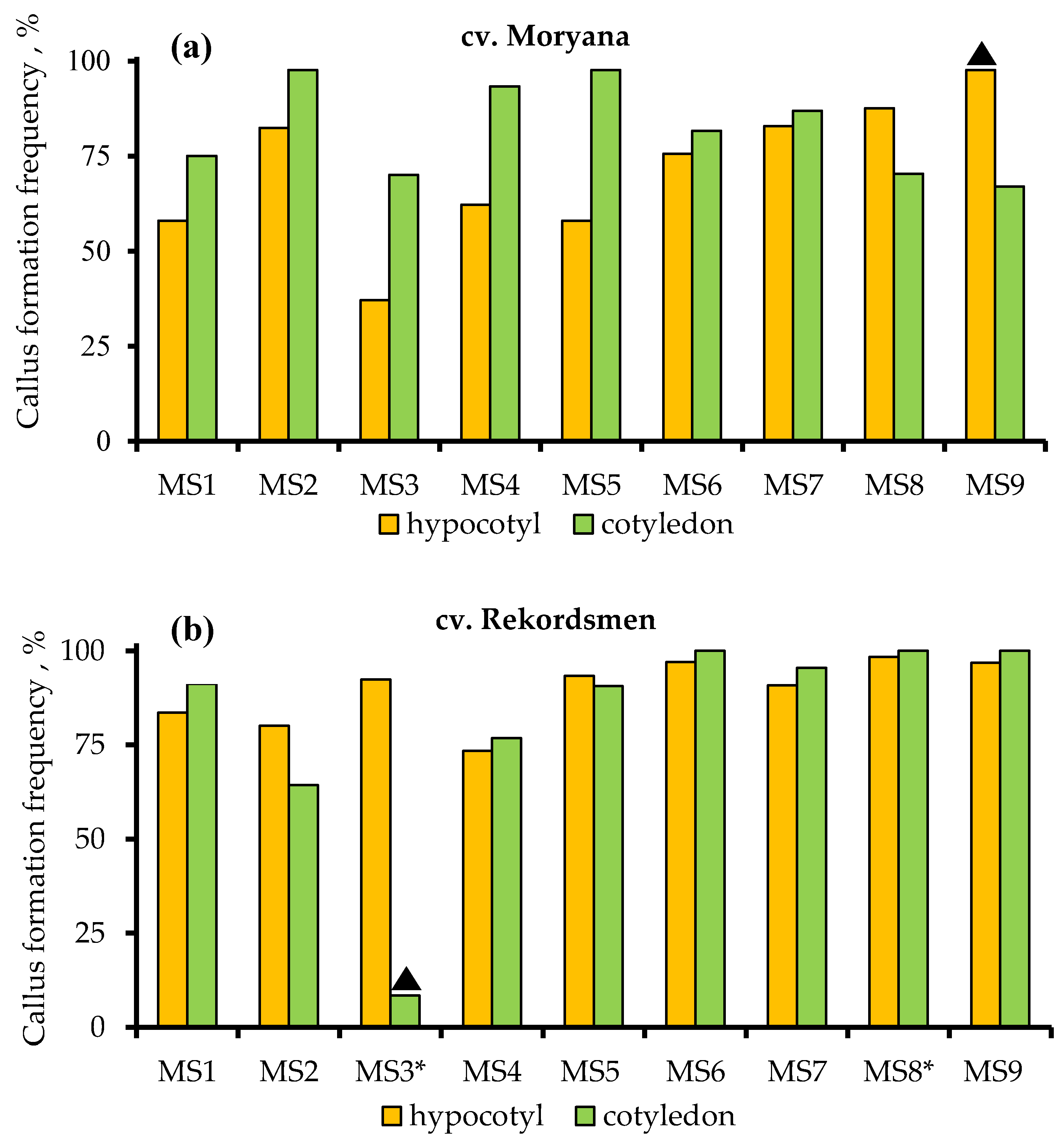
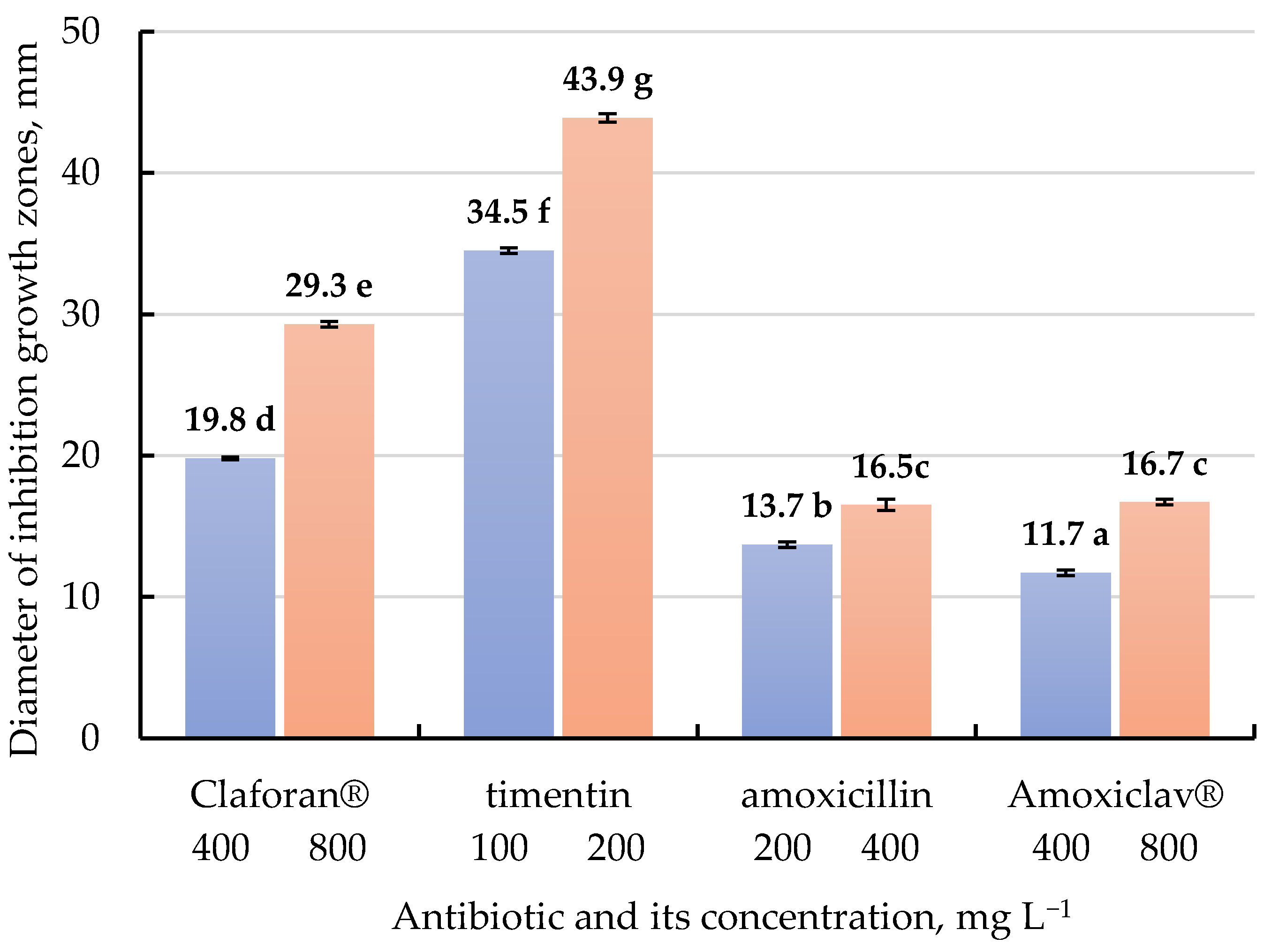
| Culture Medium | MS2 | MS3 | MS4 | MS5 | MS6 | MS7 | MS8 | MS9 | |
|---|---|---|---|---|---|---|---|---|---|
| Antibiotic | brand name | Claforan®, Sanofi-Aventis (France) | Timentin, PhytoTechnology Laboratories (USA) | Amoxicillin, PhytoTechnology Laboratories (USA) | Amoxiclav®, Lek d.d. (Slovenia) | ||||
| generic name | Cefotaxime sodium | Ticarcillin disodium + clavulanate potassium (15:1) | Amoxicillin | Amoxicillin + clavulanate potassium | |||||
| Concentration, mg L−1 | 400 | 800 | 100 | 200 | 200 | 400 | 400 | 800 | |
| Culture Medium | Antibiotic and Its Concentration, mg L−1 | Frequency of Shoot Organogenesis, % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| cv. Moryana | cv. Rekordsmen | Mean (Culture Medium) 3 | |||||||
| Hypocotyls | Cotyledons | Mean 2 | Hypocotyls | Cotyledons | Mean | ||||
| MS1 | — | 4.4 1 ab | 67.1 fghijk | 35.8 cdef | 1.5 ab | 42.7 fghij | 22.1 c | 29.0 b | |
| MS2 | Claforan® | 400 | 4.3 ab | 71.6 ijk | 38.0 cdef | 11.9 bcde | 1.2 ab | 6.6 b | 22.3 b |
| MS3 | Claforan® | 800 | 0 a | 56.8 efghij | 28.4 c | 0.8 ab | 0 a | 0.4 a | 14.4 a |
| MS4 | Timentin | 100 | 37.4 de | 77.5 jk | 57.5 g | 17.6 cdef | 46.6 ghij | 32.1 cde | 44.8 d |
| MS5 | Timentin | 200 | 16.8 bcd | 89.2 k | 53.0 efg | 26.5 defgh | 80.6 l | 53.6 fg | 53.3 de |
| MS6 | Amoxicillin | 200 | 30.2 cde | 81.6 jk | 56.0 fg | 30.6 efgh | 68.4 jkl | 49.5 efg | 52.8 de |
