Antibiotic Resistance, spa Typing and Clonal Analysis of Methicillin-Resistant Staphylococcus aureus (MRSA) Isolates from Blood of Patients Hospitalized in the Czech Republic
Abstract
1. Introduction
2. Results
2.1. Antimicrobial Susceptibility of MRSA Strains
2.2. spa Typing, Cluster Analysis and Antibiotic Susceptibility within spa CCs
2.3. spa Types and Resistance to Tetracycline
2.4. MLST, SCCmec Typing and MLST CCs in Relation to the Different spa CCs (BURP Clustering)
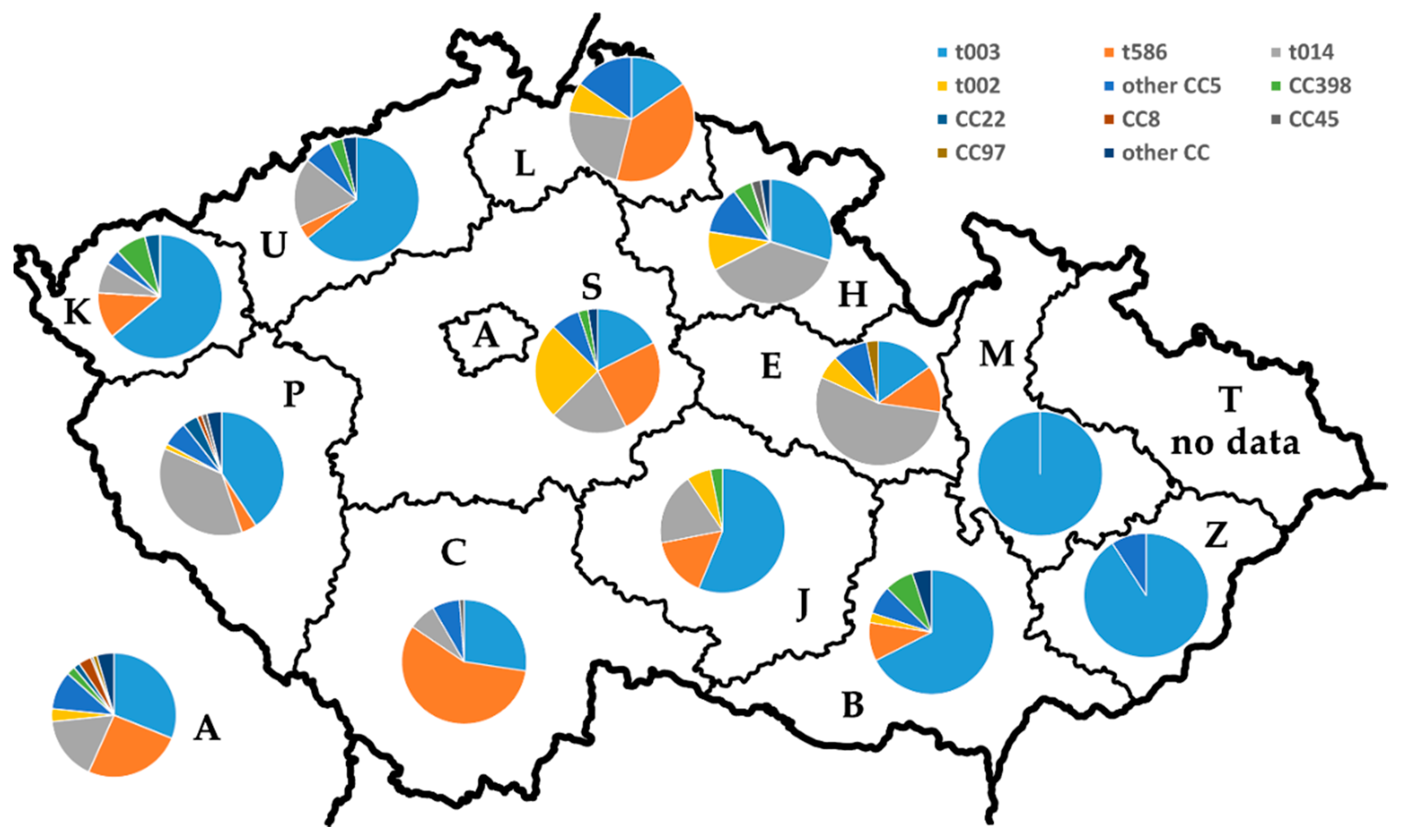
2.5. Distribution of the Major spa Types and MLST CCs among the Czech Regions
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Antibiotic Susceptibility Testing and MRSA Detection
4.3. Molecular Typing
4.3.1. mecA/mecC Detection
4.3.2. spa Typing and Based Upon Repeat Analysis (BURP)
4.3.3. Multilocus Sequence Typing (MLST)
4.3.4. SCCmec Typing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Surveillance of Antimicrobial Resistance in Europe 2016; Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net); ECDC: Stockholm, Sweden, 2017; p. 54. [Google Scholar]
- Grundmann, H.; Schouls, L.M.; Aanensen, D.M.; Pluister, G.N.; Tami, A.; Chlebowicz, M.; Glasner, C.; Sabat, A.J.; Weist, K.; Heuer, O.; et al. The dynamic changes of dominant clones of Staphylococcus aureus causing bloodstream infections in the European region: Results of a second structured survey. Euro Surveill. 2014, 19, 20987. [Google Scholar] [CrossRef]
- Surveillance Atlas of Infectious Diseases. Available online: http://atlas.ecdc.europa.eu/public/index.aspx (accessed on 10 April 2020).
