Pharmacokinetics of Meropenem in People with Cystic Fibrosis—A Proof of Concept Clinical Trial
Abstract
1. Introduction
2. Methods
Statistical Analysis
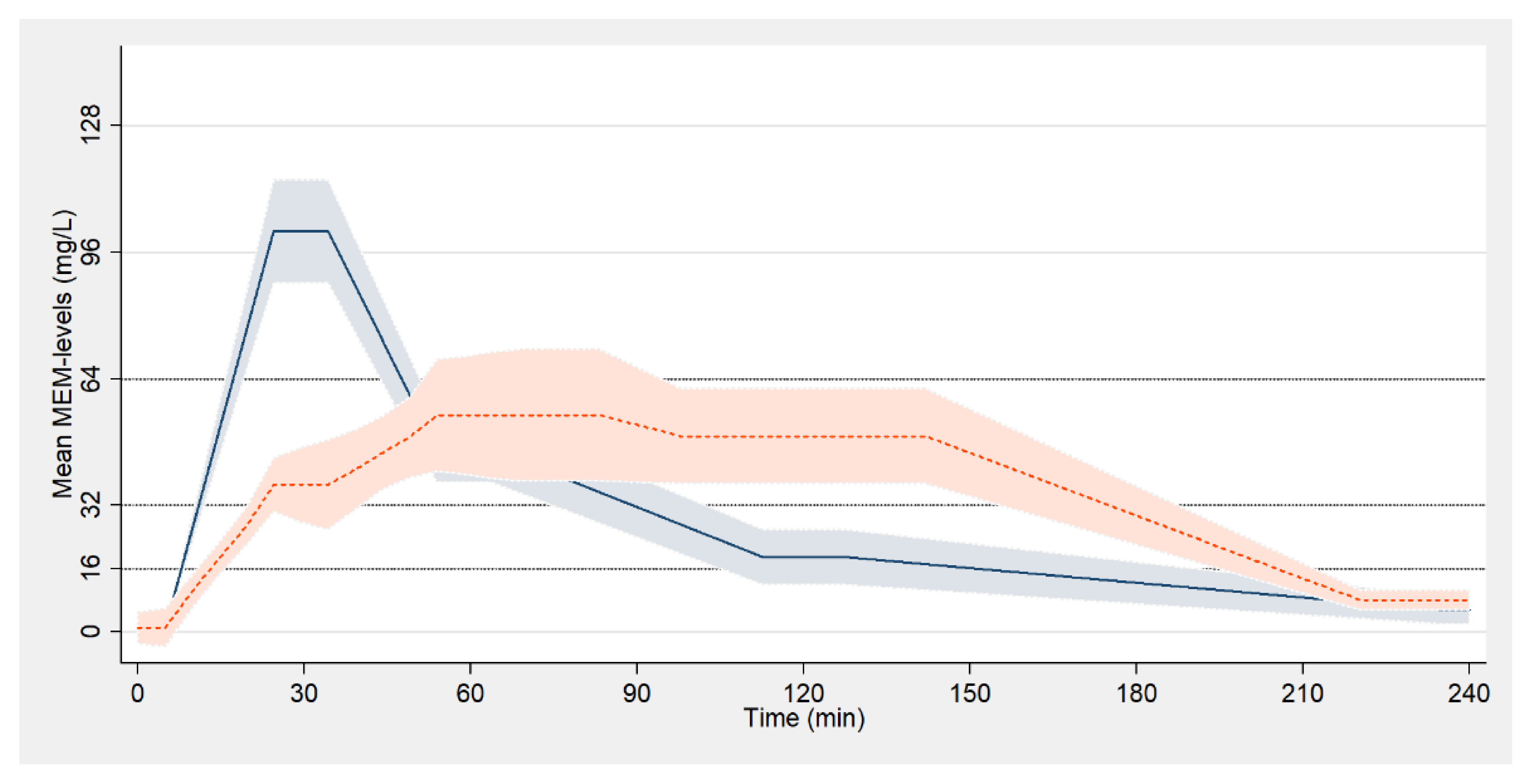
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucca, F.; Guarnieri, M.; Ros, M.; Muffato, G.; Rigoli, R.; Da Dalt, L. Antibiotic resistance evolution ofPseudomonas aeruginosain cystic fibrosis patients (2010–2013). Clin. Respir. J. 2018, 12, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- Blumer, J.L.; Saiman, L.; Konstan, M.W.; Melnick, D. The Efficacy and Safety of Meropenem and Tobramycin vs Ceftazidime and Tobramycin in the Treatment of Acute Pulmonary Exacerbations in Patients with Cystic Fibrosis. Chest 2005, 128, 2336–2346. [Google Scholar] [CrossRef] [PubMed]
- Delfino, E.; Fucile, C.; Del Bono, V.; Marchese, A.; Marini, V.; Coppo, E.; Casciaro, R.; Minicucci, L.; Giacobbe, D.R.; Martelli, A.; et al. Pharmacokinetics of high-dose extended-infusion meropenem during pulmonary exacerbation in adult cystic fibrosis patients: A case series. New Microbiol. 2018, 41, 47–51. [Google Scholar] [PubMed]
- Latzin, P.; Fehling, M.; Bauernfeind, A.; Reinhardt, D.; Kappler, M.; Griese, M. Efficacy and safety of intravenous meropenem and tobramycin versus ceftazidime and tobramycin in cystic fibrosis. J. Cyst. Fibros. 2008, 7, 142–146. [Google Scholar] [CrossRef]
- Baldwin, C.M.; Lyseng-Williamson, K.A.; Keam, S.J. Meropenem. Drugs 2008, 68, 803–838. [Google Scholar] [CrossRef] [PubMed]
- Bui, K.Q.; Ambrose, P.G.; Nicolau, D.P.; Lapin, C.D.; Nightingale, C.H.; Quintiliani, R. Pharmacokinetics of High-Dose Meropenem in Adult Cystic Fibrosis Patients. Chemotherapy 2001, 47, 153–156. [Google Scholar] [CrossRef]
- Thompson, R.Z.; Martin, C.A.; Burgess, D.R.; Rutter, W.C.; Burgess, D.S. Optimizing beta-lactam pharmacodynamics against Pseudomonas aeruginosa in adult cystic fibrosis patients. J. Cyst. Fibros. 2016, 15, 660–663. [Google Scholar] [CrossRef]
- Christensson, B.A.; Ljungberg, B.; Eriksson, L.; Nilsson-Ehle, I. Pharmacokinetics of meropenem in patients with cystic fibrosis. Eur. J. Clin. Microbiol. Infect. Dis. 1998, 17, 873–876. [Google Scholar] [CrossRef]
- VanDevanter, D.R.; O’Riordan, M.A.; Blumer, J.L.; Konstan, M.W. Assessing time to pulmonary function benefit following antibiotic treatment of acute cystic fibrosis exacerbations. Respir. Res. 2010, 11, 137. [Google Scholar] [CrossRef]
- Girón, R.M.; Cisneros, C.; Al Nakeeb, Z.; Hoyos, N.; Martínez, C.; Ancochea, J. Eficiencia del tratamiento antibiótico intravenoso domiciliario en la fibrosis quística. Med. Clín. 2006, 127, 567–571. [Google Scholar]
