Citrate Mediated Europium-Based Detection of Oxytetracycline in Citrus Tissues
Abstract
1. Introduction
2. Results
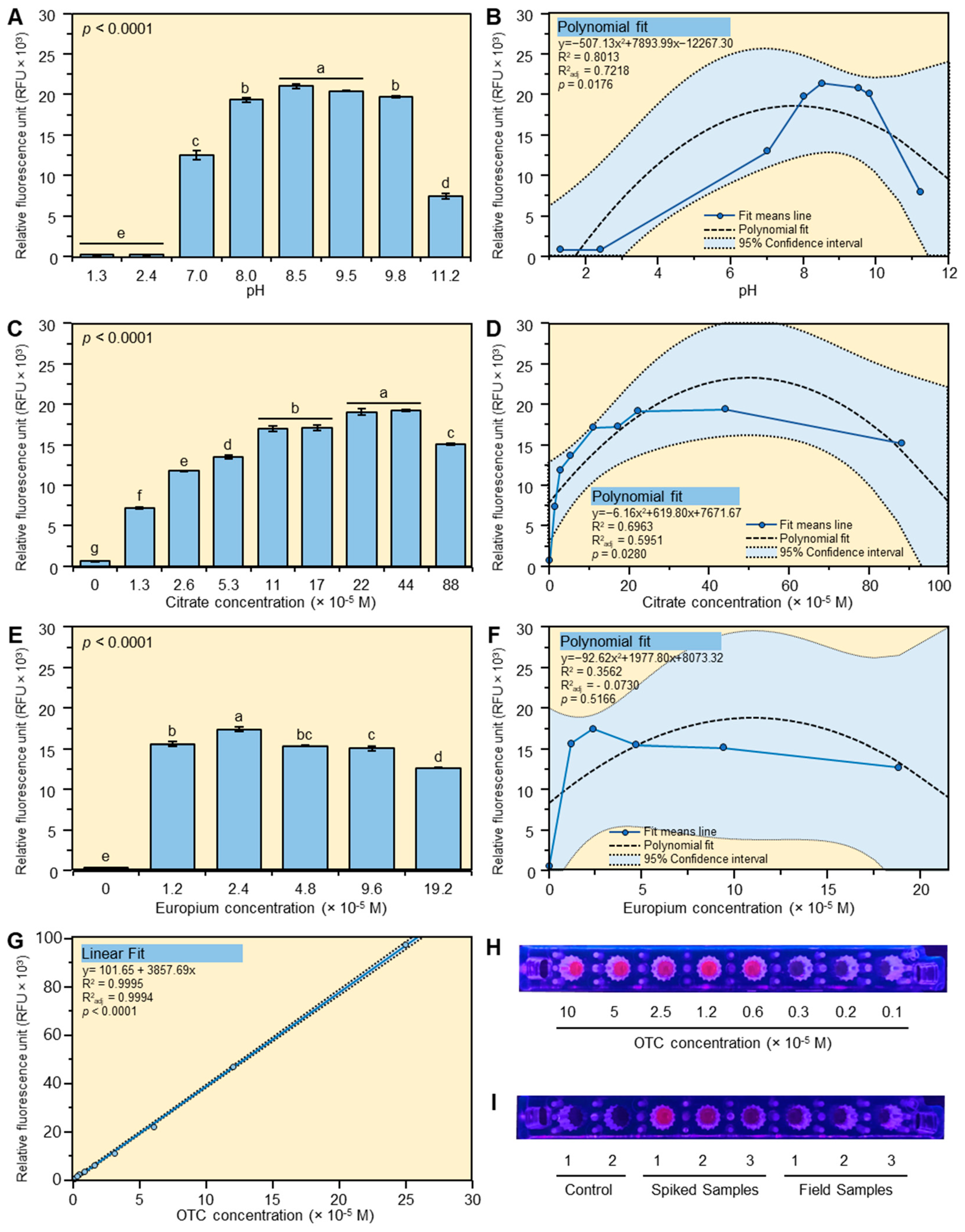
2.1. Optimization of the Europium Citrate Method
2.1.1. Effect of pH
2.1.2. Effect of Citrate
2.1.3. Effect of Europium
2.1.4. Linear Range for OTC
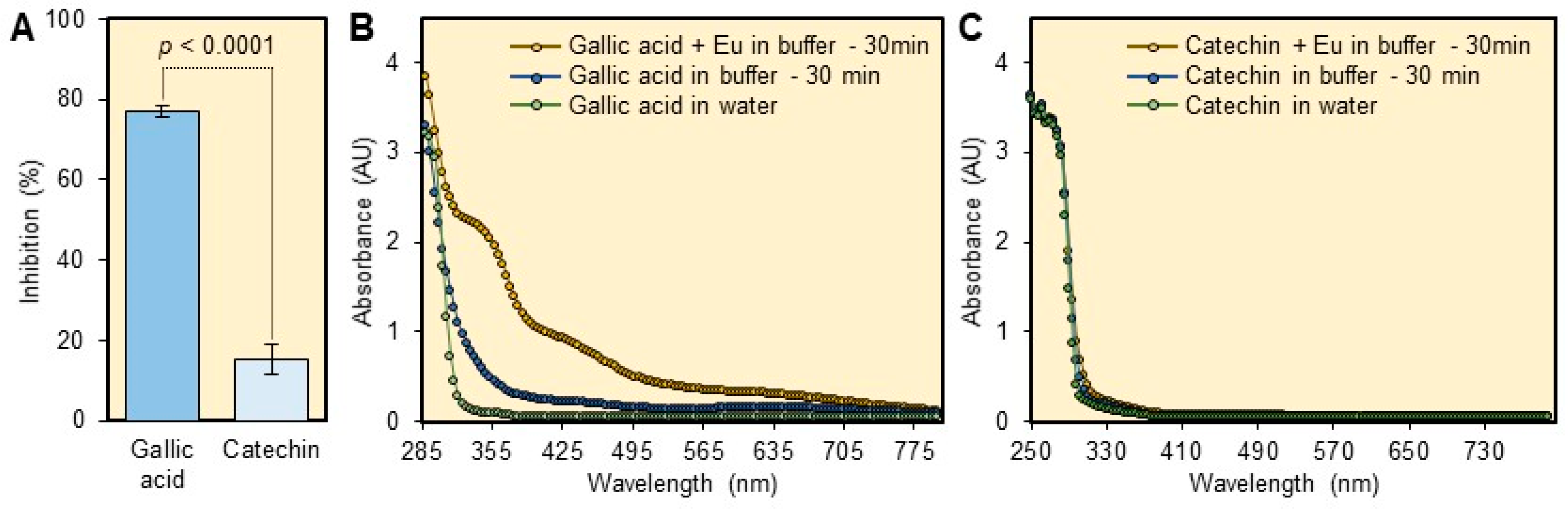
2.2. Interference of Plant Matrix
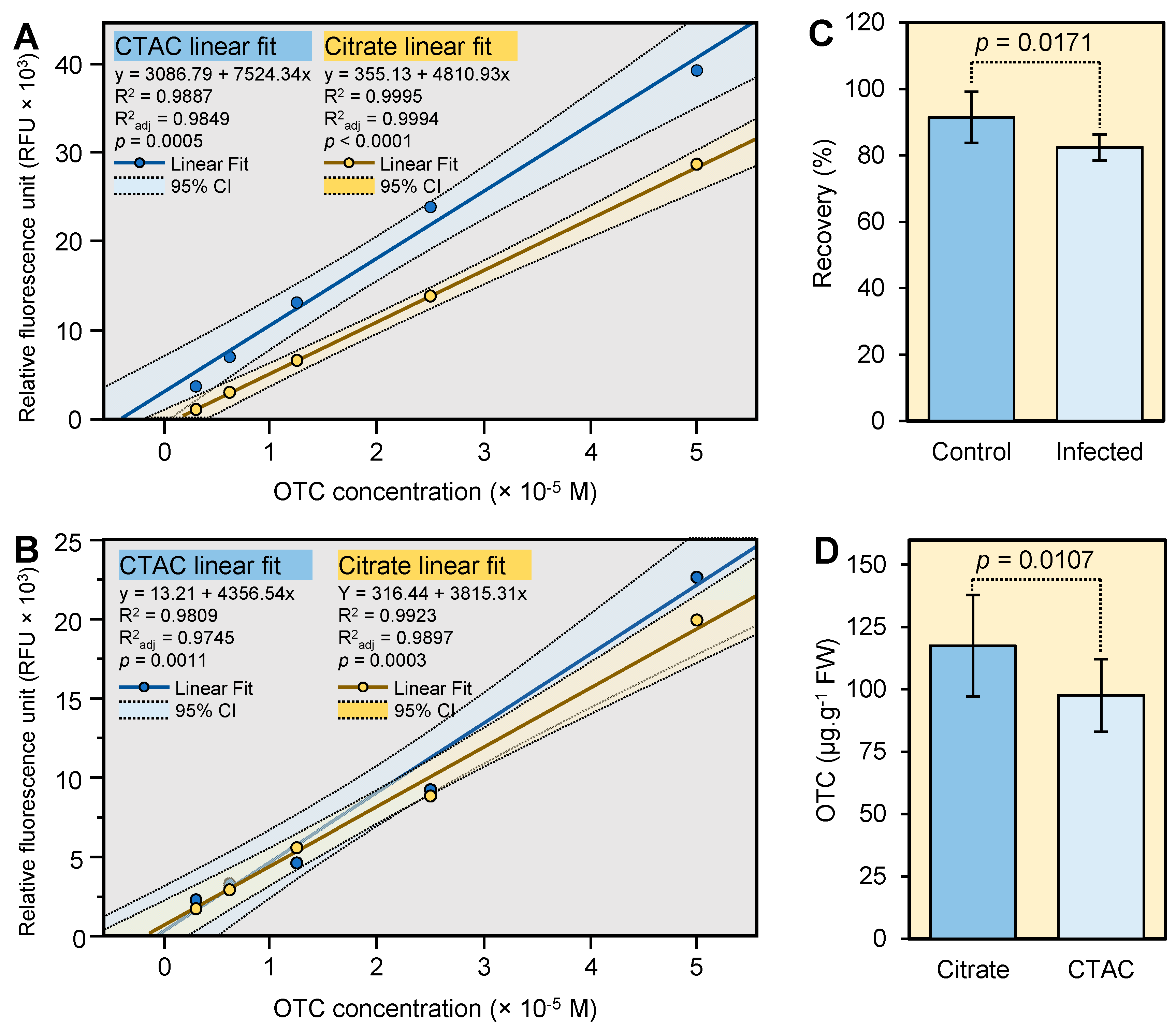
2.3. OTC Recovery from Spiked Citrus Leaves
2.4. Application of the New Method to Field’s Samples
3. Discussion
3.1. Optimization of the Method
3.2. Interference of Plant Matrix
3.3. Recovery of OTC from Spiked Leaves
3.4. Comparison between the Citrate and CTAC Method and Application of These Method to Field’s Samples
4. Material and Methods
4.1. Optimization of the Method
4.1.1. Effect of pH
4.1.2. Effect of Citrate
4.1.3. Effect of Europium
4.1.4. Effect of OTC
4.2. Inhibition of Fluorescence by Plant Matrix
4.3. Extraction of OTC from Spiked Leaves
4.4. Solid-Phase Extraction (SPE)
4.5. Fluorescence Assay
4.6. Application of the New Method to Field Samples
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yegorova, A.; Vityukova, E.; Beltyukova, S.; Duerkop, A. Determination of citrate in tablets and of oxytetracycline in serum using europium (III) luminescence. Microchem. J. 2006, 83, 1–6. [Google Scholar] [CrossRef]
