Molecular Epidemiology of Carbapenem-Resistant Acinetobacter baumannii Isolates from Northern Africa and the Middle East
Abstract
:1. Introduction
2. Results
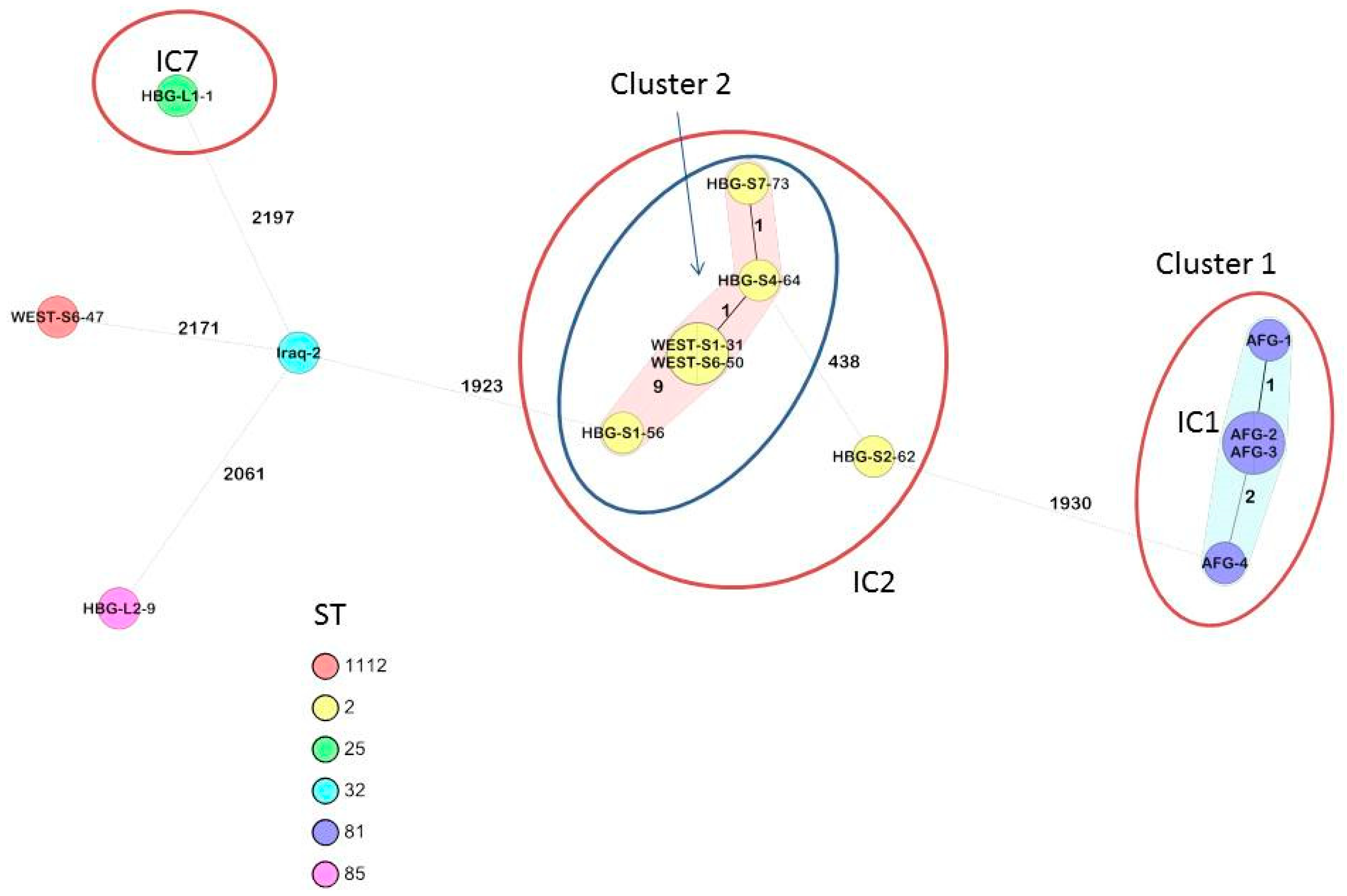
2.1. Confirmation on Species Level and Clustering with International Outbreak Strains Based on Core Genome Analysis
2.2. Identified Molecular Resistance Mechanisms and Comparison with Phenotypic Resistance Testing
3. Discussion
4. Materials and Methods
4.1. Patient Isolates
4.2. DNA Extraction and Whole Genome Sequencing
4.3. Molecular Epidemiology and Determination of Antibiotic Resistance Genes
4.4. Phenotypic Resistance Testing and Spectrometry-Based Discrimination
4.5. Ethical Clearance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Isolate WEST-S5-44 | ||||||||
| Pos. | Species | Strain | Domain | Phylum | Class | Order | Family | Z-Score |
| 1 | Acinetobacter radioresistens SH164 | SH164 | Bacteria | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | 0.99921 |
| 2 | Acinetobacter radioresistens NIPH 2130 | NIPH 2130 | Bacteria | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | 0.99869 |
| 3 | Acinetobacter radioresistens DSM 6976 = NBRC 102413 = CIP 103788 | CIP 103788 | Bacteria | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | 0.99862 |
| 4 | Acinetobacter radioresistens SK82 | SK82 | Bacteria | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | 0.99842 |
| 5 | Acinetobacter radioresistens WC-A-157 | WC-A-157 | Bacteria | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | 0.99811 |
| 6 | Acinetobacter sp. 1461402 | 1461402 | Bacteria | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | 0.99798 |
| 7 | Acinetobacter sp. 230853 | 230853 | Bacteria | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | 0.99788 |
| 8 | Acinetobacter sp. 1239920 | 1239920 | Bacteria | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | 0.99774 |
| 9 | Acinetobacter sp. 263903-1 | 263903-1 | Bacteria | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | 0.99763 |
| 10 | Acinetobacter sp. 272263 | 272263 | Bacteria | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | 0.99756 |
| Isolate Iraq-1 | ||||||||
| Pos. | Species | Strain | Domain | Phylum | Class | Order | Family | Z-Score |
| 1 | Acinetobacter lactucae ABBL098 | ABBL098 | Bacteria | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | 0.99922 |
| 2 | Acinetobacter pittii ABBL016 | ABBL016 | Bacteria | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | 0.99922 |
| 3 | Acinetobacter lactucae NRRL B-41902 | NRRL B-41902 | Bacteria | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | 0.999 |
| 4 | Acinetobacter sp. 1542444 | 1542444 | Bacteria | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | 0.99894 |
| 5 | Acinetobacter pittii ABBL148 | ABBL148 | Bacteria | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | 0.99894 |
| 6 | Acinetobacter pittii null | null | Bacteria | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | 0.99892 |
