Distribution of Extended-Spectrum β-Lactamase (ESBL)-Encoding Genes among Multidrug-Resistant Gram-Negative Pathogens Collected from Three Different Countries
Abstract
1. Introduction
2. Results
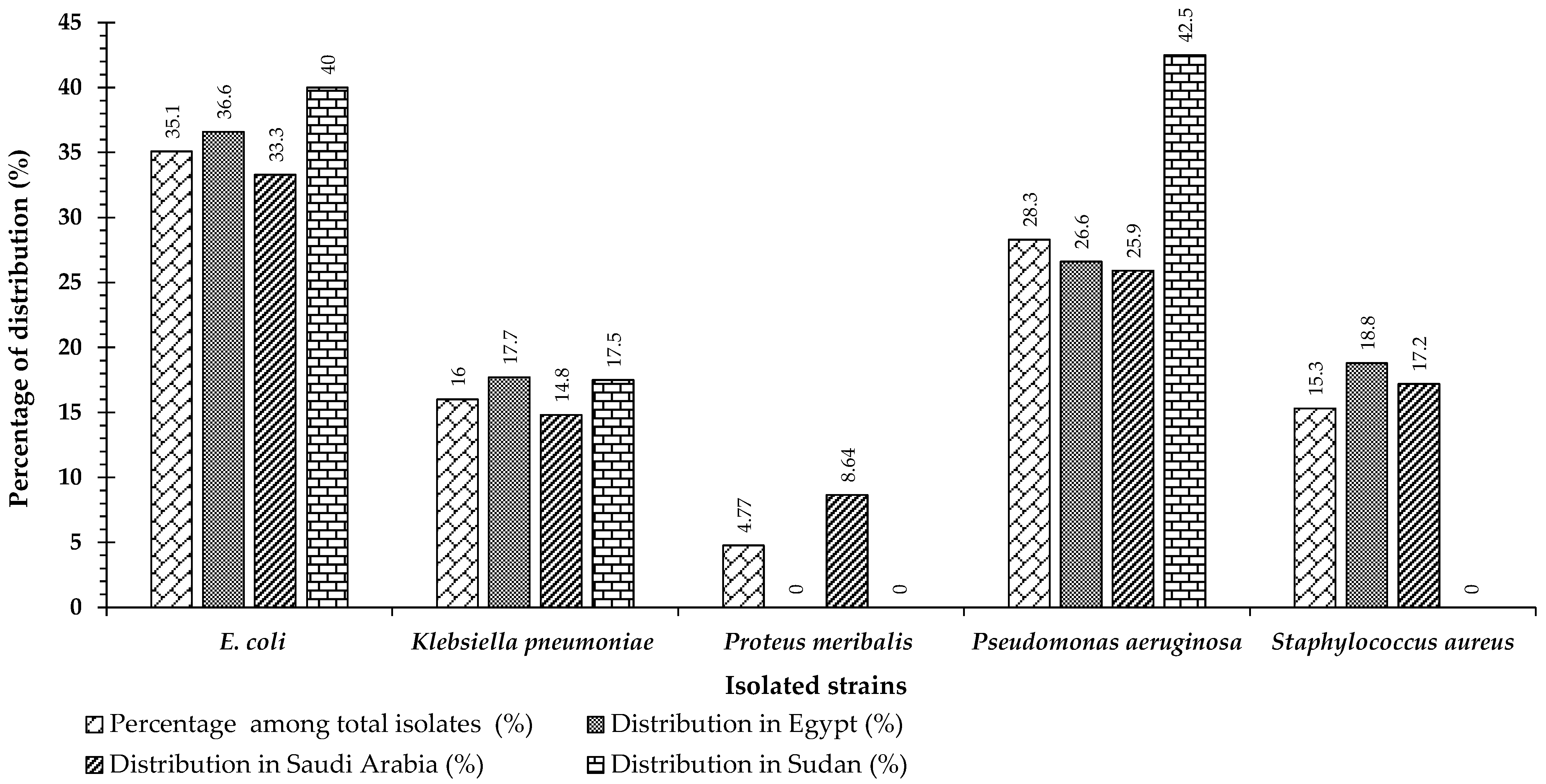
2.1. Study Cohort and Atrain Identification
2.2. Antimicrobial Susceptibility Testing
2.3. Antibiotic Susceptibility Testing of Escherichia coli Strains
2.4. Antibiotic Susceptibility of Klebsiella pneumoniae Strains
2.5. Antibiotic Susceptibility of Pseudomonas aeruginosa Strains
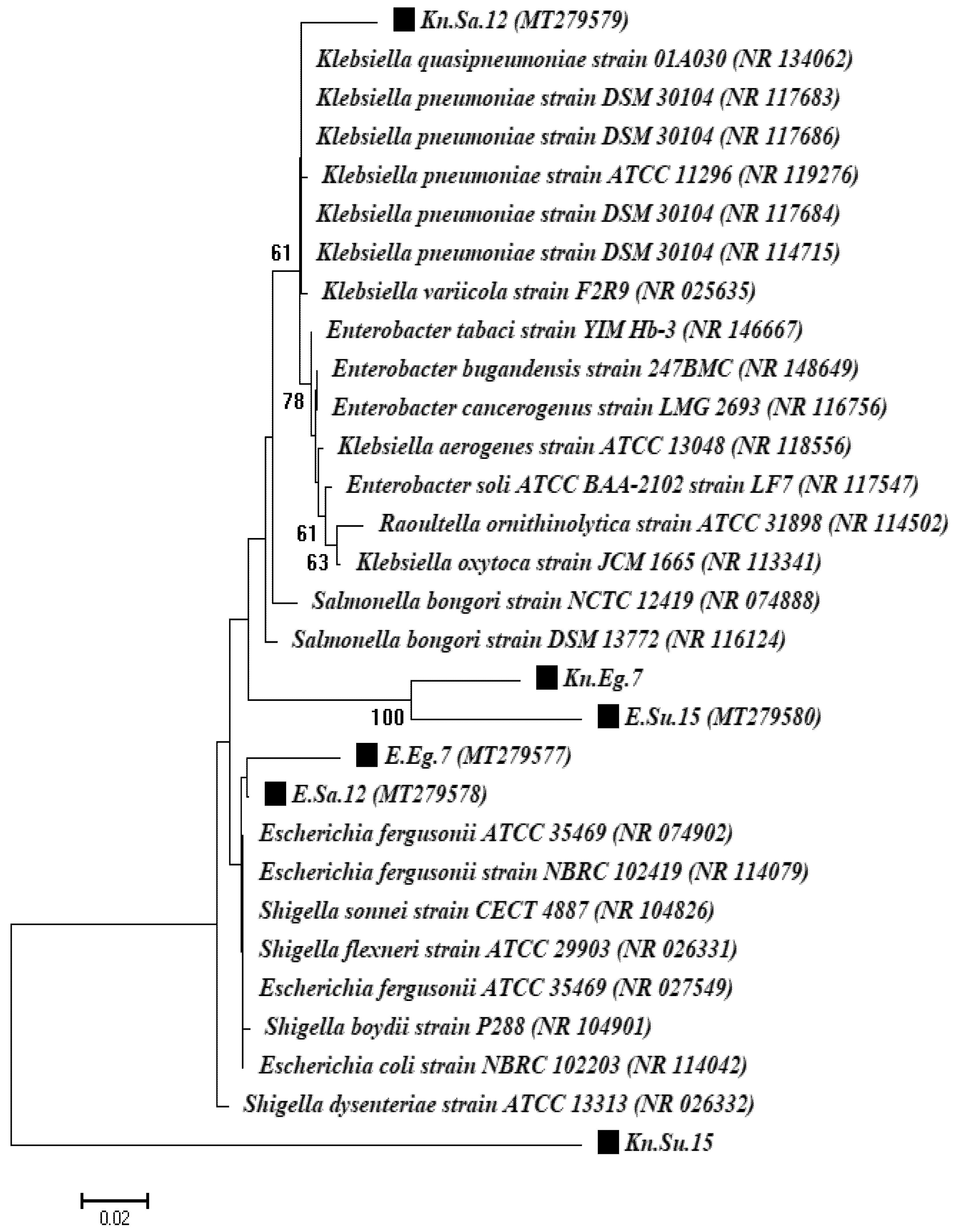
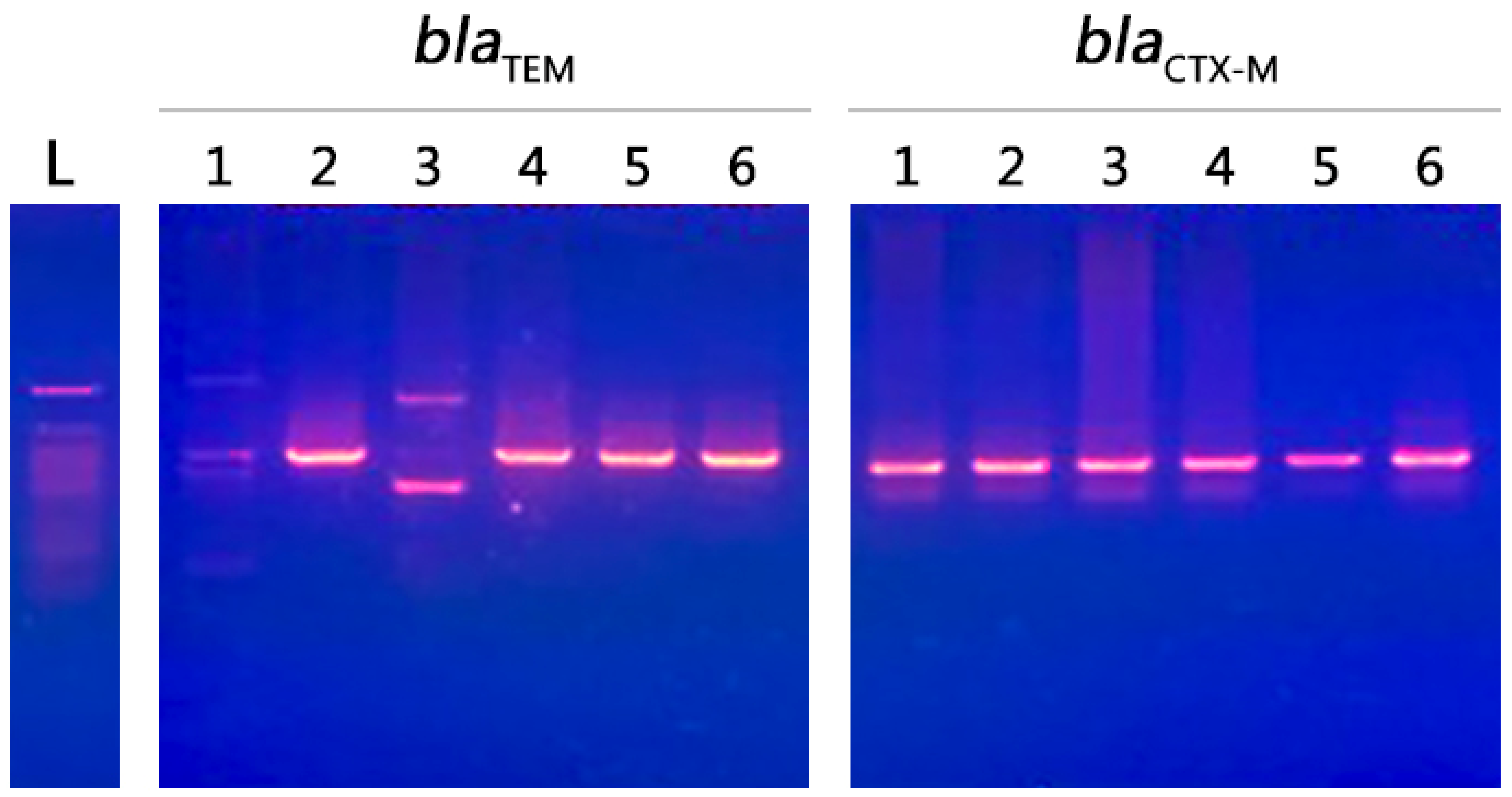
2.6. Molecular Characterization of Isolates
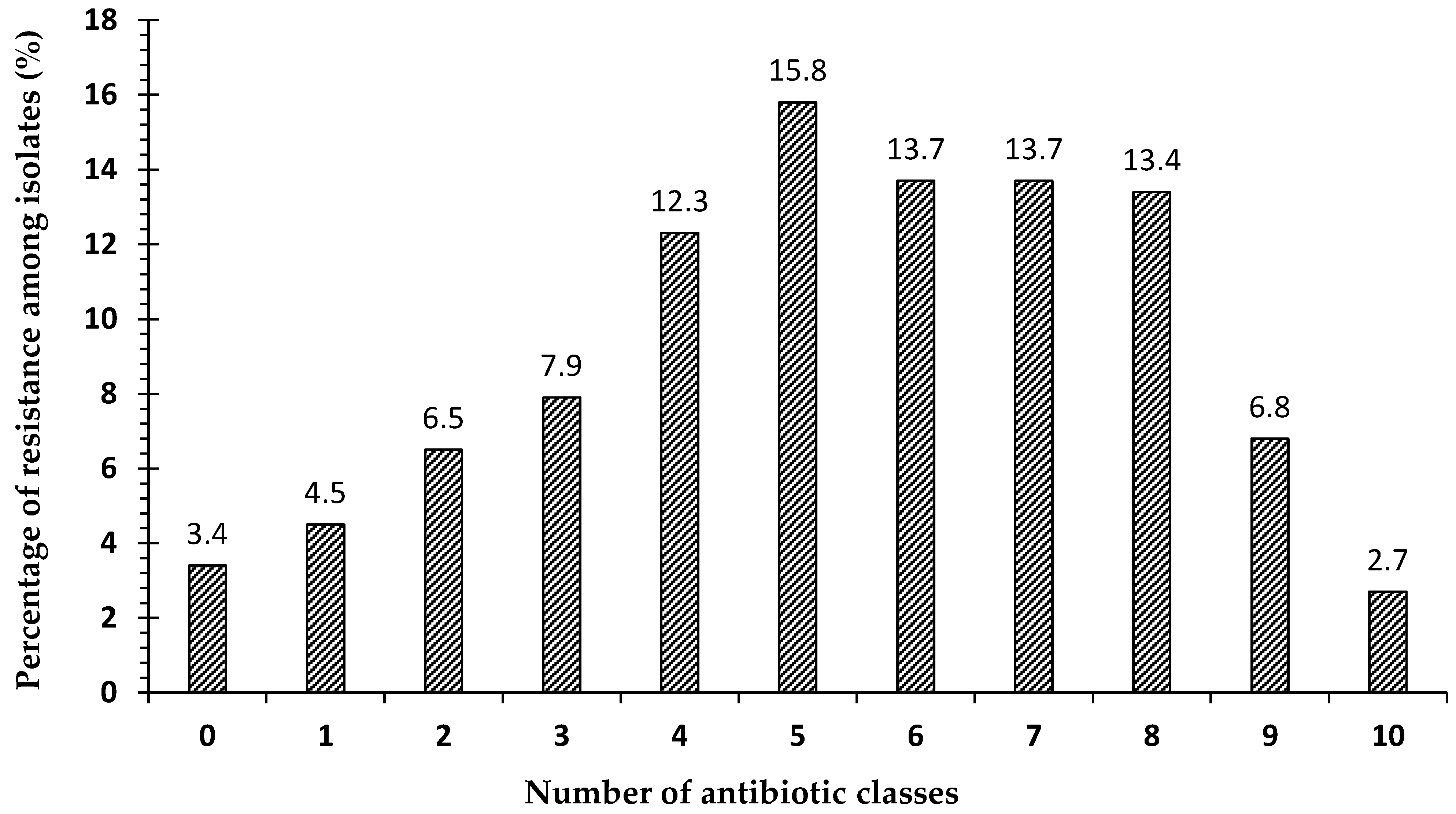
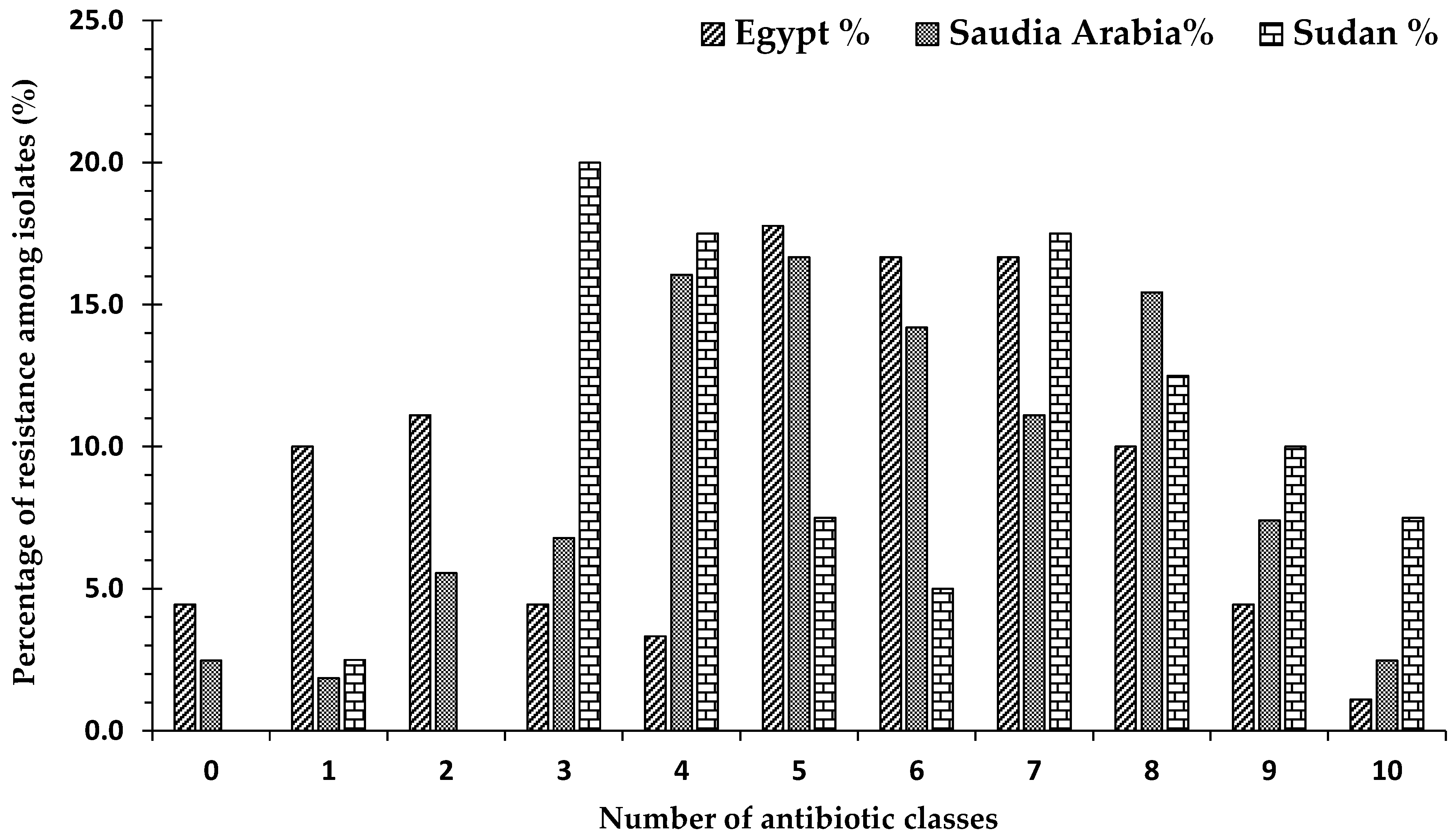
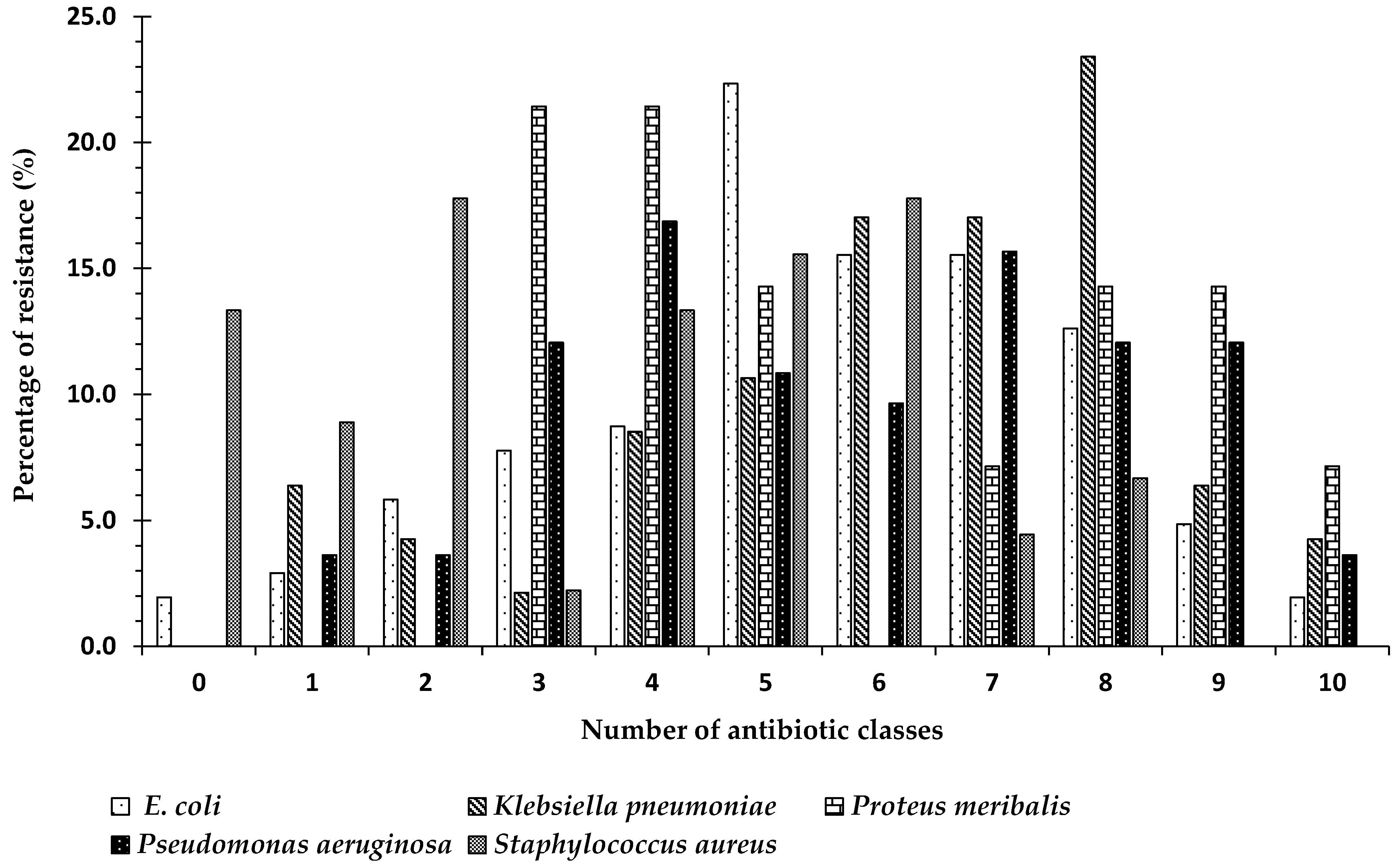
2.7. Frequency of Antibiotic Resistance
3. Discussion
4. Methods
4.1. Study Design and Specimen Collection
4.2. Isolation and Identification of Isolates
4.3. Antimicrobial Susceptibility Testing
4.4. Molecular Identification of Bacterial Isolates
4.5. Molecular Screening of Resistant Genes
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Sahm, D.F.; Thornsberry, C.; Mayfield, D.C.; Jones, M.E.; Karlowsky, J.A. Multidrug-resistant urinary tract isolates of Escherichia coli: Prevalence and patient demographics in the United States in 2000. Antimicrob. Agents Chemother. 2001, 45, 1402–1406. [Google Scholar] [CrossRef] [PubMed]
