Epidemiology of Meropenem/Vaborbactam Resistance in KPC-Producing Klebsiella pneumoniae Causing Bloodstream Infections in Northern Italy, 2018
Abstract
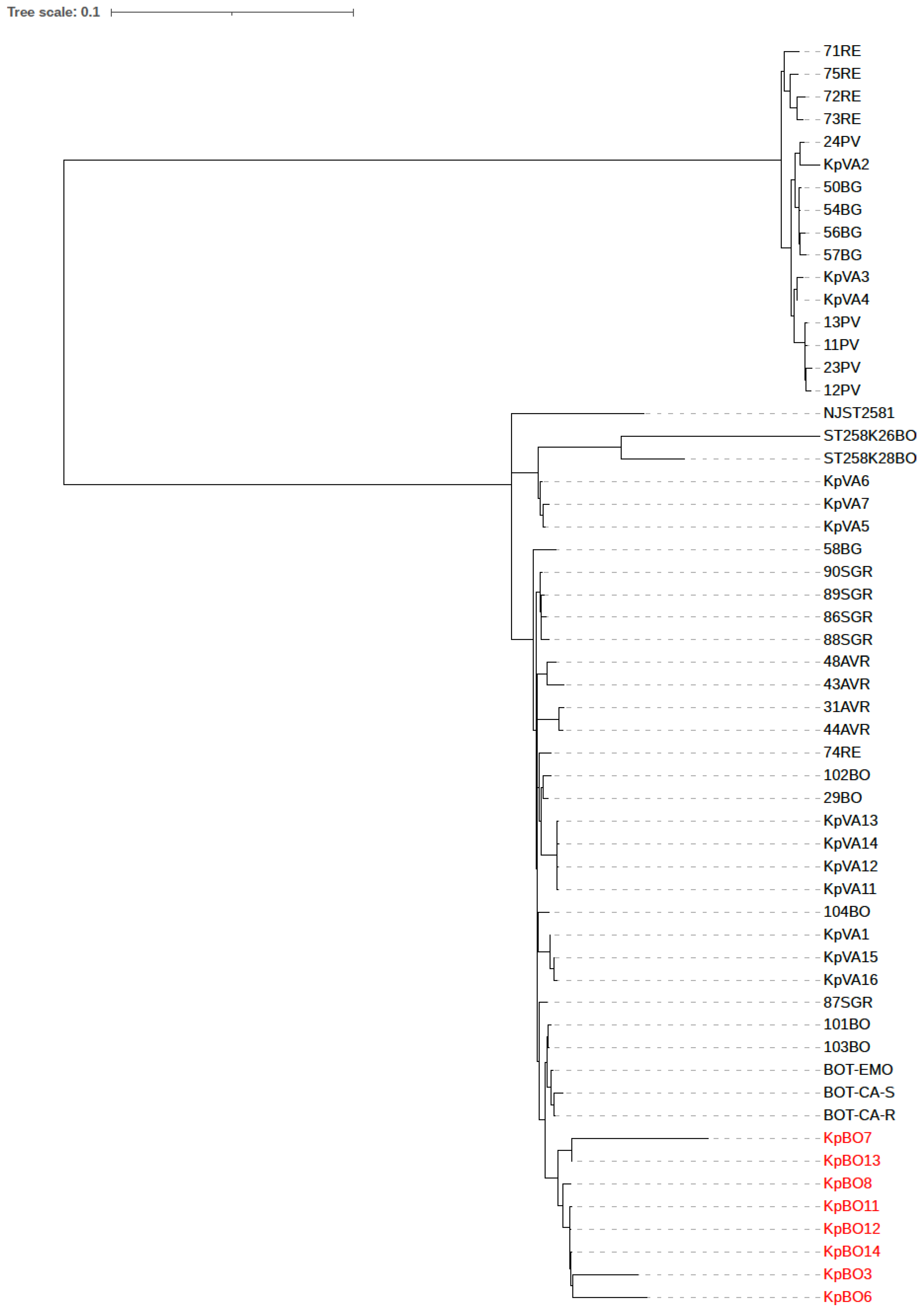
1. Introduction
2. Results
Clinical Data Analysis
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Microbiological Analysis
4.3. Whole Genome Sequencing Analysis
4.4. Data Availability
4.5. Clinical Data and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toussaint, K.A.; Gallagher, J.C. β-lactam/β-lactamase inhibitor combinations: From then to now. Ann. Pharmacother. 2015, 49, 86–98. [Google Scholar] [CrossRef]
- Petrosillo, N.; Giannella, M.; Lewis, R.; Viale, P. Treatment of carbapenem-resistant Klebsiella pneumoniae: The state of the art. Expert Rev. Anti-Infect. Ther. 2013, 11, 159–177. [Google Scholar] [CrossRef]
- Tzouvelekis, L.S.; Markogiannakis, A.; Piperaki, E.; Souli, M.; Daikos, G.L. Treating infections caused by carbapenemase-producing Enterobacteriaceae. Clin. Microbiol. Infect. 2014, 20, 862–872. [Google Scholar] [CrossRef]
- Bassetti, M.; Peghin, M. How to manage KPC infections. Ther. Adv. Infect. Dis. 2020, 7, 2049936120912049. [Google Scholar]
- Giacobbe, D.R.; Maraolo, A.E.; Viscoli, C. Pitfalls of defining combination therapy for carbapenem-resistant Enterobacteriaceae in observational studies. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1707–1709. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Drawz, S.M.; Bonomo, R.A. Three decades of β-lactamase inhibitors. Clin. Microbiol. Rev. 2010, 23, 160–201. [Google Scholar] [CrossRef] [PubMed]
- Monaco, M.; Giani, T.; Raffone, M.; Arena, F.; Garcia-Fernandez, A.; Pollini, S.; Network EuSCAPE-Italy; Grundmann, H.; Pantosti, A.; Rossolini, G.M. Colistin resistance superimposed to endemic carbapenem-resistant Klebsiella pneumoniae: A rapidly evolving problem in Italy, November 2013 to April 2014. Eur. Surveill. 2014, 19, 20939. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; van Duin, D. Novel β-lactamase inhibitors: Unlocking their potential in therapy. Drugs 2017, 77, 615–628. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Lawrence, C.K.; Adam, H.; Schweizer, F.; Zelenitsky, S.; Zhanel, M.; Lagacé-Wiens, P.R.S.; Walkty, A.; Denisuik, A.; Golden, A.; et al. Imipenem-relebactam and meropenem-vaborbactam: Two novel carbapenem-β-lactamase inhibitor combinations. Drugs 2018, 78, 65–98. [Google Scholar] [CrossRef]
