Antimicrobial Resistance in Common Respiratory Pathogens of Chronic Bronchiectasis Patients: A Literature Review
Abstract
1. Introduction
2. The Role of Pseudomonas aeruginosa and Other Microorganisms in Non-CF Bronchiectasis
3. Role of Antibiotic Therapy
3.1. Acute Exacerbation
3.2. Chronic Infection
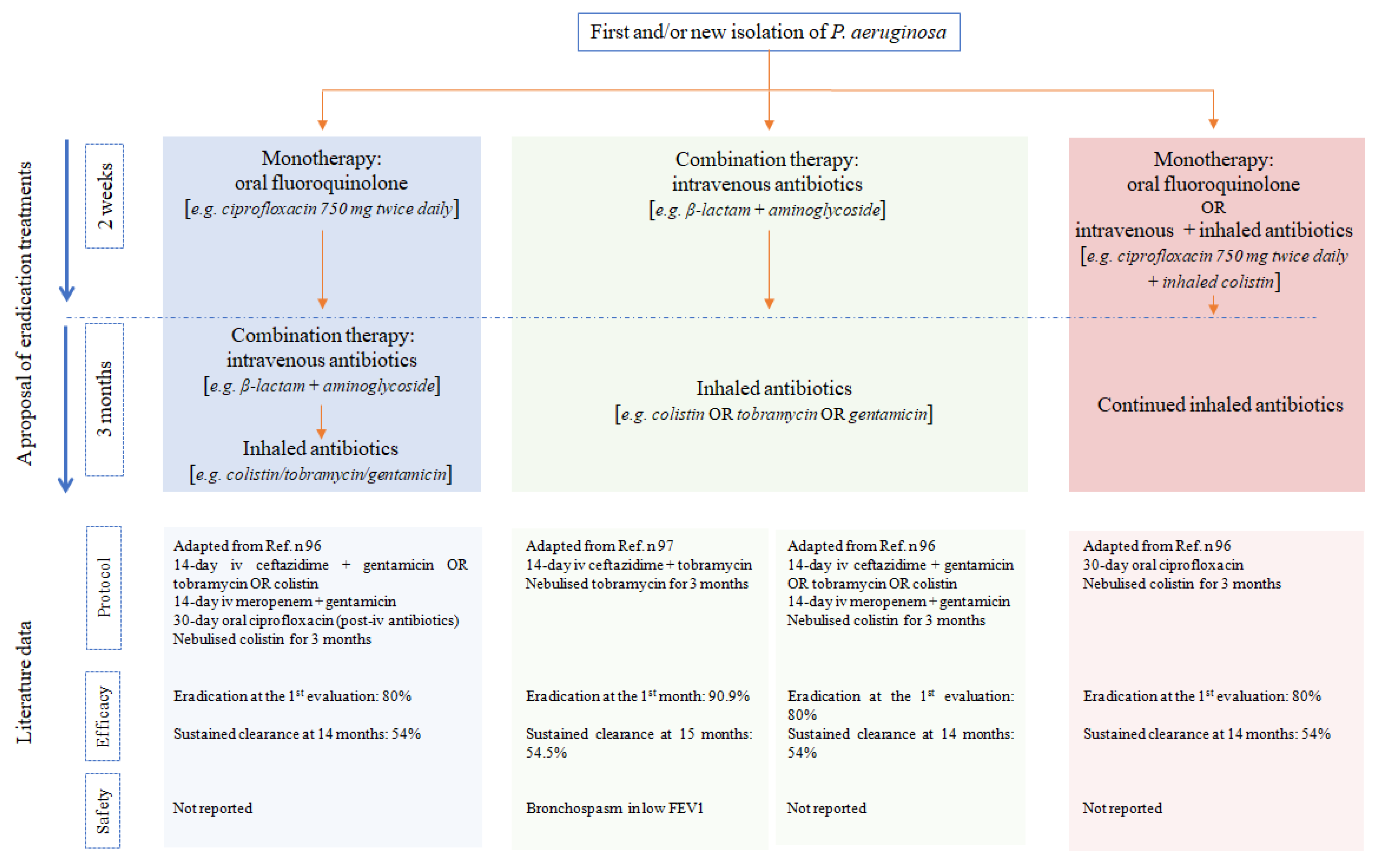
3.3. Eradication of Bronchial Infections
4. Antibiotic Resistance in Bronchiectasis
5. Five-Year View
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hill, A.T.; Sullivan, A.L.; Chalmers, J.D.; De Soyza, A.; Elborn, J.S.; Floto, R.A.; Grillo, L.; Gruffydd-Jones, K.; Harvey, A.; Haworth, C.S.; et al. British Thoracic Society Guideline for bronchiectasis in adults. Thorax 2019, 74 (Suppl. 1), 1–69. [Google Scholar] [CrossRef]
- Pasteur, M.C.; Bilton, D.; Hill, A.T. British Thoracic Society Bronchiectasis non-CF Guideline Group. British Thoracic Society guideline for non-CF bronchiectasis. Thorax 2010, 65 (Suppl. 1), i1–i58. [Google Scholar] [CrossRef]
- Cole, P.J. Inflammation: A two-edged sword—the model of bronchiectasis. Eur. J. Respir. Dis. Suppl. 1986, 147, 6–15. [Google Scholar] [PubMed]
- Bush, A.; Floto, R.A. Pathophysiology, causes and genetics of paediatric and adult bronchiectasis. Respirology 2019, 24, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Moffitt, K.L.; Suarez-Cuartin, G.; Sibila, O.; Finch, S.; Furrie, E.; Dicker, A.; Wrobel, K.; Elborn, J.S.; Walker, B.; et al. Neutrophil Elastase Activity Is Associated with Exacerbations and Lung Function Decline in Bronchiectasis. Am. J. Respir. Crit. Care Med. 2017, 195, 1384–1393. [Google Scholar] [CrossRef]
- Gramegna, A.; Amati, F.; Terranova, L.; Sotgiu, G.; Tarsia, P.; Miglietta, D.; Calderazzo, M.A.; Aliberti, S.; Blasi, F. Neutrophil elastase in bronchiectasis. Respir. Res. 2017, 18, 1–13. [Google Scholar] [CrossRef]
- Tan, H.-L.; Regamey, N.; Brown, S.; Bush, A.; Lloyd, C.M.; Davies, J.C. The Th17 Pathway in Cystic Fibrosis Lung Disease. Am. J. Respir. Crit. Care Med. 2011, 184, 252–258. [Google Scholar] [CrossRef]
- Hogg, J.C.; Chu, F.; Utokaparch, S.; Woods, R.; Elliott, W.M.; Buzatu, L.; Cherniack, R.M.; Rogers, R.M.; Sciurba, F.C.; Coxson, H.O.; et al. The nature of small-airway obstruction in chronic ob-structive pulmonary disease. N. Engl. J. Med. 2004, 350, 2645–2653. [Google Scholar] [CrossRef]
- Reid, L.M. Reduction in Bronchial Subdivision in Bronchiectasis. Thorax 1950, 5, 233–247. [Google Scholar] [CrossRef]
- Farrell, P.M.; White, T.B.; Ren, C.L.; Hempstead, S.E.; Accurso, F.; Derichs, N.; Howenstine, M.; McColley, S.A.; Rock, M.; Rosenfeld, M.; et al. Diagnosis of Cystic Fibrosis: Consensus Guide-lines from the Cystic Fibrosis Foundation. J. Pediatr. 2017, 181S, S4–S15.e1. [Google Scholar] [CrossRef] [PubMed]
- Wurzel, D.; Marchant, J.; Yerkovich, S.; Upham, J.; Petsky, H.; Smith-Vaughan, H.; Masters, B.; Buntain, H.; Chang, A. Protracted bacterial bronchitis in children: Natural history and risk factors for bronchiectasis. Paediatr. Respir. Infect. Immunol. 2015, 46, OA1994. [Google Scholar] [CrossRef]
- Welp, A.L.; Bomberger, J.M. Bacterial Community Interactions during Chronic Respiratory Disease. Front. Cell Infect. Microbiol. 2020. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7240048/ (accessed on 25 January 2021).
