Involvement of a Multidrug Efflux Pump and Alterations in Cell Surface Structure in the Synergistic Antifungal Activity of Nagilactone E and Anethole against Budding Yeast Saccharomyces cerevisiae
Abstract
1. Introduction
2. Results
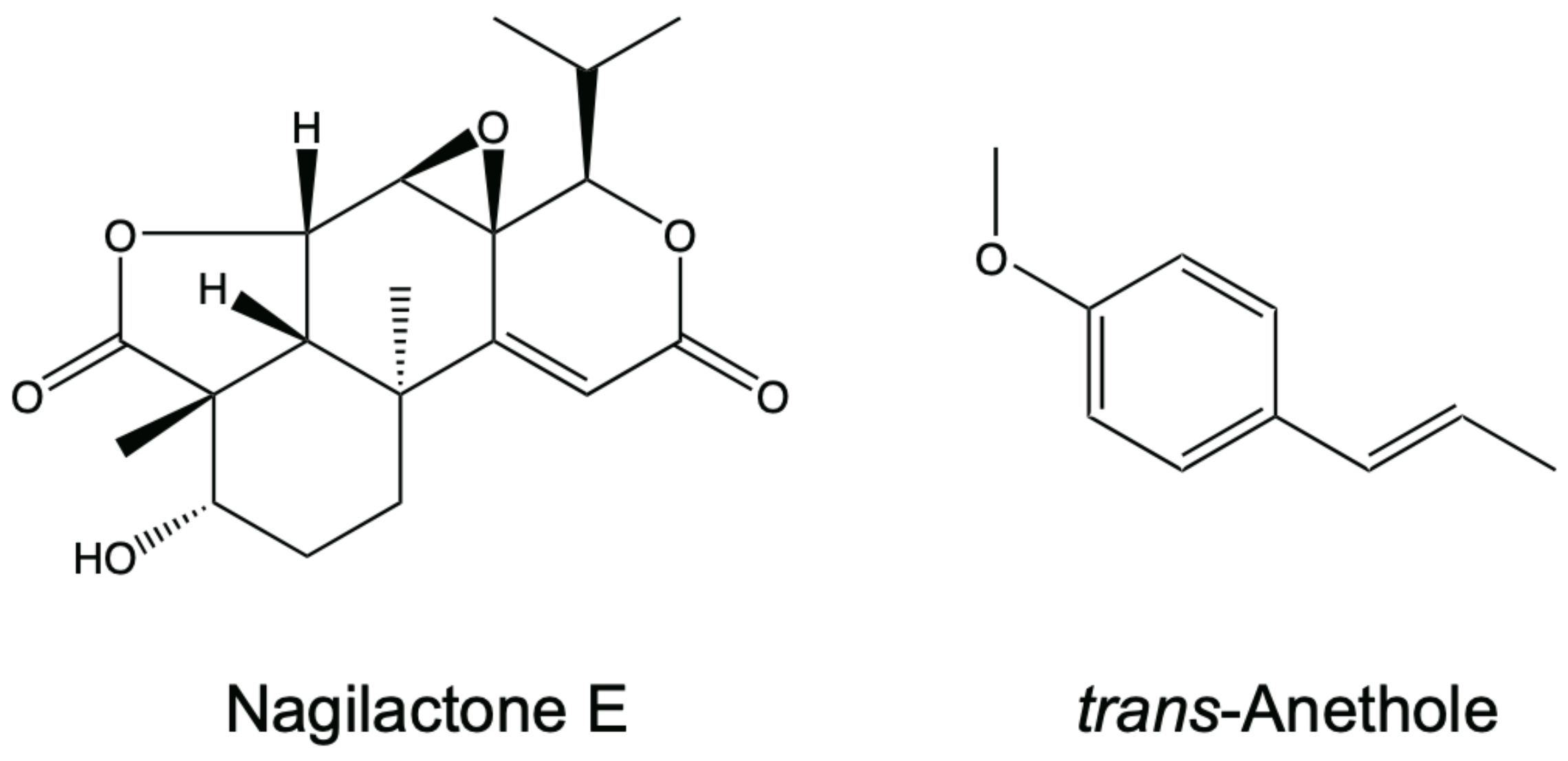
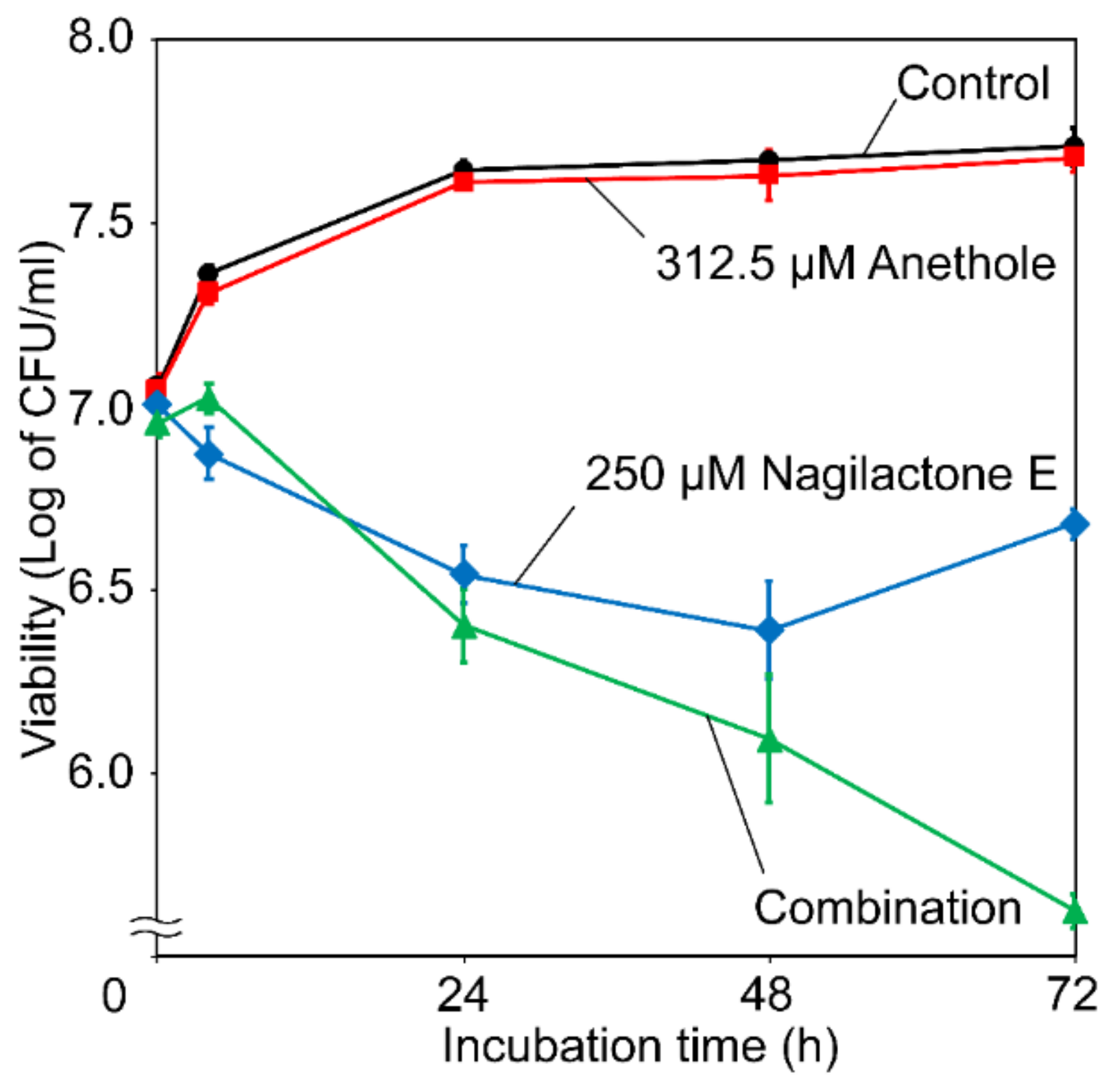
2.1. Antifungal Activity of Nagilactone E, Aenethole, and Their Combination
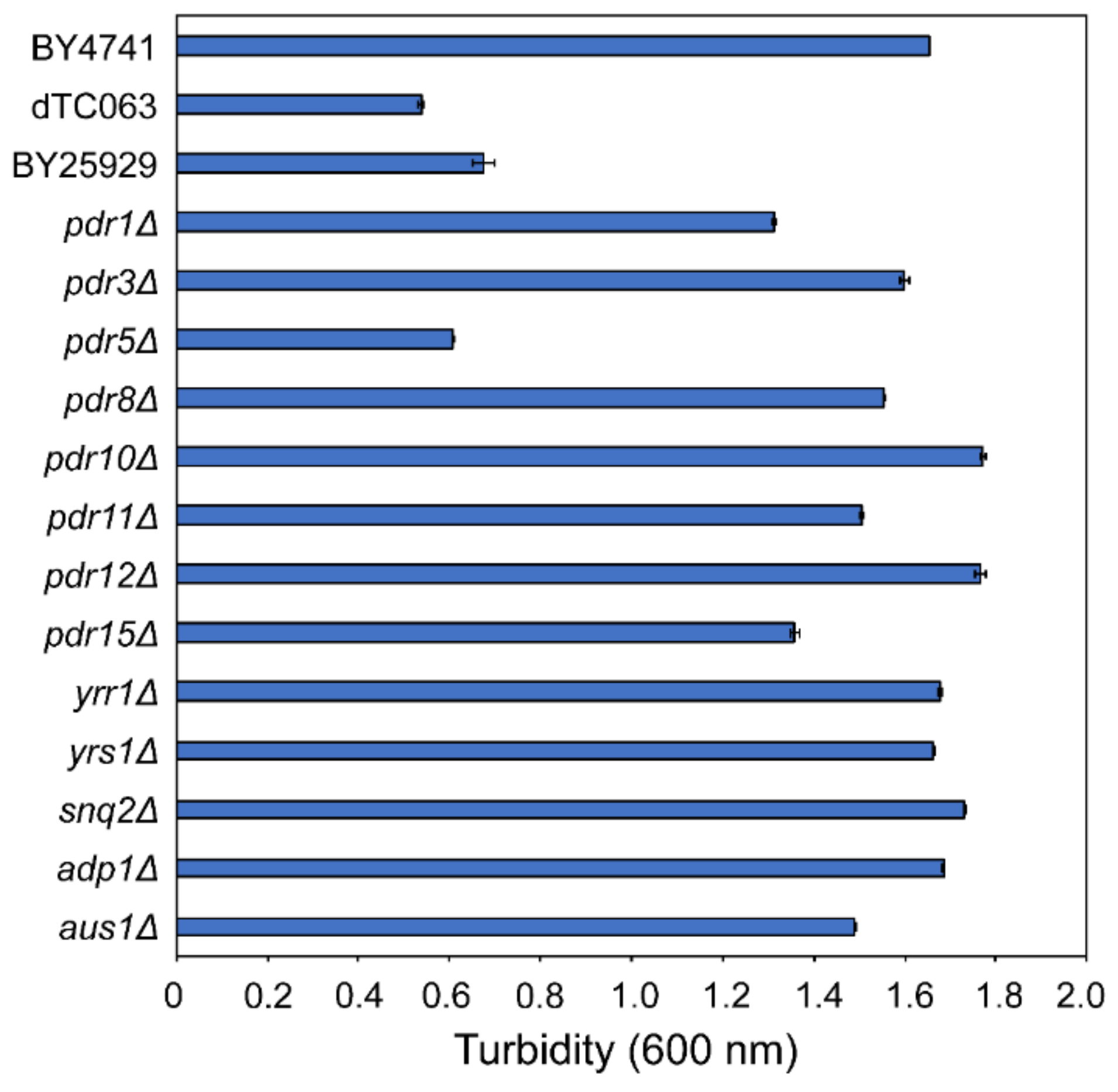
2.2. Identification of Genes Associated with Nagilactone-Efflux Using Gene-Deficient Strains
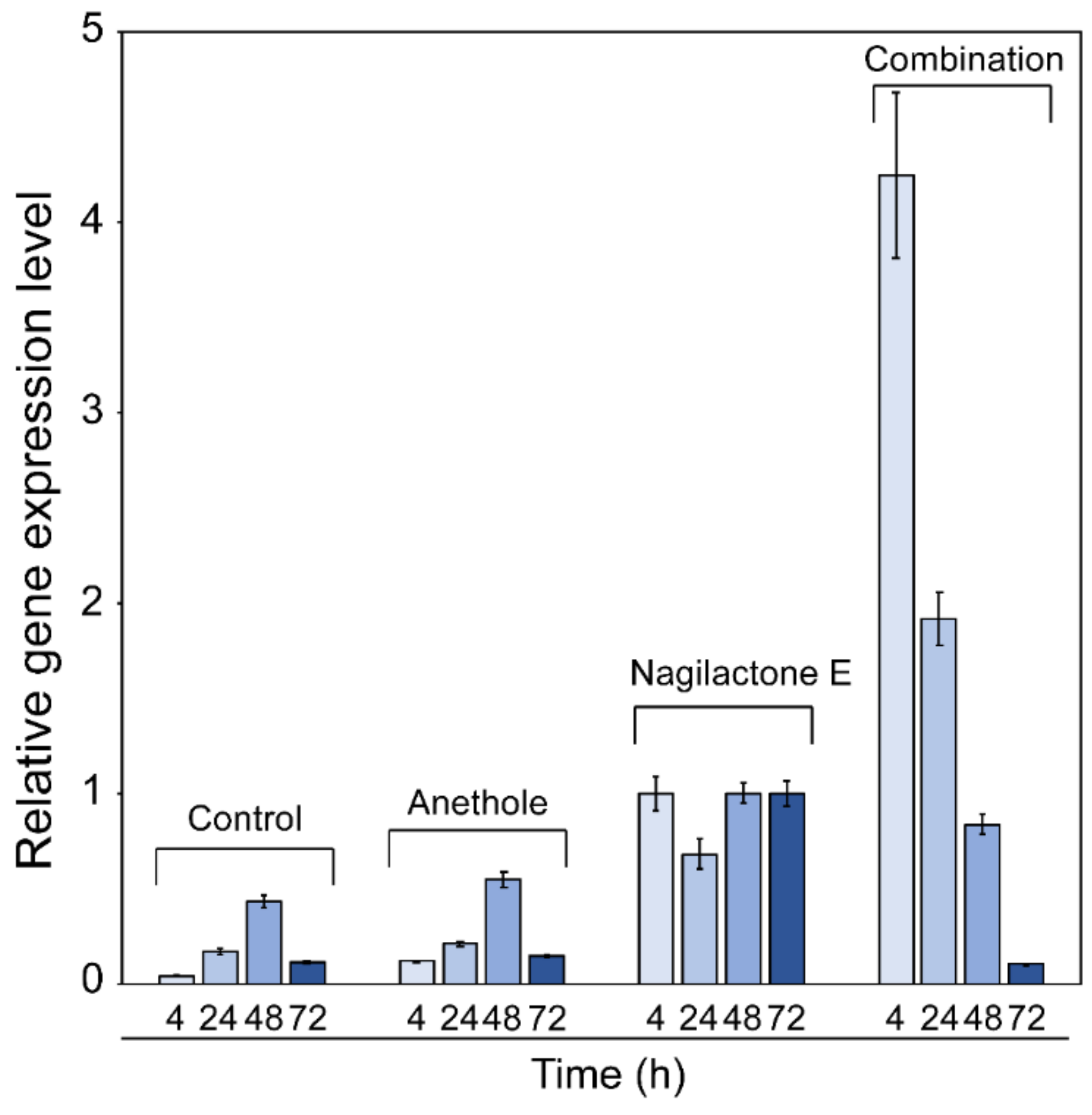
2.3. Effects of Anethole, Nagilactone E, and Their Combination on PDR5 Transcription
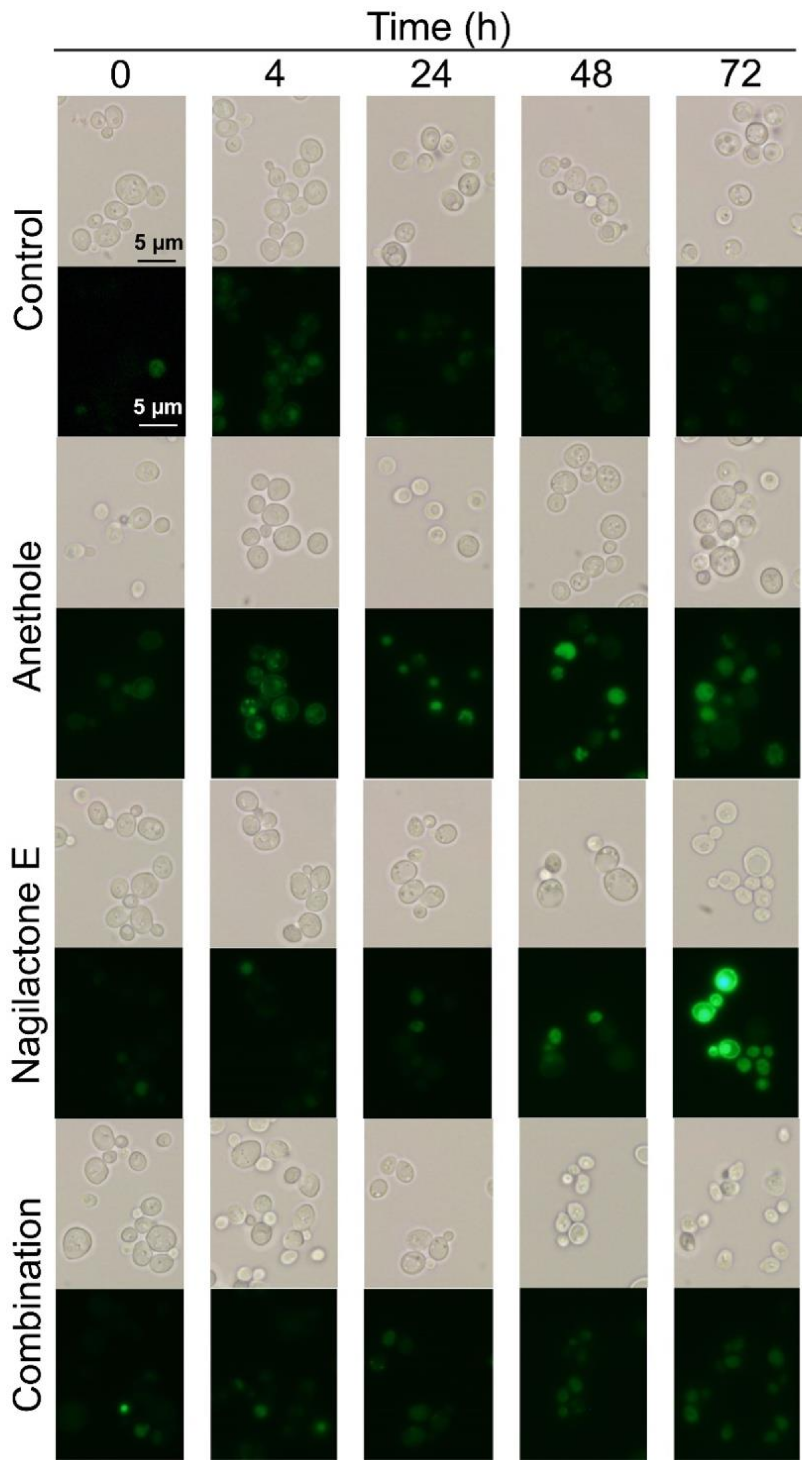
2.4. Visualization of Green Fluorescent Protein (GFP)-Tagged Pdr5p
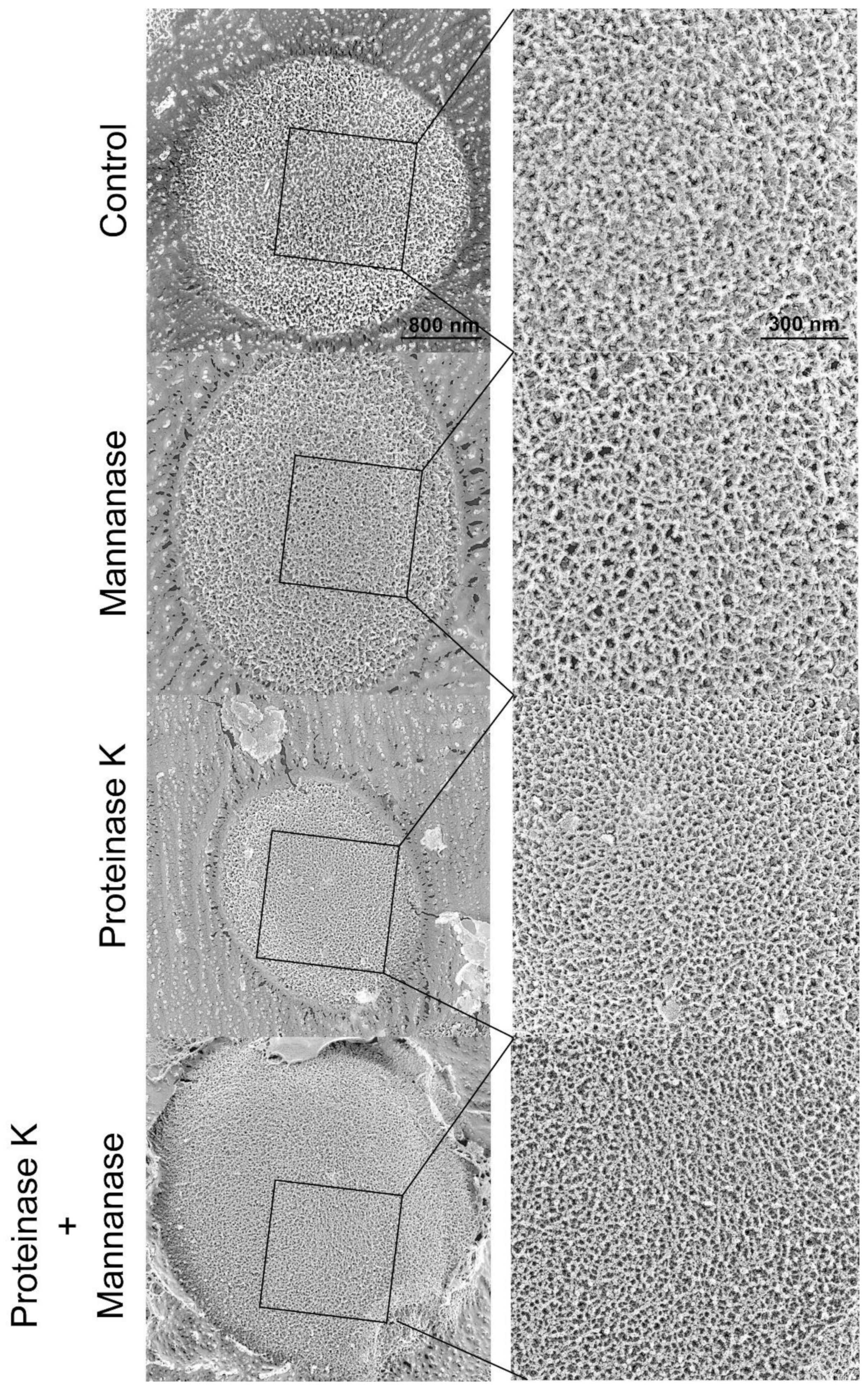
2.5. Visualization of β-Glucan and Mannan on the Cell Surface
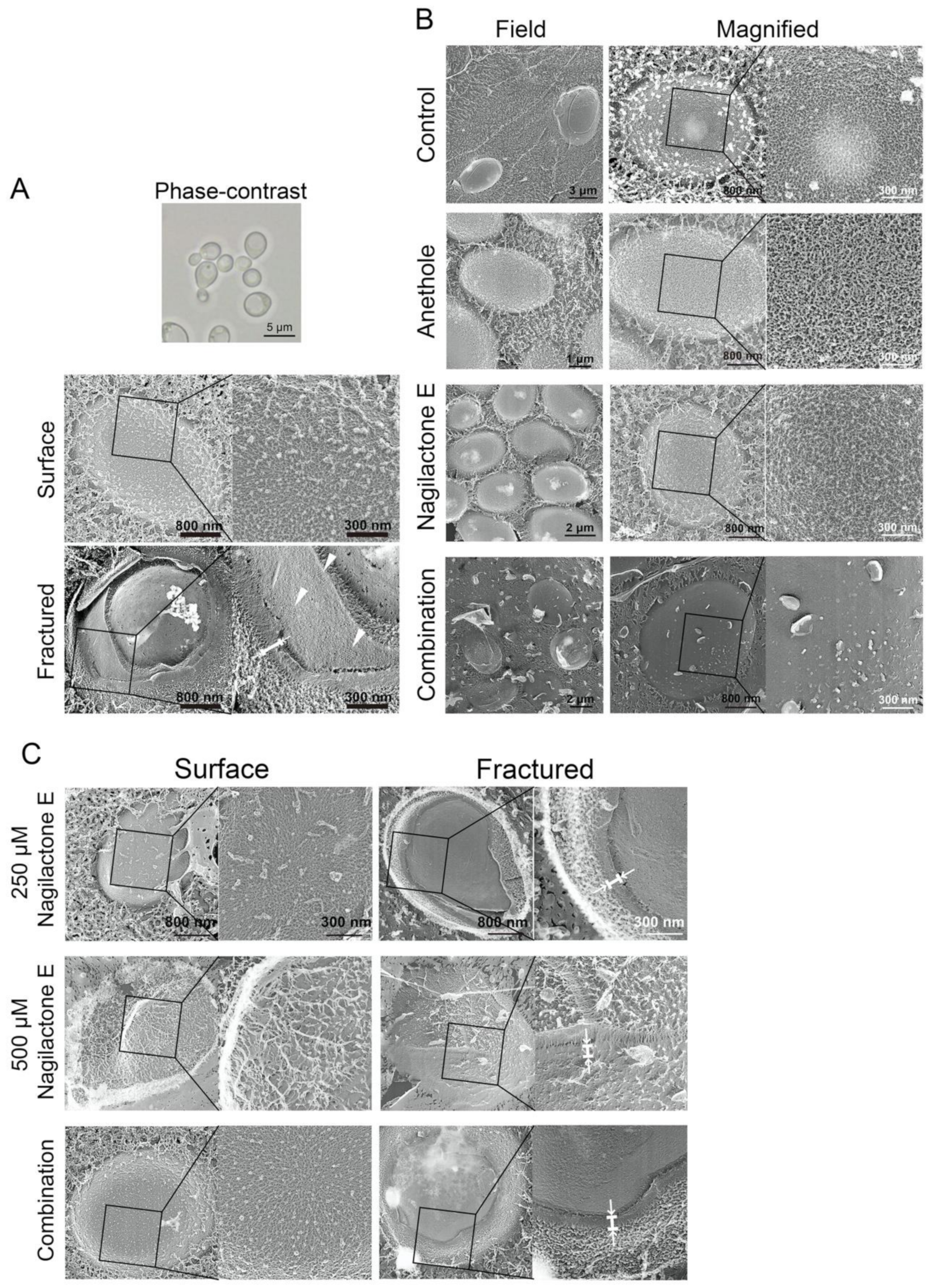
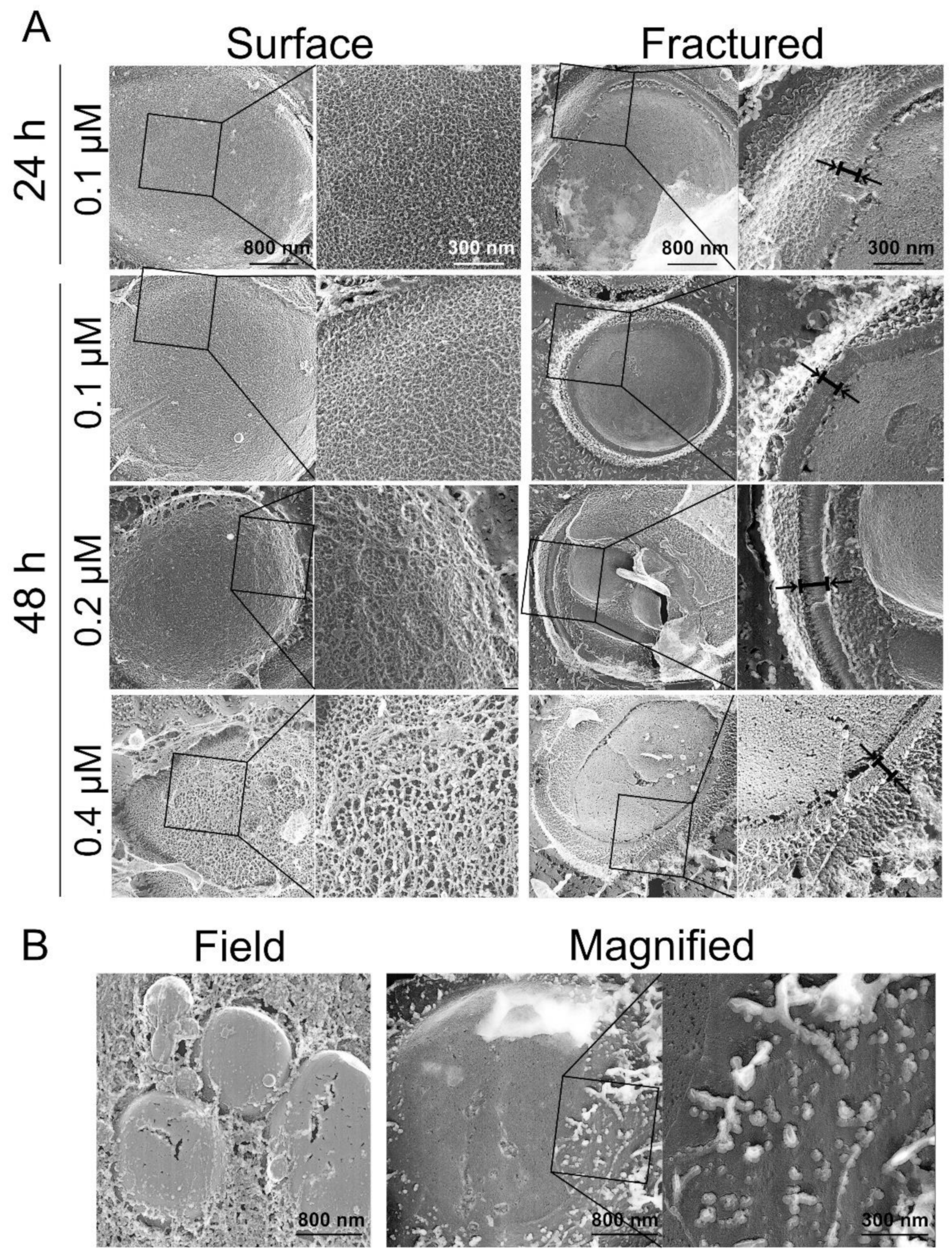
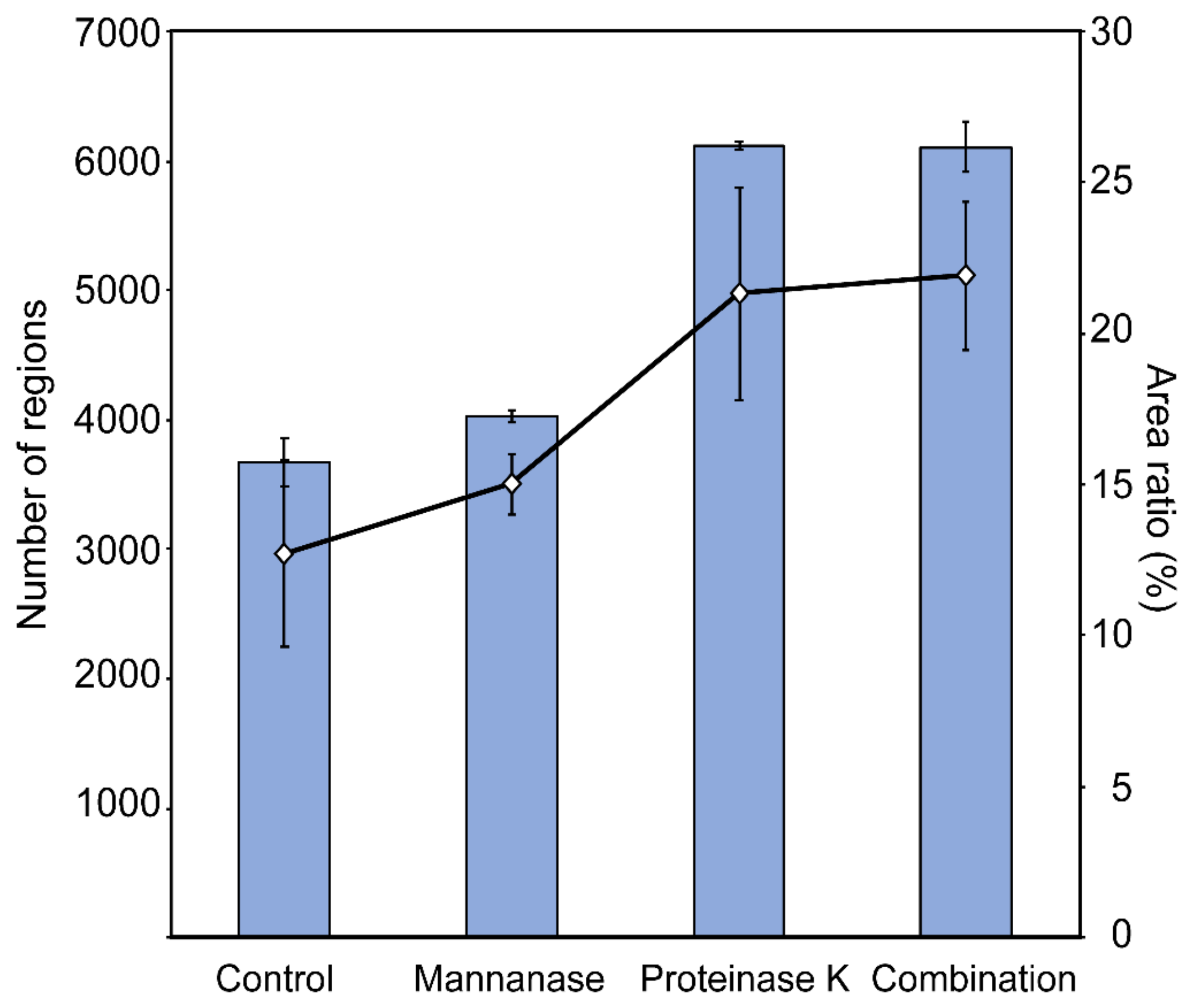
2.6. Cell Surface Structure and Thickness of Cell Wall
3. Discussion
4. Materials and Methods
4.1. Strains and Culture Conditions
4.2. Chemicals
4.3. Antifungal Susceptibility Assay
4.4. Time-Kill Assay
4.5. Measurement of Cell Turbidity in Gene Deletion Strains Related to Drug Efflux
4.6. RNA Extraction and RT-qPCR
4.7. Visualization of GFP-Pdr5p Fusion Protein
4.8. Aniline Blue Staining
4.9. Creating Spheroplasts
4.10. Visualization of the Cell Wall Using FITC-ConA
4.11. Quick-Freeze Deep-Etch Replica Electron Microscopy