- Ekkelenkamp, M.B.; Cantón, R.; Díez-Aguilar, M.; Tunney, M.M.; Gilpin, D.F.; Bernardini, F.; Dale, G.E.; Elborn, J.S.; Bayjanov, J.R.; Fluit, A. Susceptibility of Pseudomonas aeruginosa Recovered from Cystic Fibrosis Patients to Murepavadin and 13 Comparator Antibiotics. Antimicrob. Agents Chemother. 2019, 64, e01541–e015419. [Google Scholar] [CrossRef] [PubMed]
- Middlemiss, J.K.; Poole, K. Differential Impact of MexB Mutations on Substrate Selectivity of the MexAB-OprM Multidrug Efflux Pump of Pseudomonas aeruginosa. J. Bacteriol. 2004, 186, 1258–1269. [Google Scholar] [CrossRef]
- Castellani, C.; Duff, A.J.; Bell, S.C.; Heijerman, H.G.; Munck, A.; Ratjen, F.; Sermet-Gaudelus, I.; Southern, K.W.; Barben, J.; Flume, P.A.; et al. ECFS best practice guidelines: The 2018 revision. J. Cyst. Fibros. 2018, 17, 153–178. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, C.; Schulte-Hubbert, B.; Bend, J.; Abele-Horn, M.; Baumann, I.; Bremer, W.; Brunsmann, F.; Dieninghoff, D.; Eickmeier, O.; Ellemunter, H.; et al. S3-Leitlinie: Lungenerkrankung bei Mukoviszidose—Modul 2: Diagnostik und Therapie bei der chronischen Infektion mit Pseudomonas aeruginosa. Pneumologie 2018, 72, 347–392. [Google Scholar] [CrossRef]
- Rhodes, A.A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensiv. Care Med. 2017, 43, 304–377. [Google Scholar]
- De With, K.; Allerberger, F.; Amann, S.; Apfalter, P.; Brodt, H.-R.; Eckmanns, T.; Fellhauer, M.; Geiss, H.K.; Janata, O.; Krause, R.; et al. Strategies to enhance rational use of antibiotics in hospital: A guideline by the German Society for Infectious Diseases. Infection 2016, 44, 395–439. [Google Scholar] [CrossRef]
- Ambrose, P.G.; Bhavnani, S.M.; Rubino, C.M.; Louie, A.; Gumbo, T.; Forrest, A.; Drusano, G.L. Antimicrobial Resistance: Pharmacokinetics-Pharmacodynamics of Antimicrobial Therapy: It’s Not Just for Mice Anymore. Clin. Infect. Dis. 2007, 44, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef]
- Van Mansfeld, R.; De Vrankrijker, A.; Brimicombe, R.; Heijerman, H.; Van Berkhout, F.T.; Spitoni, C.; Grave, S.; Van Der Ent, C.; Wolfs, T.; Willems, R.; et al. The Effect of Strict Segregation on Pseudomonas aeruginosa in Cystic Fibrosis Patients. PLoS ONE 2016, 11, e0157189. [Google Scholar] [CrossRef]
- Koehler, D.R.; Downey, G.P.; Sweezey, N.B.; Tanswell, A.K.; Hu, J. Lung Inflammation as a Therapeutic Target in Cystic Fibrosis. Am. J. Respir. Cell Mol. Biol. 2004, 31, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Kuti, J.L.; Pettit, R.S.; Neu, N.; Cies, J.J.; Lapin, C.; Muhlebach, M.S.; Novak, K.J.; Nguyen, S.T.; Saiman, L.; Nicolau, D.P. Meropenem time above the MIC exposure is predictive of response in cystic fibrosis children with acute pulmonary exacerbations. Diagn. Microbiol. Infect. Dis. 2018, 91, 294–297. [Google Scholar] [CrossRef] [PubMed]