- Karlowsky, J.A.; Jones, M.E.; Draghi, D.C.; Thornsberry, C.; Sahm, D.F.; Volturo, G.A. Prevalence and antimicrobial susceptibilities of bacteria isolated from blood cultures of hospitalized patients in the United States in 2002. Ann. Clin. Microbiol. Antimicrob. 2004, 3, 7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van De Sande-Bruinsma, N.; Grundmann, H.; Verloo, D.; Tiemersma, E.; Monen, J.; Goossens, H.; Ferech, M.; European Antimicrobial Resistance Surveillance System Group; European Surveillance of Antimicrobial Consumption Project Group. Antimicrobial drug use and resistance in Europe. Emerg. Infect. Dis. 2008, 14, 1722–1730. [Google Scholar] [CrossRef]
- Okeke, I.N.; Aboderin, O.A.; Byarugaba, D.K.; Ojo, K.K.; Opintan, J.A. Growing problem of multidrug-resistant enteric pathogens in Africa. Emerg. Infect. Dis. 2007, 13, 1640. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Hsueh, P.-R.; Badal, R.E.; Hawser, S.P.; Hoban, D.J.; Bouchillon, S.K.; Ni, Y.; Paterson, D.L. Antimicrobial susceptibility profiles of aerobic and facultative Gram-negative bacilli isolated from patients with intra-abdominal infections in the Asia-Pacific region according to currently established susceptibility interpretive criteria. J. Infect. 2011, 62, 280–291. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Koneman, E.W.; Allen, S.D.; Janda, W.; Schreckenberger, P.; Winn, W. Diagnostic microbiology. In The Nonfermentative Gram-Negative Bacilli; Lippincott-Raven Publishers: Philedelphia, PA, USA, 1997; pp. 253–320. [Google Scholar]
- Coleman, B.L.; Louie, M.; Salvadori, M.I.; McEwen, S.A.; Neumann, N.; Sibley, K.; Irwin, R.J.; Jamieson, F.B.; Daignault, D.; Majury, A.; et al. Contamination of Canadian private drinking water sources with antimicrobial resistant Escherichia coli. Water Res. 2013, 47, 3026–3036. [Google Scholar] [CrossRef]