4.12. Image Analyses for Cell Surface Damage
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borgers, M.; Degreef, H.; Cauwenbergh, G. Fungal infections of the skin: Infection process and antimycotic therapy. Curr. Drug Targets 2005, 6, 849–862. [Google Scholar] [CrossRef]
- Hayashi, Y.; Sakan, T. Nagilactones, plant growth regulators with antiauxin-like activity, plant growth substances. In Proceedings of the 8th International Conference on Plant Growth Substances, Tokyo, Japan, 26 August–1 September 1974; pp. 525–532. [Google Scholar]
- Kubo, I.; Matsumoto, T.; Klocke, J.A. Multichemical resistance of the conifer Podocarpus gracilior (Podocarpaceae) to insect attack. J. Chem. Ecol. 1984, 10, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Russell, G.B.; Fenemore, P.G.; Singh, P. Insect-control chemicals from plants. Nagilactone C, A toxic substance from the leaves of Podocarpus nivalis and P. hallii. Aust. J. Biol. Sci. 1972, 25, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, K.; Banskota, A.H.; Kodata, S.; Shrivastava, S.P.; Strobel, G.; Gewali, M.B. An antiprolifelative norditerpene dilactone, nagilactone C, from Podocarpus nerifolius. Phytomedicine 2001, 8, 489–491. [Google Scholar] [CrossRef]
- Hayashi, Y.; Matsumoto, T.; Tashiro, T. Antitumor activity of norditerpenoid dilactones in Podocarpus plants: Structure-activity relationship on in vitro cytotoxicity against Yoshida sarcoma. Gan 1979, 70, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Sutisna, M.; Tan, K.S. Effects of nagilactones on the growth of lettuce seedlings. Phytochemistry 1991, 30, 455–456. [Google Scholar] [CrossRef]