- McManus, P.S.; Stockwell, V.O.; Sundin, G.W.; Jones, A.L. Antibiotic use in Plant Agriculture. Annu. Rev. Phytopathol. 2002, 40, 443–465. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Pierson, E.A.; Setubal, J.C.; Xu, J.; Levy, J.G.; Zhang, Y.; Li, J.; Rangel, L.T.; Martins, J. The Candidatus Liberibac-ter–Host Interface: Insights into Pathogenesis Mechanisms and Disease Control. Annu. Rev. Phytopathol. 2017, 55, 451–482. [Google Scholar] [CrossRef]
- Blaustein, R.A.; Lorca, G.L.; Teplitski, M. Challenges for Managing Candidatus Liberibacter spp. (Huanglongbing Disease Pathogen): Current Control Measures and Future Directions. Phytopathology 2018, 108, 424–435. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, C.; Powell, C.A. Application of antibiotics for control of citrus huanglongbing. Adv. Antibiot. Antibodies 2015, 1, e101. [Google Scholar]
- Hu, J.; Wang, N. Evaluation of the Spatiotemporal Dynamics of Oxytetracycline and Its Control Effect Against Citrus Huanglongbing via Trunk Injection. Phytopathology 2016, 106, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Al-Rimawi, F.; Hijaz, F.; Nehela, Y.; Batuman, O.; Killiny, N. Uptake, Translocation, and Stability of Oxytetracycline and Streptomycin in Citrus Plants. Antibiotics 2019, 8, 196. [Google Scholar] [CrossRef] [PubMed]
- Hijaz, F.; Nehela, Y.; Al-Rimawi, F.; Vincent, C.I.; Killiny, N. The Role of the Xylem in Oxytetracycline Translocation within Citrus Trees. Antibiotics 2020, 9, 691. [Google Scholar] [CrossRef] [PubMed]
