Abstract
Synbiotic (SYN) additives were assessed as an antibiotic alternative on the effects on the nonspecific immune response and disease resistance of O. niloticus to P. aeruginosa. Healthy fish (n = 120, average initial weight 18 ± 2 g) were allotted randomly into four experimental groups (3 replicates for each); 1) a control group with no additives (CON), 2) basal diet complemented with 0.1 g kg–1 diets of norfloxacin, NFLX, 3) basal diet fortified with 1 mL kg–1 diet of SYN, and 4) basal diet complemented with a mixture of NFLX and SYN, which was carried out for eight weeks. Results showed a significant increase (p < 0.01) in the serum immune parameters (total protein, globulin and albumin, nitric oxide (NO), and lysozyme activity) in the SYN group and the NFLX+SYN group compared with the CON and NFLX groups. The serum glucose, cholesterol, and triglycerides were higher in NFLX and NFLX+SYN groups than the CON and SYN groups. The catalase (CAT), superoxide dismutase, glutathione peroxidase (GPX) activities were significantly augmented in the NFLX+SYN group, followed by the SYN group compared with CON and NFLX groups. The cumulative mortality rate (CMR) of O. niloticus following the P. aeruginosa challenge was decreased in the SYN group compared to other groups. The results emphasize that synbiotic could be used as a norfloxacin alternative to enhance the related immunological parameters, including antioxidant activity and disease resistance against P. aeruginosa infection of O. niloticus.
1. Introduction
Fish diseases, especially those caused by bacterial pathogens, are important problems in the fish farm industry [1]. Pseudomonas infection has been incriminated as one of the common bacterial infections of fishes and appears as an opportunistic and stress-related disease of freshwater fish cultured under intensive environments [2]. Motile Pseudomonas species are the most prevalent fish diseases in Egypt [3], causing septicemia, especially in Nile tilapia [2]. They are highly adaptable opportunistic bacteria that survive in different environments [4] and are responsible for the ulcerative syndrome of bacterial hemorrhagic septicemia, tail and fin rot, and ascites [2].
Antibiotics are commonly used to treat fish diseases and improve their growth performance to prevent and control fish diseases [5]. Large quantities of antibiotics are also used in aquaculture for prevention [6]. Fluoroquinolones control bacterial infections that cause urinary, pulmonary, and digestive tract diseases in farmed fish [7]. Norfloxacin (NFLX), a cheap third-generation fluoroquinolone with broad-spectrum action against aerobic Gram-negative, has been used for fish disease control for several years. However, it has a few side effects in fish [8]. NFLX was the antibiotic of choice after applying an antibiotic sensitivity test against P. aeruginosa isolated from infected Nile tilapia. Chemotherapy, including antibiotics, decreases the normal flora of fish and biological filters and must be used only as an emergency measure. It can also reduce or prevent the incidence of bacterial pathogens and be hazardous for aquatic animals [5]. It could also lead to many problems as their cumulative residues affect human health [9]. Thus, other successful alternative preventive measures in aquaculture could be considered to prevent the entrance of the pathogen, enhance water quality, reduce the different stressors in the fish environment, and provide good nutrition and immunization. Furthermore, the application of feed additives like probiotics, prebiotics, and synbiotics in aquacultures enhances the fish’s health status [5].
There is a global interest in using feed additives as an alternative to chemotherapy to enhance fish growth, digestive enzyme activities, and immunity, and prevent disease occurrence [10,11]. Synbiotics are formed from a combination of non-digestible food ingredients (prebiotic) and living organisms (probiotics) that increase the growth and improve the health status among aquaculture species [12,13,14,15,16]. Synbiotics provide more beneficial effects in comparison to the addition of only probiotics or prebiotics [9] for fish and aquatic animals as they promote the establishment of live microbes in the gut and stimulate the metabolism of several beneficial bacteria, which improves the host health [9,17].
Nile tilapia (Oreochromis niloticus) is one of the most important Tilapia species widely cultured in the tropical, subtropical, and temperate regions [18]. The most important goal in the aquaculture industry is enhancing fish growth, health, and immunity during intensive culture. Fish intensification leads to aquatic disease transmission and fish mortality because microbial diseases are predicted to increase [19]. Accordingly, the search for alternatives to control potential infection of P. aeruginosa could promote the health of various fish species, promoting their growth and contributing to food safety. This study was designed to assess the potential effect of using synbiotic as a norfloxacin alternative on serum biochemical parameters, antioxidant activity, immune response, and disease resistance of O. niloticus against P. aeruginosa.
2. Materials and Methods
2.1. Feed Additives Used
The commercial product Curazole-M as a synbiotic (Rupa Vet Pharma, Cairo, Egypt) was composed of Saccharomyces cerevisiae (1.0 × 1011 CFU L–1), Echinacea (115 g L–1), mannan oligosaccharides (35 g L–1), β-glucan (25 g L–1), Vitamin E (100 g L–1), DL-methionine (30 g L–1), L-lysine (10 g L–1), choline chloride (100 g L–1), minerals (30 g L–1) and propylene glycol (75 g L–1). The antibiotic NORACIN (norfloxacin 400 mg) was procured from El-Amirya Pharma, Cairo, Egypt.
2.2. Fish and Cultural Conditions
The experiment was carried out according to the guidelines of the care and use of animals for scientific purposes research committee, and ethical approval was obtained from Zagazig University (ZU-IACUC/3/F/170/2019).