- Kubo, I.; Himejima, M. Potentiation of antifungal activity of sesquiterpene dialdehydes against Candida albicans and two other fungi. Experientia 1992, 48, 1162–1164. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Yamaguchi, Y.; Ogita, A.; Tanaka, T.; Kubo, I.; Fujita, K. Effect of nagilactoneE on cell morphology and glucan biosynthesis in budding yeast Saccharomyces cerevisiae. Fitoterapia 2018, 128, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Frost, D.J.; Brandt, K.D.; Cugier, D.; Goldman, R. A whole-cell Candida albicans assay for the detection of inhibitors towards fungal cell wall synthesis and assembly. J. Antibiot. 1995, 48, 306–310. [Google Scholar] [CrossRef]
- Hitokoto, H.; Morozumi, S.; Wauke, T.; Sakai, S.; Kurata, H. Inhibitory effects of spices on growth and toxin production of toxigenic fungi. Appl. Environ. Microbiol. 1980, 39, 818–822. [Google Scholar] [CrossRef]
- Kosalec, I.; Pepeljnjak, S.; Kuštrak, D. Antifungal activity of fluid extract and essential oil from anise fruits (Pimpinella anisum L., Apiaceae). Acta Pharm. 2005, 55, 377–385. [Google Scholar]
- Yutani, M.; Hashimoto, Y.; Ogita, A.; Kubo, I.; Tanaka, T.; Fujita, K.-I. Morphological changes of the filamentous fungus Mucor mucedo and inhibition of chitin synthase activity induced by anethole. Phytother. Res. 2011, 25, 1707–1713. [Google Scholar] [CrossRef]