- Hecker, S.J.; Reddy, K.R.; Totrov, M.; Hirst, G.C.; Lomovskaya, O.; Griffith, D.C.; King, P.; Tsivkovski, R.; Sun, D.; Sabet, M.; et al. Discovery of a cyclic boronic acid β-lactamase inhibitor (RPX7009) with utility vs class A serine carbapenemases. J. Med. Chem. 2015, 58, 3682–3692. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Sun, D.; Rubio-Aparicio, D.; Nelson, K.; Tsivkovski, R.; Griffith, D.C.; Dudley, M.N. Vaborbactam: Spectrum of beta-lactamase inhibition and impact of resistance mechanisms on activity in Enterobacteriaceae. Antimicrob. Agents Chemother. 2017, 61, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wunderink, R.G.; Giamarellos-Bourboulis, E.J.; Rahav, G.; Mathers, A.J.; Bassetti, M.; Vazquez, J.; Cornely, O.A.; Solomkin, J.; Bhowmick, T.; Bishara, J.; et al. Effect and Safety of Meropenem-Vaborbactam versus Best-Available Therapy in Patients with Carbapenem-Resistant Enterobacteriaceae Infections: The TANGO II Randomized Clinical Trial. Infect. Dis. Ther. 2018, 7, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Lapuebla, A.; Abdallah, M.; Olafisoye, O.; Cortes, C.; Urban, C.; Quale, J.; Landman, D. Activity of meropenem combined with RPX7009, a novel β-lactamase inhibitor, against Gram-negative clinical isolates in New York City. Antimicrob. Agents Chemother. 2015, 59, 4856–4860. [Google Scholar] [CrossRef] [PubMed]
- Gaibani, P.; Re, M.C.; Campoli, C.; Viale, P.L.; Ambretti, S. Bloodstream infection caused by KPC-producing Klebsiella pneumoniae resistant to ceftazidime/avibactam: Epidemiology and genomic characterization. Clin. Microbiol. Infect. 2020, 26, 516.e1–516.e4. [Google Scholar] [CrossRef]
- Dulyayangkul, P.; Wan Nur Ismah, W.A.K.; Douglas, E.J.A.; Avison, M.B. Mutation of kvrA Causes OmpK35 and OmpK36 Porin Downregulation and Reduced Meropenem-Vaborbactam Susceptibility in KPC-Producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2020, 64, e02208-19. [Google Scholar] [CrossRef]
- Chen, L.; Mathema, B.; Chavda, K.D.; DeLeo, F.R.; Bonomo, R.A.; Kreiswirth, B.N. Carbapenemase-producing Klebsiella pneumoniae: Molecular and genetic decoding. Trends Microbiol. 2014, 22, 686–696. [Google Scholar] [CrossRef]
- Sun, D.; Rubio-Aparicio, D.; Nelson, K.; Dudley, M.N.; Lomovskaya, O. Meropenem- Vaborbactam Resistance Selection, Resistance Prevention, and Molecular Mechanisms in Mutants of KPC-Producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2017, 61, e01694-17. [Google Scholar] [CrossRef]
- Garcia-Fernandez, A.; Villa, L.; Carta, C.; Venditti, C.; Giordano, A.; Venditti, M. Klebsiella pneumoniae ST258 producing KPC-3 identified in Italy carries novel plasmids and OmpK36/OmpK35 porin variants. Antimicrob. Agents Chemother. 2012, 56, 2143–2145. [Google Scholar] [CrossRef]
- Landman, D.; Bratu, S.; Quale, J. Contribution of OmpK36 to carbapenem susceptibility in KPC-producing Klebsiella pneumoniae. J. Med. Microbiol. 2009, 58, 1303–1308. [Google Scholar] [CrossRef]