| 7 | Acinetobacter pittii ABBL126 | ABBL126 | Bacteria | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | 0.99891 |
| 8 | Acinetobacter pittii ABBL019 | ABBL019 | Bacteria | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | 0.9989 |
| 9 | Acinetobacter pittii ABBL033 | ABBL033 | Bacteria | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | 0.9989 |
| 10 | Acinetobacter pittii ANC 4050 | ANC 4050 | Bacteria | Proteobacteria | Gammaproteobacteria | Pseudomonadales | Moraxellaceae | 0.99885 |
| Strain Number | Species | Imipenem | Meropenem | Amikacin | Gentamicin | Tobramycin | Ciprofloxacin | Cotrimoxazole | Colistin |
|---|---|---|---|---|---|---|---|---|---|
| Iraq-2 | A. baumannii | R (8) | I (8) | S (≤2) | R (8) | S (4) | R (≥4) | R (160) | S (0.0625) |
| AFG-1 | A. baumannii | R (≥16) | R (≥16) | S (≤2) | S (4) | S (2) | R (≥4) | R (≥320) | S (0.5) |
| AFG-2 | A. baumannii | R (≥16) | R (≥16) | S (≤2) | S (4) | S (≤1) | R (≥4) | R (≥320) | S (0.25) |
| AFG-3 | A. baumannii | R (8) | I (8) | S (8) | S (4) | S (2) | R (≥4) | R (≥320) | S (2) |
| AFG-4 | A. baumannii | R (8) | R (≥16) | S (≤2) | S (4) | S (≤1) | R (≥4) | R (≥320) | S (1) |
| HBG-L1-1 | A. baumannii | R (≥16) | R (≥16) | S (≤2) | R (≥16) | R (≥16) | R (≥4) | R (≥320) | S (0.5) |
| HBG-L2-9 | A. baumannii | R (≥16) | I (8) | S (≤2) | R (8) | R (8) | R (≥4) | R (≥320) | S (0.5) |
| WEST-S6-47 | A. baumannii | I (1) | I (4) | R (≥64) | R (8) | R (8) | R (≥4) | R (≥320) | S (1) |
| WEST-S1-31 | A. baumannii | R (≥16) | R (≥16) | S (4) | R (≥16) | R (≥16) | R (≥4) | R (≥320) | S (0.5) |
| WEST-S6-50 | A. baumannii | R (≥16) | R (≥16) | S (4) | R (≥16) | R (≥16) | R (≥4) | R (≥320) | S (1) |
| HBG-S4-64 | A. baumannii | R (≥16) | R (≥16) | S (8) | R (≥16) | R (≥16) | R (≥4) | S (≤20) | S (0.25) |
| HBG-S1-56 | A. baumannii | R (≥16) | R (≥16) | S (≤2) | S (4) | S (≤1) | R (≥4) | S (≤20) | S (0.125) |
| HBG-S7-73 | A. baumannii | R (≥16) | R (≥16) | S (4) | R (≥16) | R (≥16) | R (≥4) | S (≤20) | S (0.25) |
| HBG-S2-62 | A. baumannii | R (≥16) | R (≥16) | R (16) | R (≥16) | R (≥16) | R (≥4) | R (≥320) | S (1) |
| WEST-S5-44 | A. radioresistens | S (≤0.25) | S (≤0.25) | S (≤2) | S (≤1) | S (≤1) | I (≤0.25) | S (≤20) | S (0.5) |
| Iraq-1 | A. dijkshoorniae | S (≤0.25) | I (4) | S (≤2) | S (≤1) | S (≤1) | I (0.5) | S (≤20) | S (0.125) |
References
- Davis, K.A.; Moran, K.A.; McAllister, C.K.; Gray, P.J. Multidrug-resistant Acinetobacter extremity infections in soldiers. Emerg. Infect. Dis. 2005, 11, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, M.K. Acinetobacter in modern warfare. Int. J. Antimicrob. Agents 2012, 39, 363–375. [Google Scholar] [CrossRef]
- Turton, J.F.; Kaufmann, M.E.; Gill, M.J.; Pike, R.; Scott, P.T.; Fishbain, J.; Craft, D.; Deye, G.; Riddell, S.; Lindler, L.E.; et al. Comparison of Acinetobacter baumannii isolates from the United Kingdom and the United States that were associated with repatriated casualties of the Iraq conflict. J. Clin. Microbiol. 2006, 44, 2630–2634. [Google Scholar] [CrossRef] [Green Version]