- Killiny, N.; Hijaz, F.; Gonzalez-Blanco, P.; Jones, S.E.; Pierre, M.O.; Vincent, C.I. Effect of Adjuvants on Oxytetracycline Uptake upon Foliar Application in Citrus. Antibiotics 2020, 9, 677. [Google Scholar] [CrossRef] [PubMed]
- Hijaz, F.; Nehela, Y.; Gonzalez-Blanco, P.; Killiny, N. Development of Europium-Sensitized Fluorescence-Based Method for Sensitive Detection of Oxytetracycline in Citrus Tissues. Antibiotics 2021, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Jee, R.D. Study of micellar solutions to enhance the europium-sensitized luminescence of tetracyclines. Analyst 1995, 120, 2867–2872. [Google Scholar] [CrossRef]
- Chen, G.; Schneider, M.J.; Darwish, A.M.; Lehotay, S.J.; Freeman, D.W. Europium-sensitized luminescence determination of oxytetracycline in catfish muscle. Talanta 2004, 64, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, N.; Georges, J. Sensitive detection of tetracyclines using europium-sensitized fluorescence with EDTA as co-ligand and cetyltrimethylammonium chloride as surfactant. Analyst 2001, 126, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wu, M.; Schäferling, M.; Wolfbeis, O.S. Fluorescent Imaging of Citrate and Other Intermediates in the Citric Acid Cycle. Angew. Chem. Int. Ed. 2004, 43, 1735–1738. [Google Scholar] [CrossRef] [PubMed]
- Swapna Priya, S.; Radha, K.V. Brief review of spectrophotometric methods for the detection of tetracycline antibiotics. Int. J. Pharm. Pharm. Sci. 2014, 6, 48–51. [Google Scholar]