- Melter, O.; Santos Sanches, I.; Schindler, J.; Aires de Sousa, M.; Mato, R.; Kovárova, V.; Zemlicková, H.; Lencastre, H.D. Methicillin-resistant Staphylococcus aureus clonal types in the Czech Republic. J. Clin. Microbiol. 1999, 37, 2798–2803. [Google Scholar] [CrossRef]
- Melter, O.; Aires de Sousa, M.; Urbásková, P.; Jakubů, V.; Zemlicková, H.; Lencastre, H.D. Update on the major clonal types of methicillin-resistant Staphylococcus aureus in the Czech Republic. J. Clin. Microbiol. 2003, 41, 4998–5005. [Google Scholar] [CrossRef]
- Melter, O.; Urbásková, P.; Jakubů, V.; Macková, B.; Zemlicková, H.; Czech participants in EARSS. Emergence of EMRSA-15 clone in hospitals throughout the Czech Republic. Euro Surveill. 2006, 11, E060803-6. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, H.; Aanensen, D.M.; van den Wijngaard, C.C.; Spratt, B.G.; Harmsen, D.; Friedrich, A.W.; European Staphylococcal Reference Laboratory Working Group. Geographic distribution of Staphylococcus aureus causing invasive infections in Europe: A molecular-epidemiological analysis. PLoS Med. 2010, 7, e10000215. [Google Scholar] [CrossRef]
- Pomorska, K.; Jakubu, V.; Musilek, M.; Zemlickova, H. Spa typing of methicillin resistant Staphylococcus aureus (MRSA) strains isolated from blood from patients hospitalized in the Czech Republic: A comparative study after ten years. In Proceedings of the 29th Congress of Clinical Microbiology & Infectious Diseases (ECCMID), Amsterdam, The Netherlands, 13–16 April 2019. Poster no. P2716. [Google Scholar]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Tkadlec, J.; Capek, V.; Brajerova, M.; Smelikova, E.; Melter, O.; Bergerova, T.; Polivkova, S.; Balejova, M.; Hanslianova, M.; Fackova, D.; et al. The molecular epidemiology of methicillin-resistant Staphylococcus aureus (MRSA) in the Czech Republic. J. Antimicrob. Chemother. 2021, 76, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Neradova, K.; Fridrichova, M.; Jakubu, V.; Pomorska, K.; Zemlickova, H. Epidemiological characteristics of methicillin-resistant Staphylococcus aureus isolates from bloodstream cultures at University Hospital in the Czech Republic. Folia Microbiol. 2020, 65, 615–622. [Google Scholar] [CrossRef]
- Stock, N.K.; Petráš, P.; Melter, O.; Kapounová, G.; Vopalková, P.; Kubele, J.; Vaniš, V.; Tkadlec, J.; Bukáčková, E.; Machová, I.; et al. Importance of multifaceted approaches in infection control: A practical experience from an outbreak investigation. PLoS ONE 2016, 11, e0157981. [Google Scholar] [CrossRef] [PubMed]
- Ridom SpaServer—Frequencies. Available online: https://spa.ridom.de/frequencies.shtml (accessed on 18 December 2020).