- Hujer, K.M.; Hujer, A.M.; Hulten, E.A.; Bajaksouzian, S.; Adams, J.M.; Donskey, C.J.; Ecker, D.J.; Massire, C.; Eshoo, M.W.; Sampath, R.; et al. Analysis of antibiotic resistance genes in multidrug-resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob. Agents Chemother. 2006, 50, 4114–4123. [Google Scholar] [CrossRef] [Green Version]
- Griffith, M.E.; Lazarus, D.R.; Mann, P.B.; Boger, J.A.; Hospenthal, D.R.; Murray, C.K. Acinetobacter skin carriage among US army soldiers deployed in Iraq. Infect. Control Hosp. Epidemiol. 2007, 28, 720–722. [Google Scholar] [CrossRef] [PubMed]
- Whitman, T.J.; Qasba, S.S.; Timpone, J.G.; Babel, B.S.; Kasper, M.R.; English, J.F.; Sanders, J.W.; Hujer, K.M.; Hujer, A.M.; Endimiani, A.; et al. Occupational transmission of Acinetobacter baumannii from a United States serviceman wounded in Iraq to a health care worker. Clin. Infect. Dis. 2008, 47, 439–443. [Google Scholar] [CrossRef] [Green Version]
- Murray, C.K.; Yun, H.C.; Griffith, M.E.; Thompson, B.; Crouch, H.K.; Monson, L.S.; Aldous, W.K.; Mende, K.; Hospenthal, D.R. Recovery of multidrug-resistant bacteria from combat personnel evacuated from Iraq and Afghanistan at a single military treatment facility. Mil. Med. 2009, 174, 598–604. [Google Scholar] [CrossRef] [Green Version]
- Sensenig, R.A.; Murray, C.K.; Mende, K.; Wolf, S.E.; Chung, K.K.; Hospenthal, D.R.; Yun, H.C. Longitudinal characterization of Acinetobacter baumannii-calcoaceticus complex, Klebsiella pneumoniae, and methicillin-resistant Staphylococcus aureus colonizing and infecting combat casualties. Am. J. Infect. Control 2012, 40, 183–185. [Google Scholar] [CrossRef]
- Hospenthal, D.R.; Crouch, H.K.; English, J.F.; Leach, F.; Pool, J.; Conger, N.G.; Whitman, T.J.; Wortmann, G.W.; Robertson, J.L.; Murray, C.K. Multidrug-resistant bacterial colonization of combat-injured personnel at admission to medical centers after evacuation from Afghanistan and Iraq. J. Trauma 2011, 71 (Suppl. 1), S52–S57. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.Z.; Chahine, M.A.; Frye, J.G.; Cash, D.M.; Lesho, E.P.; Craft, D.W.; Lindler, L.E.; Nikolich, M.P. Molecular analysis of imipenem-resistant Acinetobacter baumannii isolated from US service members wounded in Iraq, 2003–2008. Epidemiol. Infect. 2012, 140, 2302–2307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, W.R.; Li, P.; Whitman, T.J.; Blyth, D.M.; Schnaubelt, E.R.; Mende, K.; Tribble, D.R. Multi-Drug-Resistant Gram-Negative Infections in Deployment-Related Trauma Patients. Surg. Infect. 2017, 18, 357–367. [Google Scholar] [CrossRef] [Green Version]