- Storch, I.; Sachar, D.; Katz, S. Pulmonary Manifestations of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2003, 9, 104–115. [Google Scholar] [CrossRef]
- Gómez Carrera, L.; Bonilla Hernan, G. Pulmonary manifestations of collagen diseases. Arch. Bronconeumol. 2013, 49, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Woodfield, G.; Nisbet, M.; Jacob, J.; Mok, W.; Loebinger, M.R.; Hansell, D.M.; Wells, A.U.; Wilson, R. Bronchiectasis in yellow nail syndrome. Respirology 2016, 22, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Mehr, S. The Immunological Investigation of a Child with Chronic Wet Cough. Paediatr. Respir. Rev. 2012, 13, 144–149. [Google Scholar] [CrossRef]
- Schussler, E.; Beasley, M.B.; Maglione, P.J. Lung Disease in Primary Antibody Deficiencies. J. Allergy Clin. Immunol. Pract. 2016, 4, 1039–1052. [Google Scholar] [CrossRef]
- Attia, E.F.; Miller, R.F.; Ferrand, R.A. Bronchiectasis and other chronic lung diseases in adolescents living with HIV. Curr. Opin. Infect. Dis. 2017, 30, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Calligaro, G.L.; Gray, D.M. Lung function abnormalities in HIV-infected adults and children. Respirology 2015, 20, 24–32. [Google Scholar] [CrossRef]
- José, R.J.; Periselneris, J.N.; Brown, J.S. Opportunistic bacterial, viral and fungal infections of the lung. Medicine 2020, 48, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Hoyo, I.; Sanclemente, G.; Cervera, C.; Cofán, F.; Ricart, M.J.; Perez-Villa, F.; Navasa, M.; Marcos, M.A.; de la Bellacasa, J.P.; Moreno, A.; et al. Opportunistic pulmonary infections in solid or-gan transplant recipients. Transplant. Proc. 2012, 44, 2673–2675. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Sethi, S.; Raman, D.S.V.; Behera, D. Eight-year study of allergic bronchopulmonary aspergillosis in an Indian teaching hospital. Mycoses 2002, 45, 295–299. [Google Scholar] [CrossRef]
- Agarwal, R.; Sehgal, I.S.; Dhooria, S.; Muthu, V.; Prasad, K.T.; Bal, A.; Aggarwal, A.N.; Chakrabarti, A. Allergic bronchopulmonary aspergillosis. Indian J. Med. Res. 2020, 151, 529–549. [Google Scholar] [CrossRef] [PubMed]
- Legendre, M.; Zaragosi, L.-E.; Mitchison, H.M. Motile cilia and airway disease. Semin. Cell Dev. Biol. 2021, 110, 19–33. [Google Scholar] [CrossRef]
- Pagin, A.; Sermet-Gaudelus, I.; Burgel, P.-R. Genetic diagnosis in practice: From cystic fibrosis to CFTR-related disorders. Archives de Pédiatrie 2020, 27 (Suppl. 1), eS25–eS29. [Google Scholar] [CrossRef]
- De L’Auscultation, M. ou Traité du Diagnostic des Maladies des Poumons et du Cœur, fondé principalement sur ce Nouveau Moyen d’Exploration. Edinb. Med. Surg. J. 1822, 18, 447–474. [Google Scholar]
- Noriega Aldave, A.P.; William Saliski, D.O. The clinical manifestations, diagnosis and management of williams-campbell syndrome. N. Am. J. Med. Sci. 2014, 6, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Krustins, E. Mounier-Kuhn syndrome: A systematic analysis of 128 cases published within last 25 years. Clin. Respir. J. 2016, 10, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Robitaille, G.A. Ehlers-Danlos Syndrome and Recurrent Hemoptysis. Ann. Intern. Med. 1964, 61, 716–721. [Google Scholar] [CrossRef]
- Zhang, S.-Q.; Xiong, X.-F.; Wu, Z.-H.; Huang, T.-T.; Cheng, D.-Y. Clinical features of asthma with comorbid bronchiectasis: A sys-tematic review and meta-analysis. Medicine 2021, 100, e23858. [Google Scholar] [CrossRef]
- Shi, L.; Wei, F.; Ma, T.; Zhou, W.; Li, M.; Wan, Y. Impact of Radiographic Bronchiectasis in COPD. Respir. Care 2020, 65, 1561–1573. [Google Scholar] [CrossRef]
- Carreto, L.; Morrison, M.; Donovan, J.; Finch, S.; Tan, G.L.; Fardon, T.; Wilson, R.; Furrie, E.; Loebinger, M.; Chalmers, J.D.; et al. Utility of routine screening for alpha-1 antitrypsin defi-ciency in patients with bronchiectasis. Thorax 2020, 75, 592–593. [Google Scholar] [CrossRef] [PubMed]
- Nicotra, M.B.; Rivera, M.; Dale, A.M.; Shepherd, R.; Carter, R. Clinical, Pathophysiologic, and Microbiologic Characterization of Bronchiectasis in an Aging Cohort. Chest 1995, 108, 955–961. [Google Scholar] [CrossRef]
- Pasteur, M.C.; Helliwell, S.M.; Houghton, S.J.; Webb, S.C.; Foweraker, J.E.; Coulden, R.A.; Flower, C.D.; Bilton, D.; Keogan, M.T. An Investigation into Causative Factors in Patients with Bronchiectasis. Am. J. Respir. Crit. Care Med. 2000, 162, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Aksamit, T.R.; O’Donnell, A.E.; Barker, A.; Olivier, K.N.; Winthrop, K.L.; Daniels, M.L.A.; Johnson, M.; Eden, E.; Griffith, D.; Knowles, M.; et al. Adult Patients with Bronchiectasis: A First Look at the US Bronchiectasis Research Registry. Chest 2017, 151, 982–992. [Google Scholar] [CrossRef]
- Izhakian, S.; Wasser, W.G.; Fuks, L.; Vainshelboim, B.; Fox, B.D.; Fruchter, O.; Kramer, M.R. Lobar distribution in non-cystic fibrosis bron-chiectasis predicts bacteriologic pathogen treatment. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Dimakou, K.; Triantafillidou, C.; Toumbis, M.; Tsikritsaki, K.; Malagari, K.; Bakakos, P. Non CF-bronchiectasis: Aetiologic approach, clinical, radiological, microbiological and functional profile in 277 patients. Respir. Med. 2016, 116, 1–7. [Google Scholar] [CrossRef]