- Strommenger, B.; Kettlitz, C.; Weniger, T.; Harmsen, D.; Friedrich, A.W.; Witte, W. Assignment of Staphylococcus isolates to groups by spa typing, SmaI macrorestriction analysis, and multilocus sequence typing. J. Clin. Microbiol. 2006, 44, 2533–2540. [Google Scholar] [CrossRef]
- Aanensen, D.M.; Feil, E.J.; Holden, M.T.G.; Dordel, J.; Yeats, C.A.; Fedosejev, A.; Goater, R.; Castillo-Ramírez, S.; Corander, J.; Colijn, C.; et al. Whole-genome sequencing for routine pathogen surveillance in public health: A Population snapshot of invasive Staphylococcus aureus in Europe. MBio 2016, 7, e00444-16. [Google Scholar] [CrossRef]
- Nübel, U.; Dordel, J.; Kurt, K.; Strommenger, B.; Westh, H.; Shukla, S.K.; Zemlicková, H.; Leblois, R.; Wirth, T.; Jombart, T.; et al. A timescale for evolution, population expansion, and spatial spread of an emerging clone of methicillin-resistant Staphylococcus aureus. PLoS Pathog. 2010, 6, e1000855. [Google Scholar] [CrossRef]
- Roberts, R.B.; Lencastre, A.D.; Eisner, W.; Severina, E.P.; Shopsin, B.; Kreiswirth, B.N.; Tomasz, A. Molecular epidemiology of methicillin-resistant Staphylococcus aureus in 12 New York hospitals. J. Infect. Dis. 1998, 178, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Pardos de la Gandara, M.; Curry, M.; Berger, J.; Burstein, D.; Della-Latta, P.; Kopetz, V.; Quale, J.; Spitzer, E.; Tan, R.; Urban, C.; et al. MRSA causing infections in hospitals in greater metropolitan New York: Major shift in the dominant clonal type between 1996 and 2014. PLoS ONE 2016, 11, e0156924. [Google Scholar] [CrossRef]
- Aires de Sousa, M.; Lencastre, H.D.; Santos Sanches, I.; Kikuchi, K.; Totsuka, K.; Tomasz, A. Similarity of antibiotic resistance patterns and molecular typing properties of methicillin-resistant Staphylococcus aureus isolates widely spread in hospitals in New York City and in a hospital in Tokyo, Japan. Microb. Drug. Resist. 2000, 6, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Conceição, T.; Aires-de-Sousa, M.; Füzi, M.; Tóth, A.; Pászti, J.; Ungvári, E.; van Leeuwen, W.B.; van Belkum, A.; Grundmann, H.; Lencastre, H.D. Replacement of methicillin-resistant Staphylococcus aureus clones in Hungary over time: A 10-year surveillance study. Clin. Microbiol. Infect. 2007, 13, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Aires-de-Sousa, M.; Correia, B.; Lencastre, H.D.; Multilaboratory Project Collaborators. Changing patterns in frequency of recovery of five methicillin-resistant Staphylococcus aureus clones in Portuguese hospitals: Surveillance over a 16-year period. J. Clin. Microbiol. 2008, 46, 2912–2917. [Google Scholar] [CrossRef]
- Zarfel, G.; Luxner, J.; Folli, B.; Leitner, E.; Feierl, G.; Kittinger, C.; Grisold, A. Increase of genetic diversity and clonal replacement of epidemic methicillin-resistant Staphylococcus aureus strains in South-East Austria. FEMS Microbiol. Lett. 2016, 363, fnw137. [Google Scholar] [CrossRef]