- Al-Kadmy, I.M.S.; Ali, A.N.M.; Salman, I.M.A.; Khazaal, S.S. Molecular characterization of Acinetobacter baumannii isolated from Iraqi hospital environment. New Microbes New Infect. 2017, 21, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Weintrob, A.C.; Murray, C.K.; Xu, J.; Krauss, M.; Bradley, W.; Warkentien, T.E.; Lloyd, B.A.; Tribble, D.R. Early Infections Complicating the Care of Combat Casualties from Iraq and Afghanistan. Surg. Infect. 2018, 19, 286–297. [Google Scholar] [CrossRef]
- Ganjo, A.R.; Maghdid, D.M.; Mansoor, I.Y.; Kok, D.J.; Severin, J.A.; Verbrugh, H.A.; Kreft, D.; Fatah, M.H.; Alnakshabandi, A.A.; Dlnya, A.; et al. OXA-Carbapenemases Present in Clinical Acinetobacter baumannii-calcoaceticus Complex Isolates from Patients in Kurdistan Region, Iraq. Microb. Drug. Resist. 2016, 22, 627–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fily, F.; Ronat, J.B.; Malou, N.; Kanapathipillai, R.; Seguin, C.; Hussein, N.; Fakhri, R.M.; Langendorf, C. Post-traumatic osteomyelitis in Middle East war-wounded civilians: Resistance to first-line antibiotics in selected bacteria over the decade 2006–2016. BMC Infect. Dis. 2019, 19, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, P.A.; Khider, A.K. Correlation of biofilm formation and antibiotic resistance among clinical and soil isolates of Acinetobacter baumannii in Iraq. Acta Microbiol. Immunol. Hung. 2019, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gaiarsa, S.; Batisti Biffignandi, G.; Esposito, E.P.; Castelli, M.; Jolley, K.A.; Brisse, S.; Sassera, D.; Zarrilli, R. Comparative Analysis of the Two Acinetobacter baumannii Multilocus Sequence Typing (MLST) Schemes. Front. Microbiol. 2019, 10, 930. [Google Scholar] [CrossRef] [Green Version]
- Yun, H.C.; Branstetter, J.G.; Murray, C.K. Osteomyelitis in military personnel wounded in Iraq and Afghanistan. J. Trauma 2008, 64 (Suppl. 2), S163–S168. [Google Scholar] [CrossRef] [Green Version]
- Calhoun, J.H.; Murray, C.K.; Manring, M.M. Multidrug-resistant organisms in military wounds from Iraq and Afghanistan. Clin. Orthop. Relat. Res. 2008, 466, 1356–1362. [Google Scholar] [CrossRef] [Green Version]
- Stuart, T.L.; Mulvey, M.; Simor, A.E.; Tien, H.C.; Battad, A.; Taylor, G.; Vayalumkal, J.V.; Weir, C.; Ofner, M.; Gravel, D.; et al. Acinetobacter baumannii in casualties returning from Afghanistan. Can. J. Infect. Control 2007, 22, 152–154. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Acinetobacter baumannii infections among patients at military medical facilities treating injured U.S. service members, 2002–2004. MMWR Morb. Mortal. Wkly. Rep. 2004, 53, 1063–1066. [Google Scholar]
- Hamzeh, A.R.; Al Najjar, M.; Mahfoud, M. Prevalence of antibiotic resistance among Acinetobacter baumannii isolates from Aleppo, Syria. Am. J. Infect. Control 2012, 40, 776–777. [Google Scholar] [CrossRef] [PubMed]
- Rafei, R.; Dabboussi, F.; Hamze, M.; Eveillard, M.; Lemarié, C.; Mallat, H.; Rolain, J.M.; Joly-Guillou, M.L.; Kempf, M. First report of blaNDM-1-producing Acinetobacter baumannii isolated in Lebanon from civilians wounded during the Syrian war. Int. J. Infect. Dis. 2014, 21, 21–23. [Google Scholar] [CrossRef] [Green Version]