| Short Infusion (30 min) | Extended Infusion (120 min) | |
|---|---|---|
| N = 6 | N = 6 | |
| Sex; n (%) | ||
| Female | 2 (33) | 3 (50) |
| Male | 4 (67) | 3 (50) |
| Genotypes; n (%) | ||
| Homozygous F508del | 2 (33) | 2 (33) |
| Heterozygous F508del | 2 (33) | 3 (50) |
| Other | 2 (33) | 1 (17) |
| Age; medium (SD) | 27.5 ± 8.04 | 26.67 ± 7.84 |
| BMI; medium (SD) | 20.07 ± 3.5 | 19.75 ± 2.71 |
| Best FEV1; medium (SD) | 36.67 ± 11.3 | 48 ± 15.67 |
| PA; n (%) | ||
| Yes | 5 (83) | 6 (100) |
| No | 1 (17) | 0 (0) |
| 3/4 MRGN PA; n (%) | 2 (33)/1 (17) | 0 (0) / 3 (50) |
| Combination therapy with tobramycin, n (%) | 5 (83) | 6 (100) |
| Laboratory results; medium (SD) | ||
| eGFR (mL/min) | 129.83 ± 6.27 | 129 ± 12.39 |
| ALT (U/L) | 26.33 ± 18.91 | 39.33 ± 27.63 |
| AST (U/L) | 36.17 ± 30.22 | 25 ± 8.74 |
| WBC (103/µL) | 11.55 ± 3.59 | 13.65 ± 3.15 |
| CRP (mg/L) | 32.81 ± 47.32 | 37.15 ± 40.21 |
| Treatment Group | |||
|---|---|---|---|
| 30 min | 120 min | p-Value | |
| Mean fT > 16 mg/L (min) | 141 ± 49 | 200 ± 21 | 0.078 |
| Mean fT > 32 mg/L (min) | 82 ± 23 | 134 ± 43 | 0.037 |
| C peak | 107 ± 20 | 59 ± 20 | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamp, J.C.; Fuge, J.; Ringshausen, F.C.; Grote-Koska, D.; Brand, K.; Graalmann, L.; Vonberg, R.-P.; Welte, T.; Rademacher, J. Pharmacokinetics of Meropenem in People with Cystic Fibrosis—A Proof of Concept Clinical Trial. Antibiotics 2021, 10, 292. https://doi.org/10.3390/antibiotics10030292
Kamp JC, Fuge J, Ringshausen FC, Grote-Koska D, Brand K, Graalmann L, Vonberg R-P, Welte T, Rademacher J. Pharmacokinetics of Meropenem in People with Cystic Fibrosis—A Proof of Concept Clinical Trial. Antibiotics. 2021; 10(3):292. https://doi.org/10.3390/antibiotics10030292
Chicago/Turabian StyleKamp, Jan C., Jan Fuge, Felix C. Ringshausen, Denis Grote-Koska, Korbinian Brand, Lukas Graalmann, Ralf-Peter Vonberg, Tobias Welte, and Jessica Rademacher. 2021. "Pharmacokinetics of Meropenem in People with Cystic Fibrosis—A Proof of Concept Clinical Trial" Antibiotics 10, no. 3: 292. https://doi.org/10.3390/antibiotics10030292
APA StyleKamp, J. C., Fuge, J., Ringshausen, F. C., Grote-Koska, D., Brand, K., Graalmann, L., Vonberg, R.-P., Welte, T., & Rademacher, J. (2021). Pharmacokinetics of Meropenem in People with Cystic Fibrosis—A Proof of Concept Clinical Trial. Antibiotics, 10(3), 292. https://doi.org/10.3390/antibiotics10030292