- Monecke, S.; Coombs, G.; Shore, A.C.; Coleman, D.C.; Akpaka, P.; Borg, M.; Chow, H.; Ip, M.; Jatzwauk, L.; Jonas, D.; et al. A field guide to pandemic, epidemic and sporadic clones of methicillin-resistant Staphylococcus aureus. PLoS ONE 2011, 6, e17936. [Google Scholar] [CrossRef]
- Haenni, M.; Châtre, P.; Dupieux-Chabert, C.; Métayer, V.; Bes, M.; Madec, J.Y.; Laurent, F. Molecular epidemiology of methicillin-resistant Staphylococcus aureus in horses, cats, and dogs over a 5-year period in France. Front. Microbiol. 2017, 8, 2493. [Google Scholar] [CrossRef] [PubMed]
- Tegegne, H.A.; Florianová, M.; Gelbíčová, T.; Karpíšková, R.; Koláčková, I. Detection and molecular characterization of methicillin-resistant Staphylococcus aureus isolated from bulk tank milk of cows, sheep, and goats. Foodborne Pathog. Dis. 2019, 16, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Tegegne, H.A.; Koláčková, I.; Karpíšková, R. Diversity of livestock associated methicillin-resistant Staphylococcus aureus. Asian Pac. J. Trop. Med. 2017, 10, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Neradova, K.; Jakubu, V.; Pomorska, K.; Zemlickova, H. Methicillin-resistant Staphylococcus aureus in veterinary professionals in 2017 in the Czech Republic. BMC Vet. Res. 2020, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Wulf, M.W.; Markestein, A.; van der Linden, F.T.; Voss, A.; Klaassen, C.; Verduin, C.M. First outbreak of methicillin-resistant Staphylococcus aureus ST398 in a Dutch hospital, June 2007. Euro Surveill. 2008, 13, 8051. [Google Scholar] [CrossRef]
- O’Neill, G.L.; Murchan, S.; Gil-Setas, A.; Aucken, H.M. Identification and characterization of phage variants of a strain of epidemic methicillin-resistant Staphylococcus aureus (EMRSA-15). J. Clin. Microbiol. 2001, 39, 1540–1548. [Google Scholar] [CrossRef]
- Faria, N.A.; Miragaia, M.; Lencastre, H.D.; Multi laboratory project collaborators. Massive dissemination of methicillin resistant Staphylococcus aureus in bloodstream infections in a high MRSA prevalence country: Establishment and diversification of EMRSA-15. Microb. Drug Resist. 2013, 19, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Hetem, D.J.; Derde, L.P.G.; Empel, J.; Mroczkowska, A.; Orczykowska-Kotyna, M.; Kozińska, A.; Hryniewicz, W.; Goossens, H.; Bonten, M.J.M.; MOSAR WP3 study group. Molecular epidemiology of MRSA in 13 ICUs from eight European countries. J. Antimicrob. Chemother. 2016, 71, 45–52. [Google Scholar] [CrossRef]
- Effelsberg, N.; Stegger, M.; Peitzmann, L.; Altinok, O.; Coombs, G.W.; Pichon, B.; Kearns, A.; Randad, P.R.; Heaney, C.D.; Bletz, S.; et al. Global epidemiology and evolutionary history of Staphylococcus aureus ST45. J. Clin. Microbiol. 2020, 59, e02198-20. [Google Scholar] [CrossRef]
- Zemlickova, H.; Fridrichová, M.; Tyllová, K.; Jakubů, V.; Machová, I. Carriage of methicillin-resistant Staphylococcus aureus in veterinary personnel. Epidemiol. Infect. 2009, 137, 1233–1236. [Google Scholar] [CrossRef]