Healthy O. niloticus fingerlings (n = 120) with an initial average weight of 18 ± 2 g were obtained from the Abassa fish farm in the Sharkia governorate, Egypt. The fish were conditioned for 15 days and fed a basic diet (Table 1) until the experiment began. Before starting the experiment, according to the CCAC, a fish health check was performed to establish that the fish were free of pathogens and disease markers [20]. Fish were assigned randomly to 12 glass tanks (96 L) with ten fish per tank filled with chlorine-free tap water. The aquaria were aerated by a central air compressor. The dissolved oxygen was 6.55 mg/L, and the water temperature and pH were 25 ± 0.5 °C and 7.8 ± 0.4, respectively; all were measured daily. Water was exchanged every other day. Ammonia-N, nitrite-N, and water hardness were measured weekly according to APHA [21]. The light period was controlled automatically as 12 light hours: 12 h darkness in the laboratory.

Table 1.
The proximate chemical composition of the basal diet (g kg–1, on dry matter basis).
2.3. Diets and Experimental Design
The fish were allotted randomly into four experimental groups (3 replicates per group) (10 fish per replicate): (1) a non-additive control group (CON), (2) basal diet complemented with 0.1 g kg–1 diet of NFLX, (3) fortified basal diet with 1 mL kg–1 diet of SYN, and (4) basal diet complemented with a mixture of NFLX and SYN. Fish were fed twice by hand until satiety at 9 am and 2 pm for eight weeks. The diet was prepared and formulated according to standard requirements for Nile tilapia [22] (Table 1). The ingredients for the meals were mixed, pelletized, dried for 24 h at room temperature, and stored in the refrigerator until use.
2.4. Blood Sampling
At the termination of the experiment (8 weeks of feeding), nine fish/group were collected and anesthetized using 100 mg L–1 benzocaine solution (Al-Nasr pharmaceutical Chemicals Co., Oubour, Qalyubia, Egypt). Blood samples (3 samples per group, 1 mL per fish) were collected from the caudal blood vessels without anticoagulant for serum separation using sterile syringes (22 Gauge-3 CC-1). The blood was coagulated for 15–20 min at 4 °C in the refrigerator before being centrifuged for 15 min at 1107× g. The separated serum was stored at –20 °C until further analysis for biochemical and immunological parameters.
2.5. Nonspecific Immune Parameters
The lysozyme activity was determined colorimetrically using the freeze-dried particles of Micrococcus lysodeikticus, labeled with Remazol brilliant blue R (blue ML) that acts as a substrate as identified originally by Ellis [23]. The release of soluble blue products was determined spectrophotometrically at 600 nm after the labeled substrate was treated with lysozyme. On incubation with hen egg lysozyme at 40 °C, the blue color was effectively released at pH 7 and ionic strength of 0.2. The assay method produced a linear dose-response curve with a minimum of 0.1 µg serum lysozyme (1 g/mL, 100 L) in the sample.
The concentration of nitric oxide (NO) was measured calorimetrically according to Schmidt et al. [24]. The colorimetric stain (azo dye) was prepared and used for 96-well plates, with an absorbance of 540 nm (±20 nm). The total assay time was 15 min, and the serum sample size was 50 µL.
2.6. Serum Biochemical Parameters
The serum total protein and albumin were measured following the methods of Biuret [25] and Reinhold [26], respectively, using a biochemistry kit by spectrophotometry (Systronics, UV–Visible spectrophotometer – 118, IndiaMART, Uttar Pradesh, India) at 540 nm. The serum globulin was calculated by subtracting serum albumin from total serum protein, as mentioned by Coles [27]. A cell-based (quantitative) assay by Trinder [28] was used for colorimetric determination of serum glucose using Abcam diagnostic kits (ab136955), Giza, Egypt. The serum cholesterol levels were determined calorimetrically using Abcam diagnostic kits (ab65390), Giza, Egypt, following Davidson and Nelson [29].
2.7. Antioxidant Activity
The catalase activity (CAT) was determined calorimetrically using Abcam diagnostic kits (ab83464) (Giza, Egypt), according to Aebi’s method [30]. The superoxide dismutase activity (SOD) was measured calorimetrically using Abcam diagnostic kits (ab65354, Giza, Egypt), according to Nishikimi et al. [31]. The content of glutathione peroxidase (GPX) was determined calorimetrically using Abcam diagnostic kits (ab102530, Giza, Egypt) according to Pascual’s method [32].
2.8. Challenge Test
P. aeruginosa was previously isolated from naturally infected fishes at the Department of Fish Diseases and Management, Faculty of Veterinary Medicine, Zagazig University, and confirmed to be pathogenic for Nile tilapia. P. aeruginosa was identified by conventional biochemical tests and the VITEK 2-C15 automated system for bacterial identification (BioMérieux, Marcy-l’Étoile, France) according to the manufacturer’s instructions. At the end of the experiment, all groups were injected intraperitoneally with the pathogenic strain of P. aeruginosa at a dose of 0.1 mL cell suspension containing 3 × 107 cells mL–1 measured using McFarland standard tubes. Fish clinical signs and mortalities were recorded for 15 days.
2.9. Statistical Analysis
The normality of distribution and homogeneity of variances between different treatments were tested using the Kolmogorov–Smirnov test and Bartlett’s test, respectively, and the assumption was achieved (p > 0.05). The data were statistically analyzed by ONE-WAY Analysis of Variance (ANOVA) using SPSS version 24. Duncan’s Multiple Range Test was utilized to assess statistical differences between the groups at a significance level of 0.05 [33]. The data were expressed as mean ± standard error (SE).