- Giani, T.; Pini, B.; Arena, F.; Conte, V.; Bracco, S.; Migliavacca, R.; AMCLI-CRE Survey Participants; Pantosti, A.; Pagani, L.; Luzzaro, F.; et al. Epidemic diffusion of KPC carbapenemase-producing Klebsiella pneumoniae in Italy: Results of the first countrywide survey. Eur. Surveill. 2011, 18, 20489. [Google Scholar]
- Gaibani, P.; Galea, A.; Fagioni, M.; Ambretti, S.; Sambri, V.; Landini, M.P. Evaluation of Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry for Identification of KPC-Producing Klebsiella pneumoniae. J. Clin. Microbiol. 2016, 54, 2609–2613. [Google Scholar] [CrossRef]
- Foschi, C.; Gaibani, P.; Lombardo, D.; Re, M.C.; Ambretti, S. Rectal screening for carbapenemase-producing Enterobacteriaceae: A proposed workflow. J. Glob. Antimicrob. Resist. 2020, 21, 86–90. [Google Scholar] [CrossRef]
- Gaibani, P.; Ambretti, S.; Tamburini, M.V.; Vecchio-Nepita, E.; Re, M.C. Clinical application of Bruker Biotyper MALDI-TOF/MS system for real-time identification of KPC production in Klebsiella pneumoniae clinical isolates. J. Glob. Antimicrob. Resist. 2018, 12, 169–170. [Google Scholar] [CrossRef]
- Gaibani, P.; Campoli, C.; Lewis, R.E.; Volpe, S.L.; Scaltriti, E.; Giannella, M.; Pongolini, S.; Berlingeri, A.; Cristini, F.; Bartoletti, M.; et al. In vivo evolution of resistant subpopulations of KPC-producing Klebsiella pneumoniae during ceftazidime/avibactam treatment. J. Antimicrob. Chemother. 2018, 73, 1525–1529. [Google Scholar] [CrossRef]
- Treangen, T.J.; Ondov, B.D.; Koren, S.; Phillippy, A.M. The Harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol. 2014, 15, 524. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL): An online tool for phylogenetic treedisplay and annotation. Bioinformatics 2011, 23, 127–128. [Google Scholar] [CrossRef]
- Feng, Y.; Zou, S.; Chen, H.; Yu, Y.; Ruan, Z. BacWGSTdb 2.0: A one-stop repository for bacterial whole-genome sequence typing and source tracking. Nucleic Acids Res. 2021, 49, 644–650. [Google Scholar] [CrossRef]
- Barrick, J.E.; Colburn, G.; Deatherage, D.E.; Traverse, C.C.; Strand, M.D.; Borges, J.J.; Knoester, D.B.; Reba, A.; Meyer, A.G. Identifying structural variation in haploid microbial genomes from short-read resequencing data using breseq. BMC Genom. 2014, 15, 1039. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure assessment) score to describe organ dysfunction/failure. On behalf of the Working group on Sepsis-related Problems of the european Society of intensive care Medicine. Intensive Care Med. 1998, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Piano, S.; Bartoletti, M.; Tonon, M.; Baldassarre, M.; Chies, G.; Romano, A.; Viale, P.; Vettore, E.; Domenicali, M.; Stanco, M.; et al. Assessment of Sepsis-3 criteria and quick SOFA in patients with cirrhosis and bacterial infections. Gut 2018, 67, 1892–1899. [Google Scholar] [CrossRef] [PubMed]