- Fujita, K.-I.; Tatsumi, M.; Ogita, A. Anethole induces apoptotic cell death accompanied by reactive oxygen species production and DNA fragmentation in Aspergillus fumigatus and Saccharomyces cerevisiae. FEBS J. 2014, 281, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.-I.; Fujita, T.; Kubo, I. Anethole, a potential antimicrobial synergist, converts a fungistatic dodecanol to a fungicidal agent. Phytother. Res. 2007, 21, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.; Kubo, I. Antimicrobial agents from Tanacetum balsamita. J. Nat. Prod. 1995, 58, 1565–1569. [Google Scholar] [CrossRef]
- Fujita, K.-I.; Ishikura, T.; Jono, Y. Anethole potentiates dodecanol’s fungicidal activity by reducing PDR5 expression in budding yeast. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Oyama, M.; Tamaki, H.; Yamaguchi, Y.; Ogita, A.; Tanaka, T.; Fujita, K.-I. Deletion of the Golgi Ca2+-ATPase PMR1 gene potentiates antifungal effects of dodecanol that depend on intracellular Ca2+ accumulation in budding yeast. FEMS Yeast Res. 2020, 20. [Google Scholar] [CrossRef]
- Mahé, Y.; Lemoine, Y.; Kuchler, K. The ATP binding cassette transporters Pdr5 and Snq2 of Saccharomyces cerevisiae can mediate transport of steroids in vivo. J. Biol. Chem. 1998, 271, 25167–25172. [Google Scholar] [CrossRef] [PubMed]
- Katzmann, D.J.; Burnett, P.E.; Golin, J.; Mahé, Y.; Moye-Rowley, W.S. Transcriptional control of the yeast PDR5 gene by the PDR3 gene product. Mol. Cel. Biol. 1994, 14, 4653–4661. [Google Scholar] [CrossRef] [PubMed]
- Lipke, P.N.; Ovalle, R. Cell wall architecture in yeast: New structure and new challenges. J. Bacteriol. 1998, 180, 3735–3740. [Google Scholar] [CrossRef]