- Nys, S.; Okeke, I.N.; Kariuki, S.; Dinant, G.J.; Driessen, C.; Stobberingh, E.E. Antibiotic resistance of faecal Escherichia coli from healthy volunteers from eight developing countries. J. Antimicrob. Chemother. 2004, 54, 952–955. [Google Scholar] [CrossRef]
- Martin, R.M.; Bachman, M.A. Colonization, infection, and the accessory genome of Klebsiella pneumoniae. Front. Cell. Infect. Microbiol. 2018, 8, 4. [Google Scholar] [CrossRef]
- Bassetti, M.; Righi, E.; Carnelutti, A.; Graziano, E.; Russo, A. Multidrug-resistant Klebsiella pneumoniae: Challenges for treatment, prevention and infection control. Expert Rev. Anti-Infect. Ther. 2018, 16, 749–761. [Google Scholar] [CrossRef]
- Paterson, D.L.; Ko, W.-C.; Von Gottberg, A.; Mohapatra, S.; Casellas, J.M.; Goossens, H.; Mulazimoglu, L.; Trenholme, G.; Klugman, K.P.; Bonomo, R.A.; et al. Antibiotic Therapy for Klebsiella pneumoniae Bacteremia: Implications of production of extended-spectrum β-Lactamases. Clin. Infect. Dis. 2004, 39, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Azab, K.S.M.; Abdel-Rahman, M.A.; Farag, M.M.S.; El-Sheikh, H.H. Identification and distribution of pathogenic bacteria in clinical specimens within Egypt, Saudi Arabia, and Sudan. Al-Azhar Bull. Sci. 2020, 10, 25–34. [Google Scholar]
- Chong, Y.; Shimoda, S.; Shimono, N. Current epidemiology, genetic evolution and clinical impact of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect. Genet. Evol. 2018, 61, 185–188. [Google Scholar] [CrossRef]