- Witte, W.; Werner, G.; Cuny, C. Subtyping of MRSA isolates belonging to a widely disseminated clonal group by polymorphism of the dru sequences in mec-associated DNA. Int. J. Med. Microbiol. 2001, 291, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Udo, E.E.; Aly, N.Y.A.; Sarkhoo, E.; Al-Sawan, R.; Al-Asar, A.S.M. Detection and characterization of an ST97-SCCmec-V community-associated meticillin-resistant Staphylococcus aureus clone in a neonatal intensive care unit and special care baby unit. J. Med. Microbiol. 2011, 60, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Rubin, I.M.; Hansen, T.A.; Klingenberg, A.M.; Petersen, A.M.; Worning, P.; Westh, H.; Bartels, M.D. A sporadic four-year hospital outbreak of a ST97-IVa MRSA with half of the patients first identified in the community. Front. Microbiol. 2018, 9, 1494. [Google Scholar] [CrossRef] [PubMed]
- Idelevich, E.A.; Seifert, H.; Sundqvist, M.; Scudeller, L.; Amit, S.; Balode, A.; Bilozor, A.; Drevinek, P.; Kocak Tufan, Z.; Koraqi, A.; et al. Microbiological diagnostics of bloodstream infections in Europe-an ESGBIES survey. Clin. Microbiol. Infect. 2019, 25, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Surveillance of Antimicrobial Resistance in Europe 2018; Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net); ECDC: Stockholm, Sweden, 2019; pp. 56, 73. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters; Version 9.0; EUCAST: Växjö, Sweden, 2019. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; pp. 136–138. [Google Scholar]
- Oliveira, D.C.; Lencastre, H.D. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 2155–2161. [Google Scholar] [CrossRef] [PubMed]
- Stegger, M.; Andersen, P.S.; Kearns, A.; Pichon, B.; Holmes, M.A.; Edwards, G.; Laurent, F.; Teale, C.; Skov, R.; Larsen, A.R. Rapid detection, differentiation and typing of methicillin-resistant Staphylococcus aureus harbouring either mecA or the new mecA homologue mecA(LGA251). Clin. Microbiol. Infect. 2012, 18, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Ridom Bioinformatics DNA Sequencing of the Spa Gene. Available online: https://www.ridom.de/doc/Ridom_spa_sequencing.pdf (accessed on 18 December 2020).
- Ridom StaphType User Guide, Version 2. Available online: https://www.ridom.de/doc/Ridom_StaphType_user_guide.pdf (accessed on 18 December 2020).
- Enright, M.C.; Day, N.P.; Davies, C.E.; Peacock, S.J.; Spratt, B.G. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 2000, 38, 1008–1015. [Google Scholar] [CrossRef]
- Milheiriço, C.; Oliveira, D.C.; Lencastre, H.D. Update to the multiplex PCR strategy for assignment of mec element types in Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 3374–3377. [Google Scholar] [CrossRef]
- Zhang, K.; McClure, J.A.; Elsayed, S.; Louie, T.; Conly, J.M. Novel multiplex PCR assay for characterization and concomitant subtyping of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 2005, 43, 5026–5033. [Google Scholar] [CrossRef]