- Menéndez, R.; Méndez, R.; Amara-Elori, I.; Reyes, S.; Montull, B.; Feced, L.; Alonso, R.; Amaro, R.; Alcaraz, V.; Fernandez-Barat, L.; et al. Systemic Inflammation during and after Bronchiectasis Exacerbations: Impact of Pseudomonas aeruginosa. J. Clin. Med. 2020, 9, 2631. [Google Scholar] [CrossRef]
- Finch, S.; McDonnell, M.J.; Abo-Leyah, H.; Aliberti, S.; Chalmers, J.D. A Comprehensive Analysis of the Impact of Pseudomonas aeruginosa Colonization on Prognosis in Adult Bronchiectasis. Ann. Am. Thorac. Soc. 2015, 12, 1602–1611. [Google Scholar] [CrossRef]
- Martinez-García, M.; Oscullo, G.; Posadas, T.; Zaldivar, E.; Villa, C.; Dobarganes, Y.; Girón, R.; Olveira, C.; Maíz, L.; García-Clemente, M.; et al. Pseudomonas aeruginosa and lung function decline in patients with bronchiectasis. Clin. Microbiol. Infect. 2021, 27, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Hilliam, Y.; Moore, M.P.; Lamont, I.L.; Bilton, D.; Haworth, C.S.; Foweraker, J.; Walshaw, M.J.; Williams, D.; Fothergill, J.L.; De Soyza, A.; et al. Pseudomonas aeruginosa adaptation and di-versification in the non-cystic fibrosis bronchiectasis lung. Eur. Respir. J. 2017, 49, 1602108. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, M.J.; Jary, H.R.; Perry, A.; MacFarlane, J.G.; Hester, K.L.; Small, T.; Molyneux, C.; Perry, J.D.; Walton, K.E.; De Soyza, A.; et al. Non cystic fibrosis bronchiectasis: A longitu-dinal retrospective observational cohort study of Pseudomonas persistence and resistance. Respiratory. Medicine 2015, 109, 716–726. [Google Scholar]
- Garcia-Clemente, M.; De La Rosa, D.; Máiz, L.; Girón, R.; Blanco, M.; Olveira, C.; Canton, R.; Martinez-García, M.A. Impact of Pseudomonas aeruginosa Infection on Patients with Chronic Inflammatory Airway Diseases. J. Clin. Med. 2020, 9, 3800. [Google Scholar] [CrossRef]
- King, P.T.; Holdsworth, S.R.; Freezer, N.J.; Villanueva, E.; Holmes, P.W. Microbiologic follow-up study in adult bronchiectasis. Respir. Med. 2007, 101, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Cabello, H.; Torres, A.; Celis, R.; El-Ebiary, M.; De La Bellacasa, J.P.; Xaubet, A.; González, J.; Agustí, C.; Soler, N. Bacterial colonization of distal airways in healthy subjects and chronic lung disease: A bronchoscopic study. Eur. Respir. J. 1997, 10, 1137–1144. [Google Scholar] [CrossRef]
- Karadag, B.; Karakoc, F.; Ersu, R.; Kut, A.; Bakac, S.; Dagli, E. Non-Cystic-Fibrosis Bronchiectasis in Children: A Persisting Problem in Developing Countries. Respiration 2005, 72, 233–238. [Google Scholar] [CrossRef]
- Edwards, E.A.; Asher, M.I.; Byrnes, C.A. Paediatric bronchiectasis in the twenty-first century: Experience of a tertiary children’s hospital in New Zealand. J. Paediatr. Child Health 2003, 39, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Marra, R.; Sgalla, G.; Richeldi, L.; Conte, E.G.; Hill, A.T. Role of Stenotrophomonas maltophilia isolation in patients with non-CF bronchiectasis. QJM Int. J. Med. 2020, 113, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Karaiskos, I.; Giamarellou, H. Multidrug-resistant and extensively drug-resistant Gram-negative pathogens: Current and emerging therapeutic approaches. Expert Opin. Pharmacother. 2014, 15, 1351–1370. [Google Scholar] [CrossRef] [PubMed]
- Klingman, K.L.; Pye, A.; Murphy, T.F.; Hill, S.L. Dynamics of respiratory tract colonization by Branhamella catarrhalis in bron-chiectasis. Am. J. Respir. Crit. Care Med. 1995, 152, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Swenson, C.E.; Sadikot, R.T. Achromobacter Respiratory Infections. Ann. Am. Thorac. Soc. 2015, 12, 252–258. [Google Scholar] [CrossRef]
- Bopaka, R.G.; El Khattabi, W.; Janah, H.; Jabri, H.; Afif, H. Bronchiectasis: A bacteriological profile. Pan Afr. Med. J. 2015, 22, 378. [Google Scholar]
- Wang, J.Y.; Hsueh, P.R.; Wang, J.T.; Lee, L.N.; Yang, P.C.; Luh, K.T. Recurrent infections and chronic colonization by an Escherichia coli clone in the respiratory tract of a patient with severe cystic bronchiectasis. J. Clin. Microbiol. 2000, 38, 2766–2767. [Google Scholar] [CrossRef] [PubMed]
- Somayaji, R.; Ramos, K.J.; Hoffman, L.R. Understanding the Role of Staphylococcus aureus in Non–Cystic Fibrosis Bronchiecta-sis: Where Are We Now? Ann. Am. Thorac. Soc. 2018, 15, 310–311. [Google Scholar] [CrossRef]
- Miao, X.-Y.; Ji, X.-B.; Lu, H.-W.; Yang, J.-W.; Xu, J.-F. Distribution of Major Pathogens from Sputum and Bronchoalveolar Lavage Fluid in Patients with Noncystic Fibrosis Bronchiectasis: A Systematic Review. Chin. Med. J. 2015, 128, 2792–2797. [Google Scholar] [CrossRef]
- Hare, K.M.; Leach, A.J.; Smith-Vaughan, H.C.; Chang, A.B.; Grimwood, K. Streptococcus pneumoniae and chronic endobronchial infections in childhood. Pediatr. Pulmonol. 2017, 52, 1532–1545. [Google Scholar] [CrossRef]
- To, K.; Cao, R.; Yegiazaryan, A.; Owens, J.; Venketaraman, V. General Overview of Nontuberculous Mycobacteria Opportunistic Pathogens: Mycobacterium avium and Mycobacterium abscessus. J. Clin. Med. 2020, 9, 2541. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, I.S.; Dhooria, S.; Prasad, K.T.; Muthu, V.; Aggarwal, A.N.; Rawat, A.; Pal, A.; Bal, A.; Garg, M.; Chakrabarti, A.; et al. Sensitization to A fumigatus in subjects with non-cystic fibrosis bronchiectasis. Mycoses 2021, 64, 412–419. [Google Scholar] [CrossRef]