- Higgins, P.; Wisplinghoff, H.; Krut, O.; Seifert, H. A PCR-based method to differentiate between Acinetobacter baumannii and Acinetobacter genomic species 13TU. Clin. Microbiol. Infect. 2007, 13, 1199–1201. [Google Scholar] [CrossRef] [PubMed]
- Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance. Microbiol. Spectr. 2016, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hamid, M.I.; Bendary, M.M.; Merwad, A.M.A.; Elsohaby, I.; Ghaith, D.M.; Alshareef, W.A. What is behind phylogenetic analysis of hospital-, community- and livestock-associated methicillin-resistant Staphylococcus aureus? Transbound. Emerg. Dis. 2019, 66, 1506–1517. [Google Scholar] [CrossRef] [PubMed]
- Abass, A.M.; Ahmed, M.E.; Ibrahim, I.G.; Yahia, S.A. Bacterial resistance to antibiotics: Current situation in Sudan. J. Adv. Microbiol. 2017, 6, 1–7. [Google Scholar] [CrossRef]
- Saeed, A.; Hamid, S.A.; Bayoumi, M.; Shanan, S.; Alouffi, S.; Alharbi, S.A.; Alshammari, F.D.; Abd, H. Elevated antibiotic resistance of Sudanese urinary tract infection bacteria. EXCLI J. 2017, 16, 1073–1080. [Google Scholar]
- Elbadawi, H.S.; Elhag, K.M.; Mahgoub, E.; Altayb, H.N.; Hamid, M.M.A. Antimicrobial resistance surveillance among Gram-negative bacterial isolates from patients in hospitals in Khartoum State, Sudan. F1000Research 2019, 8, 156. [Google Scholar] [CrossRef]
- Khalifa, H.O.; Soliman, A.M.; Ahmed, A.M.; Shimamoto, T.; Nariya, H.; Matsumoto, T.; Shimamoto, T. High prevalence of antimicrobial resistance in Gram-negative bacteria isolated from clinical settings in Egypt: Recalling for judicious use of conventional antimicrobials in developing nations. Microb. Drug Resist. 