3. Results
3.1. Nonspecific Immune Response
Table 2 demonstrates the effect of NFLX and/or SYN supplementation on the proteinogram of Nile tilapia fingerlings. The serum total protein level reduced in NFLX and NFLX + SYN groups compared with the CON group (p < 0.05). The serum globulin level significantly reduced in the NFLX group compared with other groups (p < 0.05). The serum level of albumin was insignificant in all experimental groups (p > 0.05).

Table 2.
Comparison of the proteinogram of Nile tilapia, O. niloticus, fed with diets containing antibiotic (norfloxacin, NFLX) and synbiotic (SYN).
The serum nitric oxide level was enhanced in the SYN group, followed by the NFLX+SYN group, CON, and NFLX groups (p < 0.05). The serum lysozyme activity was enhanced in the SYN group compared with other groups (p < 0.05) (Table 3).

Table 3.
Changes in the serum nitric oxide and lysozyme activities of Nile tilapia, O. niloticus, fed diets containing antibiotic (norfloxacin, NFLX) and synbiotic (SYN).
3.2. Serum Biochemical Parameters
The effect of NFLX and SYN supplementation on blood biochemical parameters is shown in Table 4. The serum glucose level increased in the NFLX group compared with other groups. The total cholesterol and triglyceride levels in the serum increased in NFLX and NFLX+SYN groups (p < 0.05), meanwhile their values were insignificant in the SYN group compared to the CON group.

Table 4.
Changes in serum glucose, total cholesterol, and triglycerides in O. niloticus fed diets supplemented with antibiotic (norfloxacin, NFLX) and synbiotic (SYN).
3.3. Antioxidant Status of Nile tilapia
Significant increases in the serum SOD, CAT, and GSH values were detected in NFLX+SYN and SYN compared to CON and NFLX groups (p < 0.01) (Table 5).

Table 5.
Changes in serum sodium dismutase (SOD), catalase (CAT), and reduced glutathione (GSH) in O. niloticus fed diets supplemented with antibiotic (Norfloxacin NFLX) and synbiotic (SYN).
3.4. Challenge with P. aeruginosa
Figure 1 shows the impact of dietary additions of NFLX and SYN on Nile tilapia resistance to P. aeruginosa challenge in terms of cumulative mortality rate (CMR). The CMR percentage was higher in the SYN group, where the fish were fed an SYN-supplemented diet (3.33%), followed by the NFLX group fed with NFLX-supplemented diet (6.66%), and then the NFLX+SYN group fed with NFLX and SYN in the diet (23.33%). The CMR of the control group was 73.33%.
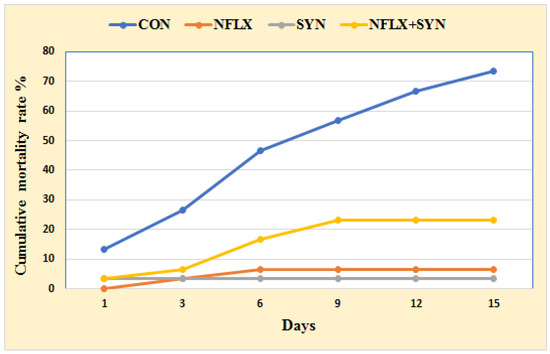
Figure 1.
The cumulative mortality rate (%) of Nile tilapia fed with an antibiotic (norfloxacin NFLX) and synbiotic (SYN) after eight weeks and experimentally challenged with P. aeruginosa.
4. Discussion
The combination of a probiotic and prebiotic is known as a synbiotic, which facilitates the colonization and survival of live microbes (probiotics) in the gut, enhancing the health status of the fish [34]. Our study illustrated the potential improvement in the immunological, biochemical, and antioxidant activities of Nile tilapia by synbiotic addition. In our study, the impact of NFLX and SYN dietary addition on the nonspecific immune response of Nile tilapia was studied. The addition of NFLX decreased the serum total protein and globulin, suggesting that NFLX acts as a stress factor on fish and leads to exhaustion of the liver and kidney during metabolism and excretion. Kori-Siakpere et al. [35] proved that the significant decrease in the serum protein due to antibiotic dietary addition indicates tissue damage caused by its toxicity, stress, and hepatic impairment, resulting in impairment of cell membranes, which permits protein to leak out.
There was an increase in the serum total protein and globulin in the SYN-supplemented diet. This may be due to the presence of protease-enzyme released from aged yeast cells (S. cerevisiae), which improves the digestibility of protein and inhibits intestinal bacteria toxins. In contrast, the cytotoxic effect of bacterial toxins causes severe hepatic and intestinal tissue damage in the control group [36]. According to Opiyo et al. [37], a high serum protein and albumin level correlate with a potent nonspecific immune response, ensuring the positive effect of a synbiotic on Nile tilapia. The current results elucidated that serum nitric oxide increased in the SYN group compared to the NFLX+SYN group. The SYN group was higher in serum lysozyme level than other groups. The hazardous consequence of using antibiotics, particularly NFLX, as a feed additive was detected on the nonspecific immune response, which could be attributed to liver dysfunction. Other studies showed different results and proved that antibiotics suppress fish immunity [38,39].