- Klis, F.M.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2002, 26, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Beyer, A.; Hollunder, J.; Nasheuer, H.P.; Wilhelm, T. Post-transcriptional expression regulation in the yeast Saccharomyces cerevisiae on a genomic scale. Mol. Cel. Proteom. 2004, 3, 1083–1092. [Google Scholar] [CrossRef]
- GoncËalves, E.; Nakic, R.Z.; Zampieri, M.; Wagih, O.; Ochoa, D.; Sauer, U.; Beltrao, P.; Saez-Rodriguez, J. Systematic analysis of transcriptional and post-transcriptional regulation of metabolism in yeast. PLoS Comput. Biol. 2017, 13, e1005297. [Google Scholar] [CrossRef]
- Hong, K.A.; Lee, S.H.; Gu, S.; Kim, E.; An, S.; Kwon, J.; Lee, J.-B.; Jang, S.K. The bent conformation of poly(A)-binding protein induced by RNA-binding is required for its translational activation function. RNA Biol. 2017, 14, 370–377. [Google Scholar] [CrossRef]
- Preiss, T.; Muckenthaler, M.; Hentze, M.W. Poly-(A)-tail-promoted translation in yeast: Implications for translational control. RNA 1998, 4, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Tarun, S.Z., Jr.; Sachs, A.B. Association of the yeast poly(A) tail binding protein with translation initiation factor eIF-4G. EMBO J. 1996, 15, 7168–7177. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Uscanga, B.; François, J.M. A study of the yeast cell wall composition and structure in response to growth conditions and mode of cultivation. Lett. Appl. Microbiol. 2003, 37, 268–274. [Google Scholar] [CrossRef]