2019, 25, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Shehab El-Din, E.M.R.; El-Sokkary, M.M.A.; Bassiouny, M.R.; Hassan, R. Epidemiology of neonatal sepsis and implicated pathogens: A study from Egypt. BioMed Res. Int. 2015, 2015, 509484. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.M.; Ahmed, S.F.; Klena, J.D.; Mohamed, Z.K.; Moussa, T.; Ghenghesh, K.S. Enteroaggregative Escherichia coli in diarrheic children in Egypt: Molecular characterization and antimicrobial susceptibility. J. Infect. Dev. Ctries. 2014, 8, 589–596. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El-Zanfaly, H.T.; Kassim, E.-S.A.-A.; Badr-Eldin, S.M. Incidence of antibiotic resistant bacteria in drinking water in Cairo. Water Air Soil Pollut. 1987, 32, 123–128. [Google Scholar] [CrossRef]
- Zowawi, H.M. Antimicrobial resistance in Saudi Arabia. An urgent call for an immediate action. Saudi Med. J. 2016, 37, 935–940. [Google Scholar] [CrossRef]
- Zowawi, H.M.; Balkhy, H.H.; Walsh, T.R.; Paterson, D.L. β-Lactamase production in key Gram-negative pathogen isolates from the Arabian Peninsula. Clin. Microbiol. Rev. 2013, 26, 361–380. [Google Scholar] [CrossRef]
- Elmofti, H.A.; Almofti, Y.; Abuelhassan, N.N.; Omer, N.N. Identification and antibiotic resistance patterns of Escherichia coli isolated from broilers farms in Bahri locality/Sudan. Acta Sci. Nutr. Health 2019, 3, 197–203. [Google Scholar] [CrossRef]
- Mekki, A.; Hassan, A.; Eldin, D.; Elsayed, M. Extended spectrum beta lactamases among multi drug resistant Escherichia coli and Klebsiella species causing urinary tract infections in Khartoum. J. Bacteriol. Res. 2010, 2, 18–21. [Google Scholar]