- Hisata, K.; Ito, T.; Matsunaga, N.; Komatsu, M.; Jin, J.; Li, S.; Watanabe, S.; Shimizu, T.; Hiramatsu, K. Dissemination of multiple MRSA clones among community-associated methicillin-resistant Staphylococcus aureus infections from Japanese children with impetigo. J. Infect. Chemother. 2011, 17, 609–621. [Google Scholar] [CrossRef]
- Boyle-Vavra, S.; Ereshefsky, B.; Wang, C.C.; Daum, R.S. Successful multiresistant community-associated methicillin-resistant Staphylococcus aureus lineage from Taipei, Taiwan, that carries either the novel staphylococcal chromosome cassette mec (SCCmec) type VT or SCCmec type IV. J. Clin. Microbiol. 2005, 43, 4719–4730. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Higuchi, W.; Zaraket, H.; Otsuka, T.; Baranovich, T.; Enany, S.; Saito, K.; Isobe, H.; Dohmae, S.; Ozaki, K.; et al. Novel characteristics of community-acquired methicillin-resistant Staphylococcus aureus strains belonging to multilocus sequence type 59 in Taiwan. Antimicrob. Agents Chemother. 2008, 52, 837–845. [Google Scholar] [CrossRef] [PubMed]
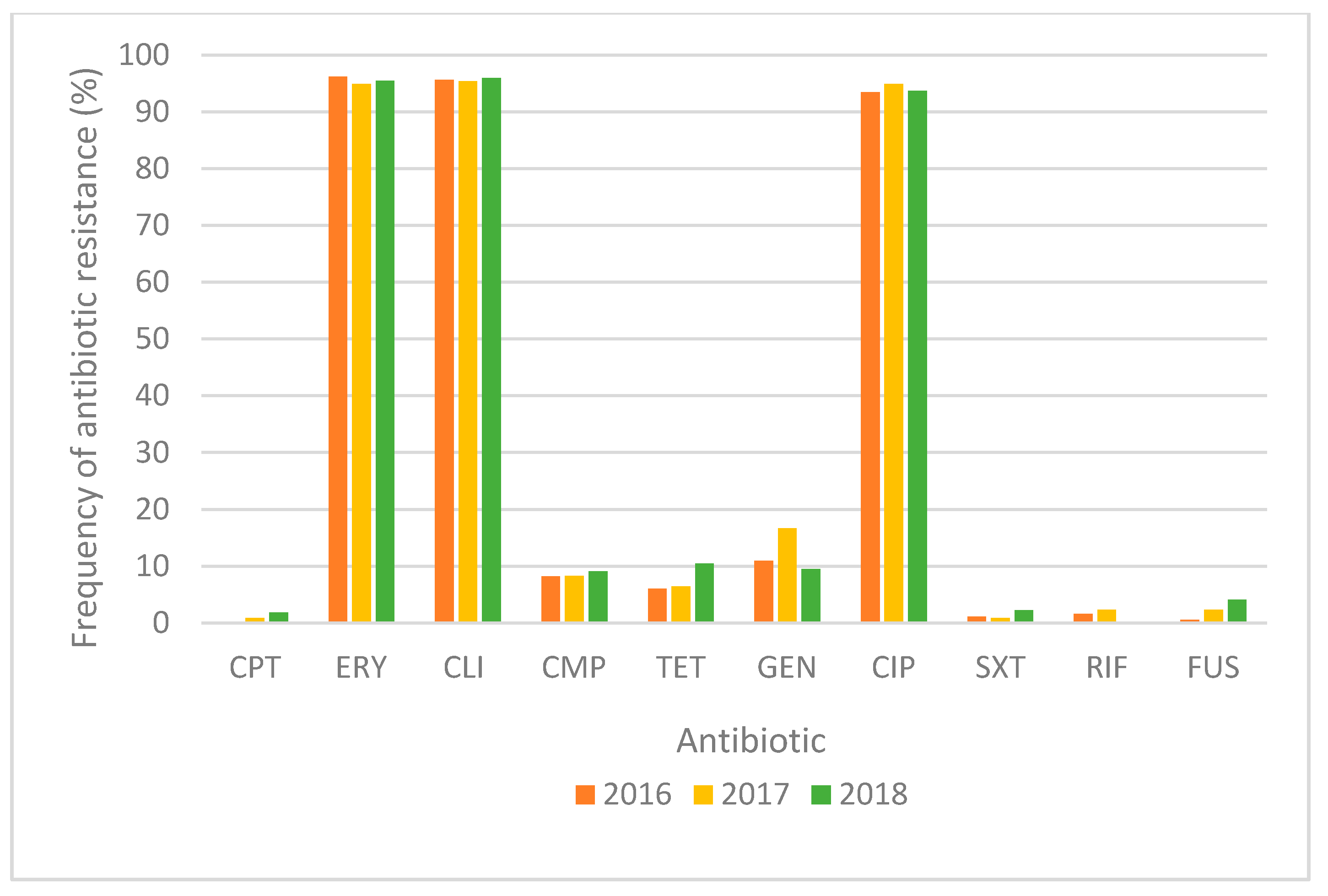
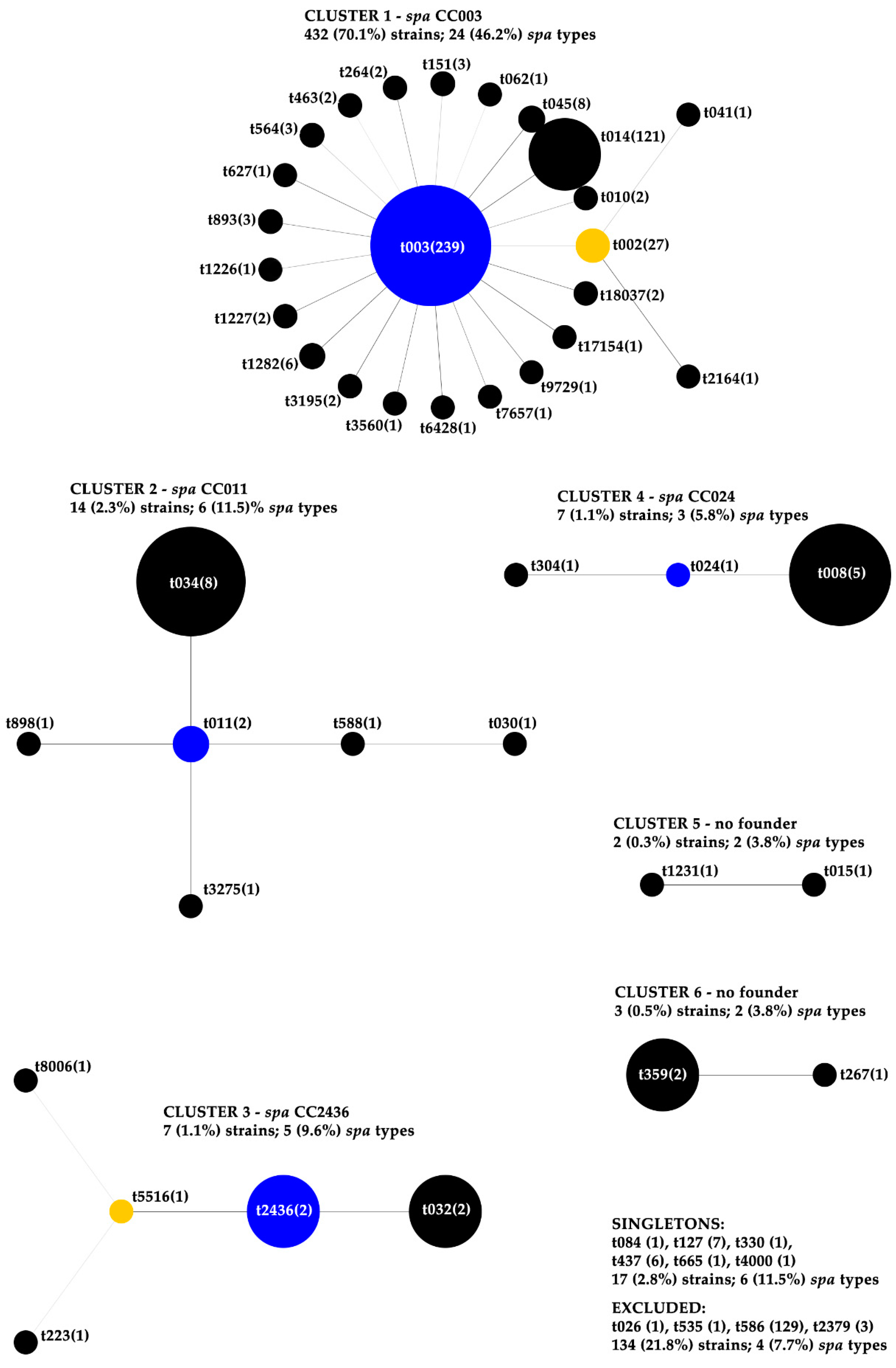
| Cluster | spa CC | No. (%) of Strains 1 | No. (%) of Strains Resistant to Antibiotics 2 | No. (%) of MDR Strains | No. of Resistant ATB (Mean Value) 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CPT | ERY | CLI | CMP | TET | GEN | CIP | SXT | RIF | FUS | |||||
| 1 | 003 | 432 (70.1) | 5 (1.2) | 426 (98.6) | 426 (98.6) | 36 (8.3) | 10 (2.3) | 35 (8.1) | 428 (99.1) | 6 (1.4) | 8 (1.9) | 10 (2.3) | 427 (98.8) | 4.2 |
| 2 | 011 | 14 (2.3) | 0 | 7 (50.0) | 12 (85.7) | 0 | 13 (92.9) | 2 (14.3) | 6 (42.9) | 1 (7.1) | 1 (7.1) | 0 | 14 (100) | 4.0 |
| 3 | 2436 | 7 (1.1) | 0 | 5 (71.4) | 5 (71.4) | 0 | 0 | 0 | 6 (85.7) | 0 | 0 | 0 | 5 (71.4) | 3.3 |
| 4 | 024 | 7 (1.1) | 0 | 5 (71.4) | 2 (28.6) | 0 | 0 | 2 (28.6) | 4 (57.1) | 0 | 0 | 1 (14.3) | 4 (57.1) | 3 |
| 5 | no founder | 2 (0.3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 6 | no founder | 3 (0.5) | 0 | 0 | 0 | 0 | 0 | 2 (66.7) | 0 | 0 | 0 | 1 (33.3) | 1 (33.3) | 2 |
| Strain | spa Type | spa Cluster | spa CC | MLST ST | MLST CC | SCCmec Type | Resistance to Antibiotics 1 |
|---|---|---|---|---|---|---|---|
| B0040949 | t003 | 1 | 003 | 225 | CC5 | II | CIP, GEN, ERY, CLI |
| B0041781 | t003 | 1 | 003 | 225 | CC5 | II | CMP, CIP, RIF, ERY, CLI |
| B0047063 | t003 | 1 | 003 | 225 | CC5 | II | CIP, ERY, CLI |
| B0034821 | t586 | excluded | excluded | 225 | CC5 | II | CMP, CIP, GEN, ERY, CLI, |
| B0038966 | t586 | excluded | excluded | 225 | CC5 | II | CIP, ERY, CLI |
| B0043165 | t586 | excluded | excluded | 225 | CC5 | II | CIP, GEN, ERY, CLI, TET |
| B0037533 | t014 | 1 | 003 | 225 | CC5 | II | CIP, GEN |
| B0040744 | t014 | 1 | 003 | 225 | CC5 | II | CIP, GEN, ERY, CLI |
| B0047366 | t014 | 1 | 003 | 225 | CC5 | II | CIP, ERY, CLI |
| B0037993 | t002 | 1 | 003 | 5 | CC5 | II | CMP, CIP, GEN, ERY, CLI |
| B0040837 | t002 | 1 | 003 | 5688 | CC5 | IV | - |