Many studies recently proved that synbiotics are essential as immune enhancera and, consequently, disease control, especially in Nile tilapia [40,41]. Our data emphasized the vital role of SYN in promoting the nonspecific immune response, especially for NO and lysozyme serum levels. This may be due to the importance of probiotics, whole yeast cell (S. cerevisiae), and yeast cell wall-derived carbohydrates increasing the phagocytic activity, which has a significant role in bacterial defenses and accounts for early activation of the inflammatory response before antibody production [42]. Synbiotic (SYN) also contains mannan-oligosaccharide, which could attach to some Gram-negative bacteria, thereby preventing it from infection, which subsequently increases fish immunity [43]. β-glucan content of SYN can increase lysozyme and nitric oxide production, promoting the immune system of fish, as stated by Adloo et al. [44] and Dawood et al. [45].
Echinacea purpurea (EP) is a globally popular herbal medicine known as an immunostimulant in humans, animals, and fish [46]. In fish, dietary supplementation with EP has been reported to enhance the immune response and resistance against bacterial infection [47,48,49] and cause a significant increase in serum lysozymes and bactericidal activity (SBA) [48], thus leading to stimulation of the fish immune system and improvement in health status. These results were concordant with Aly et al. [49], who showed the immunostimulatory effects of EP supplemented diets on O. niloticus. Furthermore, the diet fortified with EP resulted in the enhancement of serum total protein and globulins, considered as a strong innate-immune response in fishes [48,50]. These results were concordant with Oskoii et al. [47] and Khalil and Elhady [48], who reported that the total serum protein, albumin, and globulin levels were increased in EP-fed fish. The enhancement observed in the immune function of SYN-supplemented groups may be due to the polysaccharides, glycoproteins, caffeic acid derivatives, alkamides, and isobutyl amides present in Echinacea responsible for its immunomodulatory effects [48].
The NFLX group showed an increase in serum glucose, total cholesterol, and triglyceride levels. The SYN addition did not affect these levels compared with the CON group. Shalaby et al. [51] demonstrated a significant rise in serum glucose concentration in Nile tilapia administered with different levels of antibiotics compared with control fish, suggesting that fish obtained more energy to withstand stress conditions since the plasma or serum glucose level is an indicator for nonspecific stress. In addition to that, the high levels of total cholesterol and triglycerides in Nile tilapia were reported after taking different doses of antibiotics. This could be attributed to the chronic exposure of O. niloticus to NFLX, resulting in genotoxicity, which leads to physiological alterations such as energy metabolism alterations of liver tissue and muscles of fish [52,53], leading to an increase in protein and a decrease in lipid contents in O. niloticus.
Similarly, Peres et al. [54] and Gelibolu et al. [55] found no significant increase in glucose levels between groups fed with a diet supplemented with mannan-oligosaccharides and control groups. In addition, Abu-Elala et al. [36] added yeast with mannan-oligosaccharide to the fish diet and Kuhlwein et al. [56] added yeast and β-glucan to the fish diet, which showed no significant increase in serum glucose concentration after eight weeks of feeding. Adloo et al. [44], Pilarski et al. [57], and Sanchez-Martinez et al. [58] found a decrease in serum glucose levels in groups fed a diet complemented with β-glucan, indicating the ability of β-glucan to increase intestinal viscosity resulting in slow glucose absorption from the bloodstream.
Concerning the effect of NFLX and SYN on the antioxidant activity of Nile tilapia, our results showed a significant increase in the serum antioxidant enzyme levels in the NFLX+SYN group, followed by the SYN group compared to the CON and NFLX group. This may be attributed to the β-glucan content of SYN, which may enhance the antioxidant enzyme levels; glutathione peroxidase (GPX), catalase (CAT), and superoxide dismutase (SOD) compared to the control groups [45]. Many studies supported our data, such as Dalmo and Bøgwald [59], Li et al. [60], Lin et al. [61], and Adloo et al. [44]. Furthermore, Guzmán-Villanueva et al. [62] explained that the enhancement of antioxidant activity might be because β-glucan increases the circulating neutrophils in blood and stimulates phagocytic cells related to the reactivation of oxygen species. NFLX can also stimulate the suppression of cytochrome P450 monooxygenase activity in fish, as reported by Vaccaro et al. [63], thereby prompting the activity of some phase II enzymes, such as glutathione S-transferases (GST), which play a vital role in anti-oxidation through combination with glutathione (GSH). GST enzymes and GSH content activity are used to evaluate the efficiency of a drug’s metabolism, as discussed by Li et al. [64]. However, this does not prevent the harmful effect on the liver and digestive system, explaining the decrease in antioxidant enzymes in the NFLX group.
Pseudomonas septicemia is considered a critical fish disease, causing high mortality rates and economic losses among freshwater fishes [65]. The challenge test is used as an ultimate assay to assess the fish immune response [66,67,68,69,70,71]. The results displayed an increase in the survivability of fish fed a SYN diet following challenge with P. aeruginosa, indicating improvement of the immunity of fish fed a diet complemented with synbiotic and a competitive efficiency against pathogenic bacteria [40,41] as previously proven by the enhancement of several immunological markers. The highest survival rate after P. aeruginosa infection was observed in the SYN group due to the positive effect of S. cerevisiae, mannan-oligosaccharide, and β-glucan on the immune response, which resulted in increased bacterial resistance as recorded by Abu-Elala et al. [36] and Okey et al. [43] and the effectiveness of the antimicrobial agent against the pathogen.
5. Conclusions
The present results showed the beneficial effect of synbiotics (Saccharomyces cerevisiae, mannan oligosaccharides, and β-glucan) in enhancing the immune resistance of fish against P. aeruginosa infection. Synbiotics can be used as an alternative to antibiotics to improve blood biochemical parameters and antioxidant activity. The results provided data for a synbiotic application as a useful feed additive in the Nile tilapia aquaculture industry.