- Ibrahim, M.; Bilal, N.; Hamid, M. Increased multi-drug resistant Escherichia coli from hospitals in Khartoum state, Sudan. Afr. Health Sci. 2013, 12, 368–375. [Google Scholar] [CrossRef]
- Ahmed, O.B.; Omar, A.O.; Asghar, A.H.; Elhassan, M.M. Increasing prevalence of ESBL-producing Enterobacteriaceae in Sudan community patients with UTIs. Egypt. Acad. J. Biol. Sci. G Microbiol. 2013, 5, 17–24. [Google Scholar] [CrossRef]
- Satir, S.; Elkhalifa, A.; Ali, M.; Rahim, A.; Elhussein, A.; Elkhidir, I.; Enan, K. Detection of carbepenem resistance genes among selected Gram negative bacteria isolated from patients in-Khartoum state, Sudan. Clin. Microbiol. 2016, 5, 6. [Google Scholar] [CrossRef]
- Yasir, M.; Ajlan, A.M.; Shakil, S.; Jiman-Fatani, A.A.; Almasaudi, S.B.; Farman, M.; Baazeem, Z.M.; Baabdullah, R.; Alawi, M.; Al-Abdullah, N.; et al. Molecular characterization, antimicrobial resistance and clinico-bioinformatics approaches to address the problem of extended-spectrum β-lactamase-producing Escherichia coli in western Saudi Arabia. Sci. Rep. 2018, 8, 14847. [Google Scholar] [CrossRef] [PubMed]
- Hemeg, H.A. Molecular characterization of antibiotic resistant Escherichia coli isolates recovered from food samples and outpatient Clinics, KSA. Saudi J. Biol. Sci. 2018, 25, 928–931. [Google Scholar] [CrossRef]
- Aly, M.E.; Essam, T.M.; Amin, M.A. Antibiotic resistance profile of E. coli strains isolated from clinical specimens and food samples in Egypt. Int. J. Microbiol. Res. 2012, 3, 176–182. [Google Scholar]