- Koehler, J.K.; Birnbaum, W., Jr.; Hayes, T.L. Electron microscope observations on Saccharomyces cerevisiae. Cytologia 1960, 26, 301–308. [Google Scholar] [CrossRef][Green Version]
- Osumi, M. Visualization of yeast cells by electron microscopy. J. Electron. Microsc. 2012, 61, 343–365. [Google Scholar] [CrossRef] [PubMed]
- Smits, G.J.; Kapteyn, J.C.; Van Den Ende, H.; Klis, F.M. Cell wall dynamics in yeast. Curr. Opin. Microbiol. 1999, 2, 348–352. [Google Scholar] [CrossRef]
- Klis, F.M.; Sosinska, G.J.; Groot, P.W.J.; Brul, S. Covalently linked cell wall proteins of Candida albicans and their role in fitness and virulence. FEMS Yeast Res. 2009, 9, 1013–1028. [Google Scholar] [CrossRef] [PubMed]
- Tahara, O.Y.; Miyata, M.; Nakamura, T. Quick-freeze, deep-etch electron microscopy reveals the characteristic architecture of the fission yeast spore. J. Fungi 2021, 7, 7. [Google Scholar] [CrossRef]
- Pérez, P.; Cortés, C.G.J.; Cansado, J.; Ribas, C.J. Fission yeast cell wall biosynthesis and cell integrity signalling. Cell Surf. 2018, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ene, I.V.; Walker, L.A.; Schiavone, M.; Lee, K.K.; Martin-Yken, H.; Dague, E.; Gow, N.A.R.; Munro, C.A.; Brown, A.J.P. Cell wall remodeling enzymes modulate fungal cell wall elasticity and osmotic stress resistance. mBio 2015, 6, e00986-15. [Google Scholar] [CrossRef]
- Gonzalez, M.; Lipke, P.N.; Ovalle, R. GPI proteins in biogenesis and structure of yeast cell walls. Enzymes 2009, XXVI, 321–356. [Google Scholar] [CrossRef]