| MS7 | Amoxicillin | 400 | 10.9 bc | 87.0 k | 54.4 defg | 6.7 abcd | 68.4 ijkl | 37.6 cdef | 46.0 cd |
| MS8 | Amoxiclav® | 400 | 10.9 bc | 70.3 hijk | 46.1 cdefg | 77.8 kl | 100 mn | 88.9 i | 67.5 f |
| MS9 | Amoxiclav® | 800 | 6.7 ab | 67.1 ghijk | 36.9 cdef | 55.7 hijkl | 100 n | 77.9 hi | 57.4 ef |
| Mean (explant) 4 | — | 13.5 a | 74.2 b | — | 25.5 a | 56.4 b | — | — | |
| Mean (genotype) 5 | — | 43.9 a | 40.9 a | — | |||||
| Culture Medium | Antibiotic and Its Concentration, mg L−1 | Average Number of Shoots per Explant | |||||||
|---|---|---|---|---|---|---|---|---|---|
| cv. Moryana | cv. Rekordsmen | Mean (Culture Medium) 3 | |||||||
| Hypocotyls | Cotyledons | Mean 2 | Hypocotyls | Cotyledons | Mean | ||||
| MS1 | — | 1.9 1 bc | 2.0 bcde | 2.0 bcd | 1.3 ab | 2.3 bcde | 1.8 abc | 1.9 ab | |
| MS2 | Claforan® | 400 | 1.6 ab | 3.7 ghi | 2.7 bcdefg | 2.6 bcde | 1.5 abcd | 2.0 bcde | 2.4 bc |
| MS3 | Claforan® | 800 | 1.0 a | 2.3 bcdef | 1.7 ab | 1.3 ab | 1.0 a | 1.1 a | 1.4 a |
| MS4 | Timentin | 100 | 3.0 cdefghi | 5.6 j | 4.3 jkl | 2.0 abcde | 2.3 bcde | 2.2 bcdef | 3.2 cd |
| MS5 | Timentin | 200 | 2.9 cdefghi | 4.3 ij | 3.6 gh6ijk | 2.9 cde | 5.3 fgh | 4.1 ijk | 3.8 d |
| MS6 | Amoxicillin | 200 | 2.6 bcdefg | 3.5 fghi | 3.0 defghi | 3.0 de | 3.5 efg | 3.3 fghij | 3.1 cd |
| MS7 | Amoxicillin | 400 | 2.2 bcde | 3.2 efghi | 2.7 cdefg | 2.0 abcde | 9.2 i | 5.6 l | 4.2 d |
| MS8 | Amoxiclav® | 400 | 2.0 bcd | 3.1 defghi | 2.6 bcdefg | 3.2 e | 6.3 h | 4.8 kl | 3.7 d |
| MS9 | Amoxiclav® | 800 | 2.3bcdef | 4.0 hi | 3.1 efghij | 2.7 bcde | 5.3 gh | 4.0 hijk | 3.6 d |