Author Contributions
Conceptualization: G.E.-N., M.H., A.A.K., A.Y.M., S.A.A., M.M.M., and M.E.E.-s.; Methodology: G.E.-N., M.H., A.A.K., A.Y.M., S.A.A., M.M.M., and M.E.E.-s.; Software and data curation: A.Y.M.; Writing—original draft preparation: G.E.-N., M.H., A.A.K., A.Y.M., S.A.A.; Writing—reviewing and editing: A.A.K. and S.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out using the facilities and materials in Taif University Researches Supporting Project number (TURSP-2020/139), Taif University, Taif, Saudi Arabia.
Institutional Review Board Statement
The experiment was carried out according to the guidelines of the care and use of animals for scientific purposes research committee, and ethical approval was obtained from Zagazig University (ZU-IACUC/3/F/170/2019).
Acknowledgments
This work was carried out using the facilities and materials in Taif University Researches Supporting Project number (TURSP-2020/139), Taif University, Taif, Saudi Arabia. The authors extend their appreciation to the Faculty of Veterinary Medicine, Zagazig University, Egypt.
Conflicts of Interest
The authors declared that they have no conflict of interest.
References
- Saikia, D.J.; Chattopadhyay, P.; Banerjee, G.; Sarma, D. Time and Dose-Dependent Effect of Pseudomonas aeruginosa Infection on the Scales of Channapunctata (Bloch) Through Light and Electron Microscopy. Turk. J. Fish. Aquat. Sci. 2017, 17, 871–876. [Google Scholar] [CrossRef]
- El-Barbary, M.I.; Hal, A.M. Isolation and molecular characterization of some bacterial pathogens in El-Serw fish farm, Egypt. Egypt. J. Aquat. Biol. Fish. 2016, 20, 115–122, ISSN 1110–6131. [Google Scholar] [CrossRef]
- Hanna, M.I.; El-Hady, M.A.; Ahmed, H.A.; Elmeabawy, S.A.; Kenwy, A.M. A contribution to Pseudomonas aeruginosa infection in African Catfish (Clarias gariepinus). Res. J. Pharm. Biol. Chememicl Sci. 2014, 5, 575–588. [Google Scholar]
- Kholil, I.; Hossain, M.M.; Neowajh, S.; Islam, S.; Kabir, M. Comparative efficiency of some commercial antibiotics against Pseudomonas infection in fish. Int. J. Fish. Aquat. Stud. 2015, 2, 114–117. [Google Scholar] [CrossRef]
- Susmita, D.; Mondal, K.; Haque, S. A review on application of probiotic, prebiotic, and synbiotic for sustainable development of aquaculture. J. Entomol. Zool. Stud. 2017, 5, 422–429. [Google Scholar]
- Rasul, M.G.; Majumdar, B.C. Abuse of Antibiotics in Aquaculture and its Effects on Human, Aquatic Animal, and Environment. Saudi J. Life Sci. 2017, 10, 21276. [Google Scholar]
- Papich, M.G.; Riviere, J.E. Fluoroquinolones Antimicrobial Drugs, 8th ed.; Adams, H.R., Ed.; Iowa State University Press: Ames, IA, USA, 2001; pp. 898–917. [Google Scholar]
- Holmes, B.; Brogden, R.N.; Richards, D.M. Norfloxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 1985, 30, 482–513. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S.; Abdel-Daim, M.M.; Doan, H.V. Probiotic application for sustainable aquaculture. Rev. Aquac. 2018, 907–924. [Google Scholar] [CrossRef]
- Iswarya, A.; Vaseeharan, B.; Anjugam, M.; Gobi, N.; Divya, M.; Faggio, C. β-1, 3 glucan binding protein-based selenium nanowire enhances the immune status of Cyprinus carpio and protection against Aeromonas hydrophila infection. Fish Shellfish Immunol. 2018, 83, 61–75. [Google Scholar] [CrossRef]
- Wang, W.; Ishikawa, M.; Koshio, S.; Yokoyama, S.; Hossain, M.S.; Moss, A.S. Effects of dietary astaxanthin and vitamin E and their interactions on the growth performance, pigmentation, digestive enzyme activity of kuruma shrimp (Marsupenaeus japonicus). Aquat. Res. 2019, 50, 1186–1197. [Google Scholar] [CrossRef]
- Zhang, C.N.; Li, X.F.; Xu, W.N.; Jiang, G.Z.; Lu, K.L.; Wang, L.N.; Liu, W.B. Combined effects of dietary fructooligosaccharide and Bacillus licheniformis on innate immunity, antioxidant capability, and disease resistance of triangular bream Megalobrama terminalis. Fish Shellfish Immunol. 2013, 35, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- De, B.C.; Meena, D.K.; Behera, B.K.; Das, P.; das Mohapatra, P.K.; Sharma, A.P. Probiotics in fish and shellfish culture: Immunomodulatory and ecophysiological responses. Fish Physiol. Biochem. J. 2014, 40, 921–971. [Google Scholar] [CrossRef]
- Putra, A.N.; Utomo, N.B.P. Growth performance of tilapia Oreochromis niloticus fed with probiotic, prebiotic, and synbiotic in the diet. Pak. J. Nutr. 2015, 14, 263–268. [Google Scholar] [CrossRef][Green Version]