| Isolate | Patient | MIC (mg/L) | ST | Genetic Determinants | Porins | Plasmid_Replicons (InC) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MEM | CAZ/AVI | MEM/VAB | CST | Beta-lactams | Aminoglycoside | Fluoroquinolone | Sulfonamide | OmpK35 | OmpK36 | ||||
| KpBO3 | 1 | 256 | 32 | 256 | 0.25 | 512 | blaKPC-3, blaSHV-11 | aac(6’)-Ib | oqxA, oqxB, aac(6’)Ib-cr | sul1 | truncated | GD134–135 | IncFIB (K), IncFIB(pKPHS1), IncX3, ColRNAI |
| KpBO6 | 2 | 256 | 16 | 256 | 0.5 | 258 | blaKPC-3, blaSHV-12 | aadA2, aph(3’)-Ia, aac(6’)Ib-cr | oqxA, oqxB, aac(6’)Ib-cr | sul1 | truncated | GD134–135 | IncFIIK, IncFIB(K), IncX3, ColRNAI |
| KpBO7 | 3 | 256 | ≥256 | 256 | 0.5 | 1519 | blaKPC-3, blaTEM-1A, blaOXA-9, blaSHV-11 | aadA2, aph(3’)-Ia, aac(6’)Ib-cr | oqxA, oqxB, aac(6’)Ib-cr | sul1 | truncated | GD134–135 | IncFIB (pQIL), IncFIB (pKPSH1), IncFIB(K), IncFII(K), IncX3, ColRNAI, Col(BS512) |
| KpBO8 | 4 | 32 | 8 | 48 | 0.5 | 512 | blaKPC-3, blaSHV182,blaTEM-1A, blaOXA-9 | aadA2, aph(3’)-Ia, aac(6’)-Ib | oqxA, oqxB, aac(6’)Ib-cr | sul1 | truncated | GD134–135 | IncFIB (pQIL), IncFIB (pKPSH1), IncFIB(K), IncFII(K), ColRNAI |
| KpBO11 | 5 | 256 | 8 | 256 | 0.25 | 512 | blaKPC-3, blaSHV182 | aadA2, aph(3’)-Ia, aac(6’)-Ib | oqxA, oqxB, aac(6’)Ib-cr | sul1 | truncated | GD134–135 | IncFIB (pKPSH1), IncFIB(K), IncFII(K), ColRNAI, IncX3 |
| KpBO12 | 6 | 256 | 8 | 256 | 0.5 | 512 | blaKPC-3, blaSHV182 | aadA2, aph(3’)-Ia, aac(6’)-Ib | oqxB, oqxA, aac(6’)Ib-cr | sul1 | truncated | GD134–135 | IncFIB (pKPSH1), IncFIB(K), IncFII(K), ColRNAI, IncX3 |
| KpBO13 | 7 | 32 | 8 | 256 | 0.5 | 1519 | blaKPC-3, blaSHV182, blaOXA-9 | aadA2, aac(6’)-Ib | oqxB, oqxA, aac(6’)Ib-cr | sul1 | truncated | GD134–135 | IncFIB (pQIL), IncFIB (pKPSH1), IncFIB(K), IncFII(K), ColRNAI, IncX3, Col(BS512) |
| KpBO14 | 8 | 256 | 8 | 256 | 0.25 | 512 | blaKPC-3, blaSHV182 | aac(6’)-Ib | oqxA, oqxB, aac(6’)Ib-cr | - | truncated | GD134–135 | IncFIB (pKPSH1), IncFIB(K), ColRNAI, IncX3 |
| Patient | Isolate | Colonization Days’ Prior Infection | SOFA | Initial Infection | Previous Treatment (Days) | Time of Isolation after Initial Treatment (Days) | Antimicrobial Combination Therapy (Days) | Risk Factors | Clinical Outcome at 30 Days | Microbiological Outcome at 30 Days (Days) | Relapse Infection |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | KpBO3 | 0 | 13 | Abdominal infection | Ceftazidime/Avibactam (12), Meropenem (32) | 0 | Meropenem-Colistin-Tigecycline (3) | - | Failure | NA | NA |