- Cuthbertson, L.; Felton, I.; James, P.; Cox, M.J.; Bilton, D.; Schelenz, S.; Loebinger, M.R.; Cookson, W.O.; Simmonds, N.J.; Moffatt, M.F. The fungal airway microbiome in cystic fibrosis and non-cystic fibrosis bronchiectasis. J. Cyst. Fibros. 2020. [Google Scholar] [CrossRef]
- Moss, R.B. Fungi in Cystic Fibrosis and Non–Cystic Fibrosis Bronchiectasis. Semin. Respir. Crit. Care Med. 2015, 36, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.H.; Guan, W.J.; Xu, G.; Lin, Z.Y.; Tang, Y.; Lin, Z.M.; Gao, Y.; Li, H.M.; Zhong, N.S.; Zhang, G.J.; et al. The role of viral infection in pulmonary exacerbations of bron-chiectasis in adults: A prospective study. Chest 2015, 147, 1635–1643. [Google Scholar] [CrossRef]
- Metaxas, E.I.; Balis, E.; Papaparaskevas, J.; Spanakis, N.E.; Tatsis, G.; Tsakris, A. Bronchiectasis exacerbations: The role of atypical bacteria and respiratory syncytial virus. Can. Respir. J. 2015, 22, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Venning, V.; Bartlett, J.; Jayaram, L. Patients hospitalized with an infective exacerbation of bronchiectasis unrelated to cystic fibrosis: Clinical, physiological and sputum characteristics. Respirology 2017, 22, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Imam, J.S.; Duarte, A.G. Non-CF bronchiectasis: Orphan disease no longer. Respir. Med. 2020, 166, 105940. [Google Scholar] [CrossRef] [PubMed]
- Polverino, E.; Rosales-Mayor, E.; Benegas, M.; Menendez, R.; Alcaraz-Serrano, V.; Ansotegui, E.; Montull, B.; Girón, R.M.; Cisneros, C.; Vendrell, M.; et al. Pneumonic and non-pneumonic exacerbations in bronchiectasis: Clinical and microbiological differences. J. Infect. 2018, 77, 99–106. [Google Scholar] [CrossRef]
- Chen, C.L.; Huang, Y.; Yuan, J.J.; Li, H.M.; Han, X.R.; Martinez-Garcia, M.A.; de la Rosa-Carrillo, D.; Chen, R.C.; Guan, W.J.; Zhong, N.S.; et al. The Roles of Bacteria and Viruses in Bronchiec-tasis Exacerbation: A Prospective Study. Arch. Bronconeumol. 2020, 56, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Huang, Y.; Martinez-Garcia, M.A.; Yuan, J.-J.; Li, H.-M.; De La Rosa-Carrillo, D.; Han, X.-R.; Chen, R.-C.; Guan, W.-J.; Zhong, N.-S. The Role of Epstein-Barr Virus in Adults with Bronchiectasis: A Prospective Cohort Study. Open Forum Infect. Dis. 2020, 7, ofaa235. [Google Scholar] [CrossRef] [PubMed]
- Roca, M.; Verduri, A.; Corbetta, L.; Clini, E.; Fabbri, L.; Beghé, B. Mechanisms of acute exacerbation of respiratory symptoms in chronic obstructive pulmonary disease. Eur. J. Clin. Investig. 2013, 43, 510–521. [Google Scholar] [CrossRef]
- Polverino, E.; Goeminne, P.C.; McDonnell, M.J.; Aliberti, S.; Marshall, S.E.; Loebinger, M.R.; Murris-Espin, M.; Cantón, R.; Torres, A.; Dimakou, K.; et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur. Respir. J. 2017, 50, 1700629. [Google Scholar] [CrossRef]
- Kanoh, S.; Rubin, B.K. Mechanisms of Action and Clinical Application of Macrolides as Immunomodulatory Medications. Clin. Microbiol. Rev. 2010, 23, 590–615. [Google Scholar] [CrossRef]
- Vendrell, M.; De Gracia, J.; Olveira, C.; Martinez-Garcia, M.A.; Girón, R.; Máiz, L.; Cantón, R.; Coll, R.; Escribano, A.; Solé, A. Diagnóstico y tratamiento de las bronquiectasias. Arch. Bronconeumol. 2008, 44, 629–640. [Google Scholar] [CrossRef]
- NICE. Bronchiectasis (Non-Cystic Fibrosis), Acute Exacerbation: Antimicrobial Prescribing; National Institute for Health and Care Excellence: London, UK, 2018; pp. 1–18. [Google Scholar]
- Felix, L.M.; Grundy, S.; Milan, S.J.; Armstrong, R.; Harrison, H.; Lynes, D.; Spencer, S. Dual antibiotics for bronchiectasis. Cochrane Database Syst. Rev. 2018, 6, CD012514. [Google Scholar] [CrossRef] [PubMed]
- Çağdaş, D.; Kizılkan, M.P.; Tagiyev, A.; Emiralioğlu, N.; Keleş, A.; Yalçın, E.; Doğru, D.; Özçelik, U.; Kiper, N.; Tezcan, İ. Primary Immunodeficiency Disor-ders in children with Non-Cystic Fibrosis Bronchiectasis. Eur. Ann. Allergy Clin. Immunol. 2020, 52, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Metersky, M.L.; Aksamit, T.R.; Barker, A.; Choate, R.; Daley, C.L.; Daniels, L.A.; Di Mango, A.; Eden, E.; Griffith, D.; Johnson, M.; et al. The Prevalence and Significance of Staphylo-coccus aureus in Patients with Non-Cystic Fibrosis Bronchiectasis. Ann. Am. Thorac. Soc. 2018, 15, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Kwak, N.; Lee, J.H.; Kim, H.-J.; Kim, S.A.; Yim, J.-J. New-onset nontuberculous mycobacterial pulmonary disease in bronchiectasis: Tracking the clinical and radiographic changes. BMC Pulm. Med. 2020, 20, 1–7. [Google Scholar] [CrossRef]
- Janas, A.; Przybylski, P. 14- and 15-membered lactone macrolides and their analogues and hybrids: Structure, molecular mechanism of action and biological activity. Eur. J. Med. Chem. 2019, 182, 111662. [Google Scholar] [CrossRef]
- Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2019 [Internet]. European Centre for Disease Prevention and Control. 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2019 (accessed on 1 February 2021).