- Kumar, P.; Jain, K.K.; Sardar, P.; Jayant, M.; Tok, C.N. Effect of dietary on growth performance, body composition, digestive enzyme activity, and gut microbiota in Cirrhinus mrigala (Ham.) fingerling. Aquac. Nutr. 2017, 24, 921–929. [Google Scholar] [CrossRef]
- Putra, A.N.; Romdhonah, Y. Effects of dietary Bacillus NP5 and sweet potato extract on growth and digestive enzyme activity of dumbo catfish Clarias sp., University of Sultan Ageng Tirtayasa, Indonesia. J. Akuakultur Indones. 2018, 18, 80–88. [Google Scholar] [CrossRef]
- Maftei, N.M. Probiotic, Prebiotic, and Synbiotic Products in Human Health; Faculty of Medicine Pharmacy, University of Galati: Galati, Romania, 2019. [Google Scholar] [CrossRef]
- El-Sayed, A.F.M. Tilapia Culture, 2nd ed.; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Austin, B.; Austin, D.A. Aeromonadaceae representatives (motile aeromonads). In Bacterial Fish Pathogens; Springer: Dordrecht, The Netherlands, 2012; pp. 119–146. ISBN 978-94-007-4884-2. [Google Scholar]
- CCAC. Canadian Council on Animal Care Guidelines on: The Care and Use of Fish in Research, Teaching and Testing; Canadian Council on Animal Care: Ottawa, ON, Canada, 2005. [Google Scholar]
- Water Environment Federation; American Public Health Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1998; ISBN 0-87553-235-7. [Google Scholar]
- NRC. Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Ellis, A.E. Lysozyme Assays. Tech. Fish Immunol. 1990, 1, 101–103. [Google Scholar]
- Schmidt, H.H.; Seifert, R.; Bohme, E. Formation and release of nitric oxide from human neutrophils and HL-60 cells induced by a chemotactic peptide, platelet-activating factor, and leukotriene B4. FEBS Lett. 1989, 244, 357–360. [Google Scholar] [CrossRef]
- Henry, R.J. Colorimetric Determination of Total Protein, Clinical Chemistry; Harper and Row Publishers: New York, NY, USA, 1964; p. 181. [Google Scholar]
- Reinhold, R.R. Determination of serum albumin. Clin. Chem. 1953, 21, 1370–1372. [Google Scholar]
- Coles, E.H. Veterinary Clinical Pathology; W.B. Saunders Co.: Philadelphia, PA, USA, 1986; pp. 10–42. [Google Scholar]
- Trinder, P. Determination of blood glucose using 4-amino phenazone as oxygen acceptor. J. Clin. Pathol. 1969, 22, 246. [Google Scholar] [CrossRef] [PubMed]
- Davidson, I.; Nelson, D.A. The Blood: In Clinical Diagnosis—By Laboratory Methods; Davidsohn, I., Henry, J.B., Eds.; W.B. Saunders Co.: Philadelphia, PA, USA, 1977; pp. 100–310. [Google Scholar]
- Aebi, H. Colorimetric method for determination of catalase. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Nishikimi, M.; Roa, N.A.; Yogi, K. Superoxide Dismutase serum detection. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Pascual, P. Direct assay of glutathione peroxidase activity using high-performance capillary electrophoresis. J. Chromatogr. 1992, 581, 49–55. [Google Scholar] [CrossRef]
- SAS Institute, Inc. The Statical Analysis System for Windows; Version 24.0; IBM Corp: Armonk, NY, USA, 2016. [Google Scholar]
- Daniels, C.L.; Merrifield, D.L.; Boothroyd, D.P.; Davies, S.J.; Factor, J.R.; Arnold, K.E. Effect of dietary Bacillus spp. and mannan oligosaccharides (MOS) on European lobster Homarus gammarus (Linn. larvae growth performance, gut morphology, and gut microbiota. Aquaculture 2010, 304, 49–57. [Google Scholar] [CrossRef]
- Kori-Siakpere, O.; Bemigho, I.R.; Gbemi, O.M. Variations in alanine aminotransferase and aspartate aminotransferase activities in African catfish: Clarias gariepinus (Burchell, 1822) at different sublethal concentrations of potassium permanganate. Sci. Res. Essays 2010, 5, 1501–1505. [Google Scholar]
- Abu-Elala, N.; Marzouk, M.; Moustafa, M. Use of different S. cerevisiae biotic forms as immune-modulator and growth promoter for Oreochromis niloticus challenged with some fish pathogens. Int. J. Vet. Sci. Med. Diagn. 2013, 1, 21–29. [Google Scholar] [CrossRef]
- Opiyo, M.A.; Jumbe, J.; Ngugi, C.C.; Charo-Karisa, H. Dietary administration of probiotics modulates nonspecific immunity and gut microbiota of Nile tilapia (Oreochromis niloticus) cultured in low input ponds. Int. J. Vet. Sci. Med. Diagn. 2019, 7, 1–9. [Google Scholar] [CrossRef]
- He, S.; Zhou, Z.; Meng, K.; Zhao, H.; Yao, B.; Ringø, E.; Yoon, I. Effects of dietary antibiotic growth promoter and Saccharomyces cerevisiae fermentation product on production, intestinal bacterial community, and nonspecific immunity of hybrid tilapia (Oreochromis niloticus female, Oreochromis aureus male). J. Anim. Sci. 2011, 89, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, F.A.; Cerezuela, R.; Meseguer, J.; Esteban, M.A. Modulation of the immune parameters and expression of genes of gilthead seabream (Sparus aurata L.) by dietary administration of oxytetracycline. Aquaculture 2012, 334–337, 51–57. [Google Scholar] [CrossRef]