- Heydari, F.; Mammina, C.; Koksal, F. NDM-1-producing Acinetobacter baumannii ST85 now in Turkey, including one isolate from a Syrian refugee. J. Med. Microbiol. 2015, 64, 1027–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angeletti, S.; Ceccarelli, G.; Vita, S.; Dicuonzo, G.; Lopalco, M.; Dedej, E.; Blasi, A.; Antonelli, F.; Conti, A.; De Cesaris, M.; et al. Sanitary Bureau of Asylum Seekers Center of Castelnuovo di Porto. Unusual microorganisms and antimicrobial resistances in a group of Syrian migrants: Sentinel surveillance data from an asylum seekers centre in Italy. Travel Med. Infect. Dis. 2016, 14, 115–122. [Google Scholar] [CrossRef]
- Salloum, T.; Tannous, E.; Alousi, S.; Arabaghian, H.; Rafei, R.; Hamze, M.; Tokajian, S. Genomic mapping of ST85 blaNDM-1 and blaOXA-94 producing Acinetobacter baumannii isolates from Syrian Civil War Victims. Int. J. Infect. Dis. 2018, 74, 100–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammerum, A.M.; Larsen, A.R.; Hansen, F.; Justesen, U.S.; Friis-Møller, A.; Lemming, L.E.; Fuursted, K.; Littauer, P.; Schønning, K.; Gahrn-Hansen, B.; et al. Patients transferred from Libya to Denmark carried OXA-48-producing Klebsiella pneumoniae, NDM-1-producing Acinetobacter baumannii and meticillin-resistant Staphylococcus aureus. Int. J. Antimicrob. Agents 2012, 40, 191–192. [Google Scholar] [CrossRef]
- Dau, A.A.; Tloba, S.; Daw, M.A. Characterization of wound infections among patients injured during the 2011 Libyan conflict. East. Mediterr. Health J. 2013, 19, 356–361. [Google Scholar] [CrossRef]
- Mathlouthi, N.; Areig, Z.; Al Bayssari, C.; Bakour, S.; Ali El Salabi, A.; Ben Gwierif, S.; Zorgani, A.A.; Ben Slama, K.; Chouchani, C.; Rolain, J.M. Emergence of Carbapenem-Resistant Pseudomonas aeruginosa and Acinetobacter baumannii Clinical Isolates Collected from Some Libyan Hospitals. Microb. Drug. Resist. 2015, 21, 335–341. [Google Scholar] [CrossRef]
- Mathlouthi, N.; El Salabi, A.A.; Ben Jomàa-Jemili, M.; Bakour, S.; Al-Bayssari, C.; Zorgani, A.A.; Kraiema, A.; Elahmer, O.; Okdah, L.; Rolain, J.M.; et al. Early detection of metallo-β-lactamase NDM-1- and OXA-23 carbapenemase-producing Acinetobacter baumannii in Libyan hospitals. Int. J. Antimicrob. Agents 2016, 48, 46–50. [Google Scholar] [CrossRef]
- Kraiem, A.G.; Zorgani, A.; Elahmer, O.; El Salabi, A.A.; Ghenghesh, K.S. Carbapenem-resistant gram-negative bacilli in Tripoli, Libya. Am. J. Infect. Control 2016, 44, 1192–1194. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, N.; Ahmed, M.O.; Elramalli, A.K.; Daw, M.A.; Poirel, L.; Álvarez, R.; Nordmann, P. Colistin-resistant carbapenemase-producing isolates among Klebsiella spp. and Acinetobacter baumannii in Tripoli, Libya. J. Glob. Antimicrob. Resist. 2018, 13, 37–39. [Google Scholar] [CrossRef]