- Serisier, D.J.; Martin, M.L.; McGuckin, M.A.; Lourie, R.; Chen, A.C.; Brain, B.; Biga, S.; Schlebusch, S.; Dash, P.; Bowler, S.D. Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: The BLESS randomized controlled trial. JAMA 2013, 309, 1260–1267. [Google Scholar] [CrossRef]
- Altenburg, J.; De Graaff, C.S.; Stienstra, Y.; Sloos, J.H.; Van Haren, E.H.J.; Koppers, R.J.H.; Van Der Werf, T.S.; Boersma, W.G. Effect of Azithromycin Maintenance Treatment on Infectious Exacerbations Among Patients With Non–Cystic Fibrosis Bronchiectasis: The BAT randomized con-trolled trial. JAMA 2013, 309, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Jayaram, L.; Karalus, N.; Eaton, T.; Tong, C.; Hockey, H.; Milne, D.; Fergusson, W.; Tuffery, C.; Sexton, P.; et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): A randomised, double-blind, placebo-controlled trial. Lancet 2012, 380, 660–667. [Google Scholar] [CrossRef]
- Smith, S.; Rowbotham, N.J.; Charbek, E. Inhaled antibiotics for pulmonary exacerbations in cystic fibrosis. Cochrane Database Syst. Rev. 2018, 10, CD008319. [Google Scholar] [CrossRef]
- Magréault, S.; Roy, C.; Launay, M.; Sermet-Gaudelus, I.; Jullien, V. Pharmacokinetic and Pharmacodynamic Optimization of Antibiotic Therapy in Cystic Fibrosis Patients: Current Evidences, Gaps in Knowledge and Future Directions. Clin. Pharmacokinet. 2021. [Google Scholar] [CrossRef]
- Laska, I.F.; Crichton, M.L.; Shoemark, A.; Chalmers, J.D. The efficacy and safety of inhaled antibiotics for the treatment of bron-chiectasis in adults: A systematic review and meta-analysis. Lancet Respir. Med. 2019, 7, 855–869. [Google Scholar] [CrossRef]
- Sangiovanni, S.; Morales, E.I.; Fernández-Trujillo, L. Inhaled tobramycin for chronic infection with pseudomonas aeruginosa in non-cystic fibrosis bronchiectasis: A systematic review and meta-analysis. Respir. Med. 2021, 176, 106283. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.; Severn, M. Colistin for Prophylactic Use in Non-Cystic Fibrosis Bronchiectasis or COPD with Exacerbations: A Review of Clinical and Cost-Effectiveness and Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2017; (CADTH Rapid Response Reports). Available online: http://www.ncbi.nlm.nih.gov/books/NBK493389/ (accessed on 4 February 2021).