- Denning, D.W. Echinocandin antifungal drugs. Lancet 2003, 362, 1142–1151. [Google Scholar] [CrossRef]
- Kang, T.H.; Hwang, E.I.; Yun, B.S.; Park, K.D.; Kwon, B.M.; Shin, C.S.; Kim, S.U. Inhibition of chitin synthases and antifungal activities by 2′-benzoyloxycinnamaldehyde from Pleuropterus ciliinervis and its derivatives. Biol. Pharm. Bull. 2007, 30, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.-I.; Kubo, I. Antifungal activity of octyl gallate. Int. J. Food Microbiol. 2002, 79, 193–201. [Google Scholar] [CrossRef]
- Tulum, I.; Tahara, Y.O.; Miyata, M. Peptidoglycan layer and disruption processes in Bacillus subtilis cells visualized using quick-freeze, deep-etch electron microscopy. Microscopy 2019, 68, 441–449. [Google Scholar] [CrossRef] [PubMed]
| Strain | MIC a (μM) at 72 h | FIC b Index | ||
|---|---|---|---|---|
| Nagilactone E | Anethole | |||
| S. cerevisiae BY4741 | Alone | 500 | - | - |
| - | 2500 | - | ||
| Combination | 31.3 | 625 | 0.31 | |
| C. albicans NBRC1061 | Alone | 1000 | - | - |
| - | 1250 | - | ||
| Combination | 62.5 | 625 | 0.56 | |
| C. albicans IFM46910 c | Alone | >1000 | - | - |
| - | 1250 | - | ||
| Combination | 250 | 313 | <0.5 | |
| C. albicans IFM54354 c | Alone | >1000 | - | - |
| - | 1250 | - | ||
| Combination | 62.5 | 625 | <0.56 | |
| Primer Name | Sequence (5′-3′) |
|---|---|
| ACT1-F | ATGGTCGGTATGGGTCAAAA |
| ACT1-R | AACCAGCGTAAATTGGAACG |
| PDR5-F | GTTGCCTAAACCCAGGTGAA |