- Frickmann, H.; Köller, T.; Hagen, R.M.; Ebert, K.P.; Müller, M.; Wenzel, W.; Gatzer, R.; Schotte, U.; Binder, A.; Skusa, R.; et al. Molecular Epidemiology of Multidrug-Resistant Bacteria Isolated from Libyan and Syrian Patients with War Injuries in Two Bundeswehr Hospitals in Germany. Eur. J. Microbiol. Immunol. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Higgins, P.G.; Prior, K.; Harmsen, D.; Seifert, H. Development and evaluation of a core genome multilocus typing scheme for whole-genome sequence-based typing of Acinetobacter baumannii. PLoS ONE 2017, 12, e0179228. [Google Scholar] [CrossRef] [Green Version]
- Richter, M.; Rosselló-Móra, R.; Oliver Glöckner, F.; Peplies, J. JSpeciesWS: A web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics 2016, 32, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Zarrilli, R.; Pournaras, S.; Giannouli, M.; Tsakris, A. Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int. J. Antimicrob. Agents 2013, 41, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Wisplinghoff, H.; Hippler, C.; Bartual, S.G.; Haefs, C.; Stefanik, D.; Higgins, P.G.; Seifert, H. Molecular epidemiology of clinical Acinetobacter baumannii and Acinetobacter genomic species 13TU isolates using a multilocus sequencing typing scheme. Clin. Microbiol. Infect. 2008, 14, 708–715. [Google Scholar] [CrossRef] [Green Version]
- Diancourt, L.; Passet, V.; Nemec, A.; Dijkshoorn, L.; Brisse, S. The population structure of Acinetobacter baumannii: Expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS ONE 2010, 5, e10034. [Google Scholar] [CrossRef] [Green Version]
- Frickmann, H.; Essig, A.; Hagen, R.M.; Riecker, M.; Jerke, K.; Ellison, D.; Poppert, S. Rapid identification of Acinetobacter spp. by fluorescence in situ hybridization (FISH) from colony and blood culture material. Eur. J. Microbiol. Immunol. 2011, 1, 289–296. [Google Scholar] [CrossRef] [Green Version]
- Higgins, P.G.; Hagen, R.M.; Podbielski, A.; Frickmann, H.; Warnke, P. Molecular Epidemiology of Carbapenem-Resistant Acinetobacter baumannii Isolated from War-Injured Patients from the Eastern Ukraine. Antibiotics 2020, 9, 579. [Google Scholar] [CrossRef] [PubMed]
- Granzer, H.; Hagen, R.M.; Warnke, P.; Bock, W.; Baumann, T.; Schwarz, N.G.; Podbielski, A.; Frickmann, H.; Koeller, T. Molecular Epidemiology of Carbapenem-Resistant Acinetobacter Baumannii Complex Isolates from Patients that were Injured During the Eastern Ukrainian Conflict. Eur. J. Microbiol. Immunol. 2016, 6, 109–117. [Google Scholar] [CrossRef]
- Ruppitsch, W.; Pietzka, A.; Prior, K.; Bletz, S.; Fernandez, H.L.; Allerberger, F.; Harmsen, D.; Mellmann, A. Defining and Evaluating a Core Genome Multilocus Sequence Typing Scheme for Whole-Genome Sequence-Based Typing of Listeria monocytogenes. J. Clin. Microbiol. 2015, 53, 2869–2876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, J.; Zander, E.; Stefanik, D.; Higgins, P.G.; Roca, I.; Vila, J.; McConnell, M.J.; Cisneros, J.M.; Seifert, H.; MagicBullet Working Group WP4. High incidence of pandrug-resistant Acinetobacter baumannii isolates collected from patients with ventilator-associated pneumonia in Greece, Italy and Spain as part of the MagicBullet clinical trial. J. Antimicrob. Chemother. 2017, 72, 3277–3282. [Google Scholar] [CrossRef]