- Sewaka, M.; Trullas, C.; Chotiko, A.; Rodkhum, C.; Chansue, N.; Boonanuntanasarn, S.; Pirarat, N. Efficacy of synbiotic Jerusalem artichoke and Lactobacillus rhamnosus GG supplemented diets on growth performance, serum biochemical parameters, intestinal morphology, immune parameters, and protection against Aeromonas veronii in juvenile red tilapia (Oreochromis spp.). Fish Shellfish Immunol. 2019, 86, 260–268. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Eweedah, N.M.; Moustafa, E.M.; Shahin, M.G. Synbiotic effects of Aspergillus oryzae and β-Glucan on growth and oxidative and immune responses of Nile Tilapia, Oreochromis niloticus. Probiotics Antimicrob. Proteins 2020, 12, 172–183. [Google Scholar] [CrossRef]
- Gharekhani, A.; Azari, T.G.; Tukmechi, A.; Afsharnasab, M.; Agh, N. Effect of dietary supplementation with zinc enriched yeast (Saccharomyces cerevisiae) on the immunity of rainbow trout (Oncorhynchus mykiss). Iran. J. Vet. Res. 2015, 16, 278–282. [Google Scholar]
- Okey, I.B.; Gabriel, U.U.; Deekae, S.N. The Use of Synbiotics (Prebiotic and Probiotic) in Aquaculture Development. Sumer. J. Biotechnol. 2018, 1, 51–60. [Google Scholar]
- Adloo, M.N.; Soltanian, S.; Hafezieh, M.; Ghadimi, N. Effects of long term dietary administration of β-Glucan on the growth, survival, and some blood parameters of striped catfish, Pangasianodon hypophthalmus (Siluriformes: Pangasiidae). Iran. J. Ichthyol. 2015, 2, 194–200. [Google Scholar]
- Dawood, M.A.O.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; El Basuini, M.F.; Hossain, M.S.; Nhu, T.H.; Moss, A.S.; Dossou, S.; Wei, H. Dietary supplementation of β-glucan improves growth performance, the innate immune response, and stress resistance of red sea bream, Pagrus major. Aquac. Nutr. 2017, 23, 148–159. [Google Scholar] [CrossRef]
- Tang, X.L.; Fu, J.H.; Li, Z.H.; Fang, W.P.; Yang, J.Y.; Zou, J.X. Effects of a dietary administration of purple coneflower (Echinacea purpurea) on growth, antioxidant activities and 8 mRNAs expressions in crucian carp (Carassius auratus). Aquac. Res. 2016, 47, 1631–1638. [Google Scholar] [CrossRef]
- Oskoii, S.B.; Kohyani, A.T.; Parseh, A.; Salati, A.P.; Sadeghi, E. Effects of dietary administration of Echinacea purpurea on growth indices and biochemical and hematological indices in rainbow trout (Oncorhynchus mykiss) fingerlings. Fish Physiol. Biochem. 2012, 38, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.A.; ElHady, M. Effects of Echinacea purpurea and vitamin c on the health status, immune response and resistance of Oreochromis niloticus to Aeromonas sobria infection. Abbassa Int. J. Aquac. 2015, 8, 253–267. [Google Scholar]
- Aly, S.M.; Mohamed, M.F.; John, G. Echinacea as Immunostimulatory Agent in Nile Tilapia (Oreochromis niloticus) via Earthen Pond Experiment. In Proceedings of the 8th International Symposium on Tilapia in Aquaculture, Cairo, Egypt, 12–14 October 2008; pp. 1033–1041. [Google Scholar]
- Reverter, M.; Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: Current status and future perspectives. Aquaculture 2014, 433, 50–61. [Google Scholar] [CrossRef]
- Shalaby, A.M.; Khattab, Y.M.; Abdel Rahman, A.M. Effects of garlic (Allium sativum) and chloramphenicol on growth performance, physiological parameters, and survival of Nile Tilapia (Oreochromis niloticus). J. Venom. Anim. Toxins Incl. Trop. Dis. 2006, 12, 172–201. [Google Scholar] [CrossRef]
- Yang, C.; Song, G.; Lim, W. A review of the toxicity in fish exposed to antibiotics. Comp. Biochem. Physiol. 2020, 237, 108840. [Google Scholar] [CrossRef]
- El-Nobi, G.; Hassanin, M.; Khalil, A.A.; Mohammed, A.Y. Comparative efficiency of the dietary addition of synbiotic “curazol-M” and norfloxacin on the growth performance, body composition, and histological alteration of the Nile tilapia. Egypt. J. Aquatic Biol. Fish. 2021, 25, 371–582. [Google Scholar]
- Peres, H.; Santos, S.; Oliva-Teles, A. Selected plasma biochemistry parameters in gilthead seabream (Sparus aurata) juveniles. J. Appl. Ichthyol. 2013, 29, 630–636. [Google Scholar] [CrossRef]
- Gelibolu, S.; Yanar, Y.; Genc, M.A.; Genc, E. The Effect of Mannan-Oligosaccharide (MOS) as a Feed Supplement on Growth and Some Blood Parameters of Gilthead Sea Bream (Sparus aurata). Turk. J. Fish. Aquat. Sci. 2017, 18, 817–823. [Google Scholar] [CrossRef]