| PDR5-R | GTTGCCTAAACCCAGGTGAA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueda, Y.; Tahara, Y.O.; Miyata, M.; Ogita, A.; Yamaguchi, Y.; Tanaka, T.; Fujita, K.-i. Involvement of a Multidrug Efflux Pump and Alterations in Cell Surface Structure in the Synergistic Antifungal Activity of Nagilactone E and Anethole against Budding Yeast Saccharomyces cerevisiae. Antibiotics 2021, 10, 537. https://doi.org/10.3390/antibiotics10050537
Ueda Y, Tahara YO, Miyata M, Ogita A, Yamaguchi Y, Tanaka T, Fujita K-i. Involvement of a Multidrug Efflux Pump and Alterations in Cell Surface Structure in the Synergistic Antifungal Activity of Nagilactone E and Anethole against Budding Yeast Saccharomyces cerevisiae. Antibiotics. 2021; 10(5):537. https://doi.org/10.3390/antibiotics10050537
Chicago/Turabian StyleUeda, Yuki, Yuhei O. Tahara, Makoto Miyata, Akira Ogita, Yoshihiro Yamaguchi, Toshio Tanaka, and Ken-ichi Fujita. 2021. "Involvement of a Multidrug Efflux Pump and Alterations in Cell Surface Structure in the Synergistic Antifungal Activity of Nagilactone E and Anethole against Budding Yeast Saccharomyces cerevisiae" Antibiotics 10, no. 5: 537. https://doi.org/10.3390/antibiotics10050537
APA StyleUeda, Y., Tahara, Y. O., Miyata, M., Ogita, A., Yamaguchi, Y., Tanaka, T., & Fujita, K.-i. (2021). Involvement of a Multidrug Efflux Pump and Alterations in Cell Surface Structure in the Synergistic Antifungal Activity of Nagilactone E and Anethole against Budding Yeast Saccharomyces cerevisiae. Antibiotics, 10(5), 537. https://doi.org/10.3390/antibiotics10050537