- Higgins, P.G.; Wisplinghoff, H.; Krut, O.; Seifert, H. A PCR-based method to differentiate between Acinetobacter baumannii and Acinetobacter genomic species 13TU. Clin. Microbiol. Infect. 2007, 13, 1199–1201. [Google Scholar] [CrossRef] [Green Version]
- Higgins, P.G.; Lehmann, M.; Wisplinghoff, H.; Seifert, H. gyrB multiplex PCR to differentiate between Acinetobacter calcoaceticus and Acinetobacter genomic species 3. J. Clin. Microbiol. 2010, 48, 4592–4594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, P.G.; Pérez-Llarena, F.J.; Zander, E.; Fernández, A.; Bou, G.; Seifert, H. OXA-235, a novel class D β-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2013, 57, 2121–2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerezales, M.; Biniossek, L.; Gerson, S.; Xanthopoulou, K.; Wille, J.; Wohlfarth, E.; Kaase, M.; Seifert, H.; Higgins, P.G. Novel multiplex PCRs for detection of the most prevalent carbapenemase genes in Gram-negative bacteria within Germany. J. Med. Microbiol. 2021. [Google Scholar] [CrossRef]
- Kleinheinz, K.A.; Joensen, K.G.; Larsen, M.V. Applying the ResFinder and VirulenceFinder web-services for easy identification of acquired antibiotic resistance and E. coli virulence genes in bacteriophage and prophage nucleotide sequences. Bacteriophage 2014, 4, e27943. [Google Scholar] [CrossRef] [Green Version]
- Zankari, E. Comparison of the web tools ARG-ANNOT and ResFinder for detection of resistance genes in bacteria. Antimicrob. Agents Chemother. 2014, 58, 4986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Sample, Species, Country of Origin, Year of Isolation | MLST | Clonal Lineage | Antibiotic Resistance Determinants | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| STox | STpas | Sulfonamide | Phenicol | Beta-Lactam | Aminoglycoside | Macrolide | Tetracycline | Trimethoprim | Fluoroquinolone and Aminoglycoside | ||
| Iraq-2, A. baumannii, Iraq, 2010 | 1627 | 32 | n.a. | sul2-like | blaADC-25-like, blaOXA-100, blaOXA-58 | aadB-like | |||||
| AFG-1, A. baumannii, Afghanistan, 2008 | 498 | 81 | IC1 | sul2 | blaADC-25-like, blaOXA-23, blaOXA-69 | aadB-like, aph(3′)-Ia | |||||
| AFG-2, A. baumannii, Afghanistan, 2008 | 498 | 81 | IC1 | sul2 | blaADC-25-like, blaOXA-23, blaOXA-69 | aadB-like, aph(3′)-Ia | |||||
| AFG-3, A. baumannii, Afghanistan, 2008 | 498 | 81 | IC1 | sul2 | blaADC-25-like, blaOXA-23, blaOXA-69 | aadB-like, aph(3′)-Ia, aph(3′)-VIa-like | |||||
| AFG-4, A. baumannii, Afghanistan, 2008 | 498 | 81 | IC1 | sul2 | blaADC-25-like, blaOXA-23, blaOXA-69 | aadB-like, aph(3′)-Ia | |||||
| HBG-L1-1, A. baumannii, Libya, 2011 | 440 | 25 | IC7 | sul2 | blaADC-25-like, blaOXA-23, blaOXA-64 | aadB-like, aph(3′)-Ic, strA, strB | |||||