- Amer, M.M.; Mekky, H.M.; Amer, A.M.; Fedawy, H.S. Antimicrobial resistance genes in pathogenic Escherichia coli isolated from diseased broiler chickens in Egypt and their relationship with the phenotypic resistance characteristics. Vet. World 2018, 11, 1082–1088. [Google Scholar] [CrossRef]
- Mohammed, H.; Elsadek Fakhr, A.; Al Johery, S.a.E.; Hassanein, W.A.G. Spread of TEM, VIM, SHV, and CTX-M β-lactamases in imipenem-resistant Gram-negative bacilli isolated from Egyptian hospitals. Int. J. Microbiol. 2016, 2016, 8382605. [Google Scholar] [CrossRef]
- Ahmad, S.; Al-Juaid, N.F.; Alenzi, F.Q.; Mattar, E.H.; Bakheet, O.E.-S. Prevalence, antibiotic susceptibility pattern and production of extended-spectrum beta-lactamases amongst clinical isolates of Klebsiella pneumoniae at Armed Forces Hospital in Saudi Arabia. J. Coll. Physicians Surg. Pak. 2009, 19, 264–265. [Google Scholar] [PubMed]
- Azim, N.S.A.; Nofal, M.Y.; Alharbi, M.A.; Al-Zaban, M.I.; Somily, A.M. Molecular-diversity, prevalence and antibiotic susceptibility of pathogenic Klebsiella pneumoniae under Saudi Condition. Pak. J. Biol. Sci. 2019, 22, 174–179. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shawky, S.M.; Abdallah, A.; El Kholy, M. Antimicrobial activity of Colistin and Tigecycline against carbapenem resistant Klebsiella pneumoniae clinical isolates in Alexandria, Egypt. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 731–742. [Google Scholar]
- Wasfi, R.; Elkhatib, W.F.; Ashour, H.M. Molecular typing and virulence analysis of multidrug resistant Klebsiella pneumoniae clinical isolates recovered from Egyptian hospitals. Sci. Rep. 2016, 6, 38929. [Google Scholar] [CrossRef] [PubMed]