| 2 | KpBO6 | 4 | 2 | Pneumonia | Meropenem-Colistin (27), Meropenem-Tigecycline (19) | 0 | Meropenem-Colistin (24) | - | Success | Success | None |
| 3 | KpBO7 | 7 | 1 | CVC-related | Amoxicillin/Clavulanic acid (11) | 1 | Meropenem-Colistin (13) | CVVH | Success | Success | None |
| 4 | KpBO8 | 60 | 6 | CVC-related | Oxacillin (11), Ertapenem (13) | 0 | Meropenem-Ceftazidime/Avibactam (24) | CKD | Success | Success | None |
| 5 | KpBO11 | 18 | 12 | Pneumonia | Oxacillin (8), Piperacillin/Tazobactam (34) | 15 | Meropenem-Colistin (18) | - | Failure | Failure (15) | NA |
| 6 | KpBO12 | 3 | 20 | Biliary infection | Piperacillin/Tazobactam (3), Meropenem-Tigecycline (3) | 10 | Meropenem-Tigecycline (1) | CKD, CVVH | Failure | NA | NA |
| 7 | KpBO13 | 5 | 5 | Pneumonia | Piperacillin/Tazobactam (16), Meropenem-Tigecycline (29) | 1 | Meropenem-Ceftazidime/Avibactam (14) | CKD | Failure | NA | NA |
| 8 | KpBO14 | 60 | 3 | Urinary infection | Amoxicillin/Clavulanic acid (20), Meropenem (19) | 0 | Meropenem-Ceftazidime/Avibactam (14) | CKD | Success | Success | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaibani, P.; Lombardo, D.; Bussini, L.; Bovo, F.; Munari, B.; Giannella, M.; Bartoletti, M.; Viale, P.; Lazzarotto, T.; Ambretti, S. Epidemiology of Meropenem/Vaborbactam Resistance in KPC-Producing Klebsiella pneumoniae Causing Bloodstream Infections in Northern Italy, 2018. Antibiotics 2021, 10, 536. https://doi.org/10.3390/antibiotics10050536
Gaibani P, Lombardo D, Bussini L, Bovo F, Munari B, Giannella M, Bartoletti M, Viale P, Lazzarotto T, Ambretti S. Epidemiology of Meropenem/Vaborbactam Resistance in KPC-Producing Klebsiella pneumoniae Causing Bloodstream Infections in Northern Italy, 2018. Antibiotics. 2021; 10(5):536. https://doi.org/10.3390/antibiotics10050536
Chicago/Turabian StyleGaibani, Paolo, Donatella Lombardo, Linda Bussini, Federica Bovo, Beatrice Munari, Maddalena Giannella, Michele Bartoletti, Pierluigi Viale, Tiziana Lazzarotto, and Simone Ambretti. 2021. "Epidemiology of Meropenem/Vaborbactam Resistance in KPC-Producing Klebsiella pneumoniae Causing Bloodstream Infections in Northern Italy, 2018" Antibiotics 10, no. 5: 536. https://doi.org/10.3390/antibiotics10050536
APA StyleGaibani, P., Lombardo, D., Bussini, L., Bovo, F., Munari, B., Giannella, M., Bartoletti, M., Viale, P., Lazzarotto, T., & Ambretti, S. (2021). Epidemiology of Meropenem/Vaborbactam Resistance in KPC-Producing Klebsiella pneumoniae Causing Bloodstream Infections in Northern Italy, 2018. Antibiotics, 10(5), 536. https://doi.org/10.3390/antibiotics10050536