| HBG-L2-9, A. baumannii, Libya, 2011 | 1089 | 85 | n.a. | sul2 | floR-like | blaADC-25-like,blaNDM-1, blaOXA-94 | aadB-like, aph(3′)-VIa-like | mph(E), msr(E) | |||
| WEST-S6-47, A. baumannii, Syria, 2013 | 2271 | 1112 | n.a. | sul1 | cmlA1-like | blaADC-25-like, blaGES-11, blaOXA-715 | aadA2, aadB, aph(3′)-VIa-like, strA-like, strB-like, aacA4-like | dfrA7 | aac(6′)Ib-cr-like | ||
| WEST-S1-31, A. baumannii, Syria, 2013 | 218 | 2 | IC2 | sul2 | blaADC-25-like, blaOXA-23, blaOXA-66, blaTEM-1D | aph(3′)-Ic, armA, strA, strB | mph(E), msr(E) | tet(B)-like | |||
| WEST-S6-50, A. baumannii, Syria, 2013 | 218 | 2 | IC2 | sul2 | blaADC-25-like, blaOXA-23, blaOXA-66, blaTEM-1D | aph(3′)-Ic, armA, strA, strB | mph(E), msr(E) | tet(B)-like | |||
| HBG-S4-64, A. baumannii, Syria, 2013 | 218 | 2 | IC2 | blaADC-25-like, blaOXA-23, blaOXA-66, blaTEM-1D | aph(3′)-Ic, armA, strA, strB | mph(E), msr(E) | tet(B)-like | ||||
| HBG-S1-56, A. baumannii, Syria, 2013 | 195 | 2 | IC2 | blaADC-25-like, blaOXA-23, blaOXA-66, blaTEM-1D | aph(3′)-Ic, strA, strB | tet(B)-like | |||||
| HBG-S7-73, A. baumannii, Syria, 2013 | 218 | 2 | IC2 | blaADC-25-like, blaOXA-23, blaOXA-66, blaTEM-1D | aph(3′)-Ic, armA, strA, strB | mph(E), msr(E) | tet(B)-like | ||||
| HBG-S2-62, A. baumannii, Syria, 2013 | 1114 | 2 | IC2 | sul1,sul2 | cmlA1-like | blaADC-25-like, blaGES-11, blaOXA-23, blaOXA-66, blaTEM-1D | aadA2, aadB-like, aph(3′)-VIa-like, strA-like, strB-like, aacA4-like | dfrA7 | aac(6′)Ib-cr-like | ||
| WEST-S5-44, A. radioresistens, 2013 | n.a. | n.a. | n.a. | blaOXA-815 | |||||||
| Iraq-1, A. dijkshoorniae, 2010 | 1605 | 1141 | n.a. | blaOXA-819 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higgins, P.G.; Hagen, R.M.; Kreikemeyer, B.; Warnke, P.; Podbielski, A.; Frickmann, H.; Loderstädt, U. Molecular Epidemiology of Carbapenem-Resistant Acinetobacter baumannii Isolates from Northern Africa and the Middle East. Antibiotics 2021, 10, 291. https://doi.org/10.3390/antibiotics10030291
Higgins PG, Hagen RM, Kreikemeyer B, Warnke P, Podbielski A, Frickmann H, Loderstädt U. Molecular Epidemiology of Carbapenem-Resistant Acinetobacter baumannii Isolates from Northern Africa and the Middle East. Antibiotics. 2021; 10(3):291. https://doi.org/10.3390/antibiotics10030291
Chicago/Turabian StyleHiggins, Paul G., Ralf Matthias Hagen, Bernd Kreikemeyer, Philipp Warnke, Andreas Podbielski, Hagen Frickmann, and Ulrike Loderstädt. 2021. "Molecular Epidemiology of Carbapenem-Resistant Acinetobacter baumannii Isolates from Northern Africa and the Middle East" Antibiotics 10, no. 3: 291. https://doi.org/10.3390/antibiotics10030291
APA StyleHiggins, P. G., Hagen, R. M., Kreikemeyer, B., Warnke, P., Podbielski, A., Frickmann, H., & Loderstädt, U. (2021). Molecular Epidemiology of Carbapenem-Resistant Acinetobacter baumannii Isolates from Northern Africa and the Middle East. Antibiotics, 10(3), 291. https://doi.org/10.3390/antibiotics10030291