- Friedman, M.; Jürgens, H.S. Effect of pH on the Stability of Plant Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Chen, H.; Zhang, Y.; Chang, Y. Photodegradation of gallic acid under UV irradiation: Insights regarding the pH effect on direct photolysis and the ROS oxidation-sensitized process of DOM. Chemosphere 2014, 99, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Hijaz, F.; Al-Rimawi, F.; Manthey, J.A.; Killiny, N. Phenolics, flavonoids and antioxidant capacities in Citrus species with different degree of tolerance to Huanglongbing. Plant. Signal. Behav. 2020, 15, 1752447. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hijaz, F.; Nehela, Y.; Batuman, O.; Killiny, N. Citrate Mediated Europium-Based Detection of Oxytetracycline in Citrus Tissues. Antibiotics 2021, 10, 566. https://doi.org/10.3390/antibiotics10050566
Hijaz F, Nehela Y, Batuman O, Killiny N. Citrate Mediated Europium-Based Detection of Oxytetracycline in Citrus Tissues. Antibiotics. 2021; 10(5):566. https://doi.org/10.3390/antibiotics10050566
Chicago/Turabian StyleHijaz, Faraj, Yasser Nehela, Ozgur Batuman, and Nabil Killiny. 2021. "Citrate Mediated Europium-Based Detection of Oxytetracycline in Citrus Tissues" Antibiotics 10, no. 5: 566. https://doi.org/10.3390/antibiotics10050566
APA StyleHijaz, F., Nehela, Y., Batuman, O., & Killiny, N. (2021). Citrate Mediated Europium-Based Detection of Oxytetracycline in Citrus Tissues. Antibiotics, 10(5), 566. https://doi.org/10.3390/antibiotics10050566