| Mean (explant) 4 | — | 2.0 a | 3.5 b | — | 2.0 a | 4.0 b | — | — | |
| Mean (genotype) 5 | — | 2.8 a | 3.0 a | — | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varlamova, N.V.; Dolgikh, Y.I.; Blinkov, A.O.; Baranova, E.N.; Khaliluev, M.R. Effects of Different β-Lactam Antibiotics on Indirect Tomato (Solanum lycopersicum L.) Shoot Organogenesis and Agrobacterium tumefaciens Growth Inhibition In Vitro. Antibiotics 2021, 10, 660. https://doi.org/10.3390/antibiotics10060660
Varlamova NV, Dolgikh YI, Blinkov AO, Baranova EN, Khaliluev MR. Effects of Different β-Lactam Antibiotics on Indirect Tomato (Solanum lycopersicum L.) Shoot Organogenesis and Agrobacterium tumefaciens Growth Inhibition In Vitro. Antibiotics. 2021; 10(6):660. https://doi.org/10.3390/antibiotics10060660
Chicago/Turabian StyleVarlamova, Nataliya V., Yuliya I. Dolgikh, Andrey O. Blinkov, Ekaterina N. Baranova, and Marat R. Khaliluev. 2021. "Effects of Different β-Lactam Antibiotics on Indirect Tomato (Solanum lycopersicum L.) Shoot Organogenesis and Agrobacterium tumefaciens Growth Inhibition In Vitro" Antibiotics 10, no. 6: 660. https://doi.org/10.3390/antibiotics10060660
APA StyleVarlamova, N. V., Dolgikh, Y. I., Blinkov, A. O., Baranova, E. N., & Khaliluev, M. R. (2021). Effects of Different β-Lactam Antibiotics on Indirect Tomato (Solanum lycopersicum L.) Shoot Organogenesis and Agrobacterium tumefaciens Growth Inhibition In Vitro. Antibiotics, 10(6), 660. https://doi.org/10.3390/antibiotics10060660