| B0042384 | t002 | 1 | 003 | 5 | CC5 | II | CIP, ERY, CLI |
| B0033841 | t535 | excluded | excluded | 225 | CC5 | II | CIP, ERY, CLI |
| B0039941 | t2379 | excluded | excluded | 225 | CC5 | II | CIP, ERY, CLI |
| B0040619 | t2379 | excluded | excluded | 225 | CC5 | II | CIP, ERY, CLI |
| B0047250 | t2379 | excluded | excluded | 225 | CC5 | II | CIP, ERY, CLI |
| B0038416 | t011 | 2 | 011 | 398 | CC398 | V | CIP, TET |
| B0046007 | t011 | 2 | 011 | 398 | CC398 | IV | CIP, GEN, SXT, TET |
| B0037087 | t034 | 2 | 011 | 398 | CC398 | V | CLI, TET |
| B0043212 | t034 | 2 | 011 | 398 | CC398 | V | CIP, CLI, TET |
| B0044843 | t034 | 2 | 011 | 398 | CC398 | V | ERY, CLI, TET |
| B0034866 | t032 | 3 | 2436 | 22 | CC22 | IV | CIP |
| B0039472 | t032 | 3 | 2436 | 22 | CC22 | IV | CIP, ERY, CLI |
| B0036602 | t2436 | 3 | 2436 | 22 | CC22 | IV | CIP, ERY, CLI |
| B0040230 | t2436 | 3 | 2436 | 22 | CC22 | IV | CIP, ERY, CLI |
| B0034699 | t008 | 4 | 024 | 8 | CC8 | nt | ERY |
| B0043674 | t008 | 4 | 024 | 8 | CC8 | IV | CIP, ERY |
| B0043848 | t008 | 4 | 024 | 8 | CC8 | IV | CIP, ERY, CLI |
| B0045550 | t4000 | singleton | singleton | 72 | CC8 | nt | GEN, FUS |
| B0043746 | t015 | 5 | no founder | 45 | CC45 | IV | - |
| B0044462 | t1231 | 5 | no founder | 45 | CC45 | IV | - |
| B0032812 | t026 | excluded | excluded | 45 | CC45 | IV | - |
| B0033429 | t330 | singleton | singleton | 45 | CC45 | IV | ERY, CLI |
| B0040776 | t267 | 6 | no founder | 97 | CC97 | V | GEN |
| B0037227 | t359 | 6 | no founder | 97 | CC97 | V | GEN, FUS |
| B0048151 | t359 | 6 | no founder | 97 | CC97 | IV | - |
| B0048051 | t084 | singleton | singleton | 1535 | CC15 | V | GEN, FUS, TET |
| B0042118 | t127 | singleton | singleton | 1 | CC1 | IV | ERY, CLI, TET |
| B0040853 | t437 | singleton | singleton | 59 | CC59 | VT | CMP, ERY, CLI, TET |
| B0033532 | t665 | singleton | singleton | 1472 | CC30 | IV | CIP, ERY, TET |
| Region | No. of Particip. Laboratories | No. of Isolates 1 | No. of spa Types | No. (%) of Isolates | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC5 | CC398 | CC22 | CC8 | CC45 | CC97 | Other CCs | ||||||||
| t003 | t586 | t014 | t002 | Other | ||||||||||
| Prague | 9 | 180 | 29 | 56 (31.1) | 46 (25.6) | 30 (16.7) | 6 (3.3) | 18 (10) | 4 (2.2) | 3 (1.7) | 6 (3.3) | 1 (0.6) | 2 (1.1) | 8 (4.4) |
| Central Bohemian | 4 | 40 | 8 | 7 (17.5) | 10 (25) | 8 (20) | 10 (25) | 3 (7.5) | 1 (2.5) | 0 | 0 | 0 | 0 | 1 (2.5) |
| South Bohemian | 4 | 84 | 9 | 23 (27.4) | 48 (57.1) | 6 (7.1) | 0 | 6 (7.1) | 0 | 0 | 0 | 1 (1.2) | 0 | 0 |
| Pilsen | 2 | 76 | 15 | 31 (40.8) | 3 (3.9) | 28 (36.8) | 1 (1.3) | 5 (6.6) | 0 | 3 (3.9) | 1 (1.3) | 1 (1.3) | 0 | 3 (3.9) |
| Karlovy Vary | 1 | 25 | 7 | 16 (64) | 3 (12) | 2 (8) | 0 | 1 (4) | 2 (8) | 1 (4) | 0 | 0 | 0 | 0 |
| Usti nad Labem | 2 | 28 | 7 | 18 (64.2) | 1 (3.6) | 5 (17.8) | 0 | 2 (7.1) | 1 (3.6) | 0 | 0 | 0 | 0 | 1 (3.6) |
| Liberec | 1 | 13 | 5 | 2 (15.4) | 5 (38.5) | 3 (23) | 1 (7.7) | 2 (15.4) | 0 | 0 | 0 | 0 | 0 | 0 |