- De Soyza, A.; Aksamit, T.; Bandel, T.J.; Criollo, M.; Elborn, J.S.; Operschall, E.; Polverino, E.; Roth, K.; Winthrop, K.L.; Wilson, R. RESPIRE 1: A phase III placebo-controlled ran-domised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur. Respir. J. 2018, 51, 1702052. [Google Scholar] [CrossRef]
- Aksamit, T.; De Soyza, A.; Bandel, T.J.; Criollo, M.; Elborn, J.S.; Operschall, E.; Polverino, E.; Roth, K.; Winthrop, K.L.; Wilson, R. RESPIRE 2: A phase III placebo-controlled ran-domised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur. Respir. J. 2018, 51, 1702053. [Google Scholar] [CrossRef]
- Haworth, C.S.; Bilton, D.; Chalmers, J.D.; Davis, A.M.; Froehlich, J.; Gonda, I.; Thompson, B.; Wanner, A.; O’Donnell, A.E. Inhaled liposomal ciprofloxacin in patients with non-cystic fibrosis bronchiectasis and chronic lung infection with Pseudomonas aeruginosa (ORBIT-3 and ORBIT-4): Two phase 3, randomised controlled trials. Lancet Respir. Med. 2019, 7, 213–226. [Google Scholar] [CrossRef]
- Murray, M.P.; Govan, J.R.; Doherty, C.J.; Simpson, A.J.; Wilkinson, T.S.; Chalmers, J.D.; Greening, A.P.; Haslett, C.; Hill, A.T. A randomized controlled trial of nebu-lized gentamicin in non-cystic fibrosis bronchiectasis. Am. J. Respir. Crit. Care Med. 2011, 183, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.F.; O’Donnell, A.E.; Flume, P.; Thompson, P.J.; Ruzi, J.D.; De Gracia, J.; Boersma, W.G.; De Soyza, A.; Shao, L.; Zhang, J.; et al. Aztreonam for inhalation solution in patients with non-cystic fibrosis bronchiectasis (AIR-BX1 and AIR-BX2): Two randomised double-blind, placebo-controlled phase 3 trials. Lancet Respir. Med. 2014, 2, 738–749. [Google Scholar] [CrossRef]
- Severiche-Bueno, D.; Gamboa, E.; Reyes, L.F.; Chotirmall, S.H. Hot topics and current controversies in non-cystic fibrosis bron-chiectasis. Breathe 2019, 15, 286–295. [Google Scholar] [CrossRef]
- Kocurek, E.G.; Jagana, R. Noncystic fibrosis bronchiectasis management: Opportunities and challenges. Curr. Opin Pulm. Med. 2019, 25, 192–200. [Google Scholar] [CrossRef]
- Kaehne, A.; Milan, S.J.; Felix, L.M.; Sheridan, E.; Marsden, P.A.; Spencer, S. Head-to-head trials of antibiotics for bronchiectasis. Cochrane Database Syst. Rev. 2018, 9, CD012590. [Google Scholar] [CrossRef]
- Spencer, S.; Felix, L.M.; Milan, S.J.; Normansell, R.; Goeminne, P.C.; Chalmers, J.D.; Donovan, T. Oral versus inhaled antibiotics for bronchi-ectasis. Cochrane Database Syst. Rev. 2018, 3, CD012579. [Google Scholar]
- White, L.; Mirrani, G.; Grover, M.; Rollason, J.; Malin, A.; Suntharalingam, J. Outcomes of Pseudomonas eradication therapy in patients with non-cystic fibrosis bronchiectasis. Respir. Med. 2012, 106, 356–360. [Google Scholar] [CrossRef]
- Orriols, R.; Hernando, R.; Ferrer, A.; Terradas, S.; Montoro, B. Eradication Therapy against Pseudomonas aeruginosa in Non-Cystic Fibrosis Bronchiectasis. Respiration 2015, 90, 299–305. [Google Scholar] [CrossRef]
- Orriols, R.; Roig, J.; Ferrer, J.; Sampol, G.; Rosell, A.; Ferrer, A.; Vallano, A. Inhaled antibiotic therapy in non-cystic fibrosis patients with bronchiectasis and chronic bronchial infection by Pseudomonas aeruginosa. Respir. Med. 1999, 93, 476–480. [Google Scholar] [CrossRef]
- Dhand, R. The Rationale and Evidence for Use of Inhaled Antibiotics to ControlPseudomonas aeruginosaInfection in Non-cystic Fibrosis Bronchiectasis. J. Aerosol Med. Pulm. Drug Deliv. 2018, 31, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Shorr, A.F.; Myers, D.E.; Huang, D.B.; Nathanson, B.H.; Emons, M.F.; Kollef, M.H. A risk score for identifying methicillin-resistant Staphylococcus aureus in patients presenting to the hospital with pneumonia. BMC Infect. Dis. 2013, 13, 268. [Google Scholar] [CrossRef] [PubMed]
- Menéndez, R.; Méndez, R.; Polverino, E.; Rosales-Mayor, E.; Amara-Elori, I.; Reyes, S.; Sahuquillo-Arce, J.M.; Fernández-Barat, L.; Alcaraz, V.; Torres, A. Risk factors for multidrug-resistant pathogens in bronchiectasis exacerbations. BMC Infect. Dis. 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Masekela, R.; Green, R.J. The Role of Macrolides in Childhood Non-Cystic Fibrosis-Related Bronchiectasis. Mediat. Inflamm. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, G.-Y.; He, Q.; Xiang-Lian, L.; Ya-Nan, Y.; Si-Te, F. Prolonged treatment with macrolides in adult patients with non-cystic fibrosis bronchiectasis: Meta-analysis of randomized controlled trials. Pulm. Pharmacol. Ther. 2014, 29, 80–88. [Google Scholar] [CrossRef]
- Wang, Z.; Bafadhel, M.; Haldar, K.; Spivak, A.; Mayhew, D.; Miller, B.E.; Tal-Singer, R.; Johnston, S.L.; Ramsheh, M.Y.; Barer, M.R.; et al. Lung microbiome dynamics in COPD exacerbations. Eur. Respir. J. 2016, 47, 1082–1092. [Google Scholar] [CrossRef]
- Rogers, G.B.; Bruce, K.D.; Martin, M.L.; Burr, L.D.; Serisier, D.J. The effect of long-term macrolide treatment on respiratory microbi-ota composition in non-cystic fibrosis bronchiectasis: An analysis from the randomised, double-blind, placebo-controlled BLESS trial. Lancet Respir. Med. 2014, 2, 988–996. [Google Scholar] [CrossRef]
- Drobnic, M.E.; Suñé, P.; Montoro, J.B.; Ferrer, A.; Orriols, R. Inhaled Tobramycin in Non—Cystic Fibrosis Patients with Bronchiectasis and Chronic Bronchial Infection with Pseudomonas Aeruginosa. Ann. Pharmacother. 2005, 39, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Haworth, C.S.; Foweraker, J.E.; Wilkinson, P.; Kenyon, R.F.; Bilton, D. Inhaled Colistin in Patients with Bronchiectasis and ChronicPseudomonas aeruginosaInfection. Am. J. Respir. Crit. Care Med. 2014, 189, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, S.; Aladegbami, B.; Burday, M.; Levi, M.; Marras, S.A.E.; Shah, D.; El-Hajj, H.H.; Kramer, F.R.; Alland, D. Rapid Universal Identification of Bacterial Pathogens from Clinical Cultures by Using a Novel Sloppy Molecular Beacon Melting Temperature Signature Technique. J. Clin. Microbiol. 2009, 48, 258–267. [Google Scholar] [CrossRef]