- Kuhlwein, H.; Merrifield, D.L.; Rawling, M.D.; Foey, A.D.; Davies, S.J. Effects of dietary β-(1,3)(1,6)-D-glucan supplementation on growth performance, intestinal morphology, and haemato-immunological profile of mirror carp (Cyprinus carpio L.). J. Anim. Physiol. Anim. Nutr. 2013, 98, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Pilarski, F.; Oliveira, C.A.F.; Souza, F.P.B.D.; Zanuzzo, F.S. Different β-glucans improve the growth performance and bacterial resistance in Nile tilapia. Fish Shellfish Immunol. 2017, 70, 25–29. [Google Scholar] [CrossRef]
- Sánchez-Martínez, J.G.; Rábago-Castro, J.L.; Vázquez-Sauceda, M.L.; Pérez-Castañeda, R.; Blanco-Martínez, Z.; Benavides-González, F. Effect of β-glucan dietary levels on immune response and hematology of channel catfish Ictalurus punctatus juveniles. Lat. Am. J. Aquat. Res. 2017, 45, 690–698. [Google Scholar] [CrossRef]
- Dalmo, R.A.; Bøgwald, J. β-glucans as conductors of immune symphonies. Fish Shellfish Immunol. 2008, 25, 384–396. [Google Scholar] [CrossRef]
- Li, P.; Wen, Q.; Gatlin, D.M. Dose-dependent influences of dietary β-1, 3-glucan on innate immunity and disease resistance of hybrid striped bass Morone chrysops × Morone saxatilis. Aquac. Res. 2009, 40, 1578–1584. [Google Scholar] [CrossRef]
- Lin, S.; Pan, Y.; Luo, L.; Luo, L. Effects of dietary β-1, 3-glucan, chitosan or raffinose on the growth, innate immunity, and resistance of koi (Cyprinus carpio koi). Fish Shellfish Immunol. 2011, 31, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Villanueva, L.T.; Tovar-Ramírez, D.; Gisbert, E.; Cordero, H.; Guardiola, F.A.; Cuesta, A.; Meseguer, J.; Ascencio-Valle, F.; Esteban, M.A. Dietary administration of β-1, 3/1, 6-glucan and probiotic strain Shewanella putrefaciens, single or combined, on gilthead seabream growth, immune responses and gene expression. Fish Shellfish Immunol. 2014, 39, 34–41. [Google Scholar] [CrossRef]
- Vaccaro, E.; Giorgi, M.; Longo, V.; Mengozzi, G.; Gervasi, P.G. Inhibition of cytochrome P450 enzymes by enrofloxacin in the sea bass. Aquat. Toxicol. 2003, 62, 27–33. [Google Scholar] [CrossRef]
- Li, S.W.; Wang, D.; Liu, H.B.; Lu, T.Y. Effects of norfloxacin on the drug metabolism enzymes of two sturgeon species (Acipenser schrencki and Acipenser ruthenus). J. Appl. Ichthyol. 2013, 29, 1204–1207. [Google Scholar] [CrossRef]
- Abdel-Tawab, A.A.; Maarouf, A.A.; Ahmed, N.M.G. Detection of virulence factors of Pseudomonas species isolated from freshwater fish by PCR. Benha Vet. Med. J. 2016, 30, 199–207. [Google Scholar] [CrossRef]
- Kollner, B.G.; Kotterba, U. Evaluation of immune functions of rainbow trout, how can environmental influences be detected? Toxicological. Letter. 2002, 131, 83–95. [Google Scholar] [CrossRef][Green Version]
- Amer, S.A.; Ahmed, S.A.; Ibrahim, R.E.; Al-Gabri, N.A.; Osman, A.; Sitohy, M. Impact of partial substitution of fish meal by methylated soy protein isolates on the nutritional, immunological, and health aspects of Nile tilapia, Oreochromis niloticus fingerlings. Aquaculture 2020, 518, 734871. [Google Scholar] [CrossRef]
- Ibrahim, R.E.; Ahmed, S.A.; Amer, S.A.; Al-Gabri, N.A.; Ahmed, A.I.; Abdel-Warith, A.-W.A.; Younis, E.-S.M.; Metwally, A.E. Influence of vitamin C feed supplementation on the growth, antioxidant activity, immune status, tissue histomorphology, and disease resistance in Nile tilapia, Oreochromis niloticus. Aquac. Rep. 2020, 18, 100545. [Google Scholar] [CrossRef]
- Ibrahim, R.E.; Amer, S.A.; Farroh, K.Y.; Al-Gabri, N.A.; Ahmed, A.I.; El-Araby, D.A.; Ahmed, S.A. The effects of chitosan-vitamin C nanocomposite supplementation on the growth performance, antioxidant status, immune response, and disease resistance of Nile tilapia (Oreochromis niloticus) fingerlings. Aquaculture 2020, 534, 736269. [Google Scholar] [CrossRef]
- Al-Khalaifah, H.; Khalil, A.A.; Amer, S.A.; Shalaby, S.I.; Badr, H.A.; Farag, M.F.; Altohamy, D.E.; Abdel Rahman, A.N. Effects of Dietary Doum Palm Fruit Powder on Growth, Antioxidant Capacity, Immune Response, and Disease Resistance of African Catfish, Clarias gariepinus (B.). Animals 2020, 10, 1407. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.A.; Osman, A.; Al-Gabri, N.A.; Elsayed, S.A.; El-Rahman, A.; Ghada, I.; Elabbasy, M.T.; Ahmed, S.A.; Ibrahim, R.E. The Effect of Dietary Replacement of Fish Meal with Whey Protein Concentrate on the Growth Performance, Fish Health, and Immune Status of Nile Tilapia Fingerlings, Oreochromis niloticus. Animals 2019, 9, 1003. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).