| Hradec Kralove | 3 | 40 | 9 | 12 (30) | 0 | 15 (37.5) | 4 (10) | 5 (12.5) | 2 (5) | 0 | 0 | 1 (2.5) | 0 | 1 (2.5) |
| Pardubice | 3 | 33 | 8 | 5 (15.2) | 4 (12.1) | 18 (54.5) | 2 (6.06) | 3 (9.1) | 0 | 0 | 0 | 0 | 1 (3.0) | 0 |
| Vysocina | 4 | 32 | 5 | 18 (56.3) | 5 (15.6) | 6 (18.8) | 2 (6.2) | 0 | 1 (3.1) | 0 | 0 | 0 | 0 | 0 |
| South Moravian | 2 | 40 | 10 | 27 (67.5) | 4 (10.0) | 0 | 1 (2.5) | 3 (7.5) | 3 (7.5) | 0 | 0 | 0 | 0 | 2 (5.0) |
| Olomouc | 1 | 14 | 1 | 14 (100.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Zlin | 1 | 11 | 2 | 10 (90.9) | 0 | 0 | 0 | 1 (9.1) | 0 | 0 | 0 | 0 | 0 | 0 |
| Year of the Study | Reference | ||||
|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | |||
| No. of reported staphylococcal isolates | EARS-Net | 1887 | 1944 | 2244 | [39] |
| No. of MRSA (%) | 263 (13.9) | 257 (13.2) | 304 (13.6) | ||
| No. of single-patient MRSA strains sent to NRL for ATB from the laboratories participating in EARS-Net (% are calculated from the number of MRSA isolates reported in EARS-Net) | NRL for ATB | 182 (69.2) | 216 (84.0) | 220 (72.4) | this study |
| Number of participating laboratories in our study | 31 | 37 | 36 | ||
| Population sample representativeness | EARS-Net | high | high | high | [39] |
| Hospital sample representativeness | high | high | high | ||
| Isolate sample representativeness | high | high | high | ||
| Blood culture sets/1000 patient days | 18.0 | 18.0 | 17.0 | ||
| Estimated national population coverage (%) | 85 | 85 | 81 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pomorska, K.; Jakubu, V.; Malisova, L.; Fridrichova, M.; Musilek, M.; Zemlickova, H. Antibiotic Resistance, spa Typing and Clonal Analysis of Methicillin-Resistant Staphylococcus aureus (MRSA) Isolates from Blood of Patients Hospitalized in the Czech Republic. Antibiotics 2021, 10, 395. https://doi.org/10.3390/antibiotics10040395
Pomorska K, Jakubu V, Malisova L, Fridrichova M, Musilek M, Zemlickova H. Antibiotic Resistance, spa Typing and Clonal Analysis of Methicillin-Resistant Staphylococcus aureus (MRSA) Isolates from Blood of Patients Hospitalized in the Czech Republic. Antibiotics. 2021; 10(4):395. https://doi.org/10.3390/antibiotics10040395
Chicago/Turabian StylePomorska, Katarina, Vladislav Jakubu, Lucia Malisova, Marta Fridrichova, Martin Musilek, and Helena Zemlickova. 2021. "Antibiotic Resistance, spa Typing and Clonal Analysis of Methicillin-Resistant Staphylococcus aureus (MRSA) Isolates from Blood of Patients Hospitalized in the Czech Republic" Antibiotics 10, no. 4: 395. https://doi.org/10.3390/antibiotics10040395
APA StylePomorska, K., Jakubu, V., Malisova, L., Fridrichova, M., Musilek, M., & Zemlickova, H. (2021). Antibiotic Resistance, spa Typing and Clonal Analysis of Methicillin-Resistant Staphylococcus aureus (MRSA) Isolates from Blood of Patients Hospitalized in the Czech Republic. Antibiotics, 10(4), 395. https://doi.org/10.3390/antibiotics10040395