- Srinivas, N.; Jetter, P.; Ueberbacher, B.J.; Werneburg, M.; Zerbe, K.; Steinmann, J.; Van der Meijden, B.; Bernardini, F.; Lederer, A.; Dias, R.L.; et al. Peptidomimetic antibiotics target out-er-membrane biogenesis in pseudomonas aeruginosa. Science 2010, 327, 1010–1013. [Google Scholar] [CrossRef]
- Milla, C.E.; Chmiel, J.F.; Accurso, F.J.; VanDevanter, D.R.; Konstan, M.W.; Yarranton, G.; Geller, D.E.; KB001 Study Group. Anti-PcrV antibody in cystic fibrosis: A novel approach targeting Pseudomonas aeruginosa airway infection. Pediatr. Pulmonol. 2014, 49, 650–658. [Google Scholar] [CrossRef] [PubMed]
| Nicotra et al. (1995) (123 pts) Ref. n33 | Pasteur et al. (2000) (150 pts) Ref. n34 | Aksamit et al. (2017) (1406 pts) Ref. n35 | Dimakou et al. (2016) (205 pts) Ref. n37 | Martinez-García et al (2020) * (849 pts) Ref. n40 | McDonnell et al. (2015) (155 pts) Ref. n42 | King et al. (2007) (89 pts) Ref. n44 | Cabello et al. (1997) (17 pts) Ref. n45 | Venning et al. (2017) * (65 pts) Ref. n63 | |
|---|---|---|---|---|---|---|---|---|---|
| Haemophilus influenza | 37 [30] | 52 [35] | 116 [8] | 26 [13] | [14] | 89 [57] | 42 [47] | 10 [42] | [15] |
| Streptococcus pneumoniae | 13 [11] | 20 [13] | 49 [3] | 17 [8] | [5] | 51 [33] | 6 [7] | 0 [0] | N/R |
| Staphylococcus aureus | 9 [7] | 21 [14] | 170 [12] | N/R | [4] | 35 [23] | 3 [4] | 4 [17] | [3] |
| Pseudomonas aeruginosa | 38 [31] | 46 [31] | 470 [33] | 88 [43] | [26] | 76 [49] | 11 [12] | 1 [4] | [32] |
| Mycobacteria | 49 [40] | 0 [0] | 657 [50] | 2 [1] | [2] | 5 [3] | 2 [2] | N/R | [<3] |
| No organism | N/R | 34 [23] | 93 [7] | 78 [38] | N/R | N/R | 19 [21] | N/R | [17] |
| Microrganism | Recommended First-Line Treatment (14 Days) | Recommended Second-Line Treatment (14 Days) | ||
|---|---|---|---|---|
| Hemophilus influenzae—beta lactamase positive | Amoxicillin/clavulanic acid 625 mg 1 tablet three times a day *~ | Doxycycline 100 mg twice a day *~ | ||
| Ciprofloxacin 500 mg or 750 mg twice a day *~† | ||||
| Amoxicillin/clavulanic acid 825 mg 1 tablet three times a day † | Ceftriaxone 2 g once a day (intravenous) *~ | |||
| Moraxella catarrhalis | Amoxicillin/clavulanic acid 625 mg 1 tablet three times a day * | Clarithromycin 500 mg twice a day * | ||
| Doxycycline 100 mg twice a day * | ||||
| Ciprofloxacin 500 mg or 750 mg twice a day * | ||||
| Streptococcus pneumoniae | Amoxicillin 500 mg three times a day * | Doxycycline 100 mg twice a day * | ||
| Staphylococcus aureus (MSSA) | Flucloxacillin 500 mg four times a day * | Clarithromycin 500 mg twice a day * | ||
| Doxycycline 100 mg twice a day * | ||||
| Amoxicillin/clavulanic acid 625 mg 1 tablet three times a day * | ||||
| Staphylococcus aureus (MRSA) | Oral route | Doxycycline 100 mg twice a day * | Third-line linezolid 600 mg twice a day * | |
| Rifampicin (<50 kg) 450 mg once a day * | ||||
| Rifampicin (>50 kg) 600 mg once a day * | ||||
| Trimethoprim 200 mg twice a day * | ||||
| Intravenous route | Vancomycin 1 g twice a day * | Linezolid 600 mg twice a day * | ||
| Teicoplanin 400 mg once a day * | ||||
| Pseudomonas aeruginosa | Oral ciprofloxacin 500 mg twice a day * | Monotherapy | intravenous ceftazidime 2 g three times a day * | |
| Oral ciprofloxacin 750 mg twice a day in more severe infections *† | piperacillin with tazobactam 4.5 g three times a day * | |||
| aztreonam 2 g three times a day * | ||||
| meropenem 2 g three times a day * | ||||
| Dual therapy | Previous drugs combined with gentamicin or tobramycin or colistin 2 mU three times a day (under 60 kg, 50 000–75 000 U/kg daily in 3 divided doses) * |
| If ≥ 3 Exarcerbations/Year [I Step] | If ≥ 3 Exarcerbations/Year Despite I Step Treatment [II Step] | If ≥ 5 Exacerbations/Year Despite II Step Treatment [III Step] | |
|---|---|---|---|
| Pseudomonas aeuriginosa | long term inhaled anti-pseudomonal OR long term macrolide * | ||
| Other potentially pathogenic microorganisms | long term macrolides * OR long term oral targeted antibiotic OR long term inhaled targeted antibiotic | long term macrolide * AND long term inhaled antibiotic | Regular intravenous antibioticevery 2–3 months |
| No pathogen | long term macrolides * | ||
| Trial (Ref n) | Inclusion Criteria | Intervention | Duration | Primary end Point | Main Results | Antibiotics Resistance (MDR) |
|---|---|---|---|---|---|---|
| BLESS (79) | ≥ pulmonary exarcerbations requiring supplemental systemic antibiotic therapy in the preceding 12 months and daily sputum production | Erythromycin 400mg every 12 h versus placebo | 12 months | The mean rate of PDPEs per patient per year, analyzed by intention to treat | Significant reduction of PDPEs in the erythromycin group | Median % of macrolide resistant oropharyngeal streptococci: 25.6 |
| Age 20–85 years | No difference for the emergence of new sputum pathogens | |||||
| BAT (80) | ≥3 LRTIs treated with oral or i.v. antibiotics and ≥1 sputum culture yielding one or more bacterial respiratory pathogens in the previous year | Azythromycin 250 mg daily versus placebo | 12 months | N° of infectious exacerbations during the 52-week treatment period. | Zero exacerbations in the azithromycin group | % of macrolid resistance in the azithromycin group: 88% versus 26% in placebo group |
| ≥18 years | ||||||
| EMBRACE (81) | ≥1 pulmonary exarcerbation requiring antibiotic treatment in the past year ≥18 years | Azythromycin 500 mg days week | 6 months of treatment, followed up for another 6 months | Rate of event-based exacerbations in the first 6 months | 62% relative reduction with azithromycin in the 6-month treatment period. 42% relative reduction in the 12-month period. | Not routinely undertaken, but two (4%) patients in the azithromycin group developed macrolide-resistant Streptococcus pneumoniae at 6 months |
| FEV1 before bronchodilation | No significant changes | |||||
| SGRQ total score at the end of the treatment period | No significant changes |
| 1st Author or Trial (Ref n) | Inclusion Criteria | Sputum Bacteriology | Intervention | Duration | Primary End Point | Main Results | Antibiotics Resistance (MDR) |
|---|---|---|---|---|---|---|---|
| RESPIRE 1 and 2 (87, 88) | ≥2 exacerbations in the previous 12 months | P. aeruginosa, H. influenzae, M. catarrhalis, S. aureus, S. pneumoniae, S. maltophilia, B. cepacia | Ciprofloxacin DPI 32.5 mg every 12 h | 1 year, 14 days on/off (12 active cycles) or 28 days on/off (six active cycles) | (1) time to first exacerbation AND (2) frequency of exacerbations | Ciprofloxacin DPI 14 days on/off delayed time to 1st exacerbation AND significantly reduced frequency of exacerbations by 39% | % of patients with ≥1 isolate from sputum with an elevated MIC at any time-point: 54.0% for ciprofloxacin DPI 14 days on/off and 53.9% for ciprofloxacin DPI 28 days on/off versus 36.2% for placebo |
| ORBIT-3 and -4 (89) | ≥ pulmonary exacerbations treated with antibiotics in the preceding 12 months AND history of chronic P aeruginosa lung infection | P. aeruginosa | ARD-3150 (liposome encapsulated ciprofloxacin 135 mg and free ciprofloxacin 54 mg) | 1 year, on/off regimen (six active cycles) | Occurance of pulmonary exacerbations | Reduction of pulmonary exacerbations of all severity in ORBIT-4, but not in ORBIT-3, compared with placebo | 32% of patients treated with ARD-3150 and 18% of patients treated with placebo had a P. aeruginosa isolate for which the ciprofloxacin MIC had increased by > 2 times |
| Murray (90) | Chronically infected sputum AND ≥2 exacerbations in the past year AND ability to tolerate nebulized gentamicin AND FEV1 > 30% predicted AND not currently receiving long-term antibiotics | Any PPM | Gentamicin 80 mg every 12 h | 1 year, continuous regimen | ≥1 log unit reduction in sputum bacterial density | Bacterial density significantly reduced in the gentamicin group. At follow-up: bacterial density was similar in both groups | No difference for the emergence of gentamicin indeterminately resistant or resistant strains |
| AIR-BX 1 and 2 (91) | History of positive sputum or bronchoscopic culture for target Gram-negative organism or treatment of exacerbation AND chronic sputum production AND FEV1 ≥ 20% predicted | P. aeruginosa, Achromobacter, Burkholderia, Citrobacter, Enterobacter, Escherichia, Klebsiella, Moraxella, Proteus, Serratia, Stenotrophomonas | Aztreonam solution 75 mg every 8 h | 4 months, 28 days on/off (two active cycles) | Δ in QOL-B-RSS (baseline to week 4; high scores represent few symptoms) | QOL-B-RSS numerically increased in all groups in both studies at weeks 4 and 12. No significant differences | Increases of ≥4 fold in the MIC of aztreonam: (A) in AIR-BX1: 15% of AZLI-treated patients versus 6% of placebo after 4 weeks; 35% versus 11% after 12 weeks; and 23% versus 14% of placebo after 4 weeks off -treatment. (B) AIR-BX2: 23% of AZLI-treated patients versus 7% after 4 weeks; 34%, versus 11% after 12 weeks; and 20% versus 6% after 4 weeks off -treatment |
| Orriols (97) | Recruitment after the 1st isolation of P. aeruginosa in sputum | P. aeruginosa | Nebulized tobramycin 300 mg every 12 h + i.v. ceftazidime | 14 days during the first 4 weeks, then randomization and treatment for 3 months | Bacterial eradication in sputum | % of patients free of P. aeruginosa: (A) in the 1st month: 90.9% in tobramycin group versus 76.5% in placebo. (B) At the end of study: 54.5% in tobramycin group versus 29.4% in placebo | No tobramycin-resistant P. aeruginosa |
| Drobnic (106) | ≥3 positive sputum cultures for tobramycin-sensitive P. aeruginosa during 6 months prior to the study | P. aeruginosa | Tobramycin 300 mg every 12 h | 6 months | N° of exacerbations AND days of hospital admissions | No significant differences in the frequency of pulmonary exacerbations. Days of hospital admission significantly fewer in the tobramycin period | 2 months after ending the study, all patients remained colonized by tobramycin-susceptible PA (MIC < 8 µg/mL) |
| Haworth (107) | ≥2 positive respiratory tract cultures for P. aeruginosa in the preceding 12 months AND within 21 days of completing a course of antipseudomonal antibiotics for the treatment of an exacerbation | P. aeruginosa | Colistin 1 million IU every 12 h | 6 months, continuous regimen | Time to exacerbation | The median time to exacerbation was 165 days in the colistin group versus 111 days in the placebo group | No colistin-resistant strains of P. aeruginosa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inchingolo, R.; Pierandrei, C.; Montemurro, G.; Smargiassi, A.; Lohmeyer, F.M.; Rizzi, A. Antimicrobial Resistance in Common Respiratory Pathogens of Chronic Bronchiectasis Patients: A Literature Review. Antibiotics 2021, 10, 326. https://doi.org/10.3390/antibiotics10030326
Inchingolo R, Pierandrei C, Montemurro G, Smargiassi A, Lohmeyer FM, Rizzi A. Antimicrobial Resistance in Common Respiratory Pathogens of Chronic Bronchiectasis Patients: A Literature Review. Antibiotics. 2021; 10(3):326. https://doi.org/10.3390/antibiotics10030326
Chicago/Turabian StyleInchingolo, Riccardo, Chiara Pierandrei, Giuliano Montemurro, Andrea Smargiassi, Franziska Michaela Lohmeyer, and Angela Rizzi. 2021. "Antimicrobial Resistance in Common Respiratory Pathogens of Chronic Bronchiectasis Patients: A Literature Review" Antibiotics 10, no. 3: 326. https://doi.org/10.3390/antibiotics10030326
APA StyleInchingolo, R., Pierandrei, C., Montemurro, G., Smargiassi, A., Lohmeyer, F. M., & Rizzi, A. (2021). Antimicrobial Resistance in Common Respiratory Pathogens of Chronic Bronchiectasis Patients: A Literature Review. Antibiotics, 10(3), 326. https://doi.org/10.3390/antibiotics10030326