- El-Badawy, M.F.; Tawakol, W.M.; El-Far, S.W.; Maghrabi, I.A.; Al-Ghamdi, S.A.; Mansy, M.S.; Ashour, M.S.; Shohayeb, M.M. Molecular identification of Aminoglycoside-modifying enzymes and plasmid-mediated Quinolone resistance genes among Klebsiella pneumoniae clinical isolates recovered from Egyptian patients. Int. J. Microbiol. 2017, 2017, 8050432. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.D.; Taee, S.; Dezfuli, A.A.; Meghdadi, H.; Shafie, F. Investigation of the prevalence of genes conferring resistance to carbapenems in Pseudomonas aeruginosa isolates from burn patients. Infect. Drug Resist. 2019, 12, 1153–1159. [Google Scholar] [CrossRef]
- Alkeshan, Y. Antimicrobial Resistance Pattern of Pseudomonas aeruginosa in Regional Tertiary Care Hospitals of Saudi Arabia. IOSR J. Dent. Med. Sci. 2014, 5, 54–62. [Google Scholar]
- Khan, M.A.; Faiz, A. Antimicrobial resistance patterns of Pseudomonas aeruginosa in tertiary care hospitals of Makkah and Jeddah. Ann. Saudi Med. 2016, 36, 23–28. [Google Scholar] [CrossRef]
- Gad, G.F.; El-Domany, R.A.; Zaki, S.; Ashour, H.M. Characterization of Pseudomonas aeruginosa isolated from clinical and environmental samples in Minia, Egypt: Prevalence, antibiogram and resistance mechanisms. J. Antimicrob. Chemother. 2007, 60, 1010–1017. [Google Scholar] [CrossRef]
- Elshafiee, E.A.; Nader, S.M.; Dorgham, S.M.; Hamza, D.A. Carbapenem-resistant Pseudomonas aeruginosa originating from farm animals and people in Egypt. J. Vet. Res. 2019, 63, 333–337. [Google Scholar] [CrossRef]
- Farhan, S.M.; Ibrahim, R.A.; Mahran, K.M.; Hetta, H.F.; El-Baky, R.M.A. Antimicrobial resistance pattern and molecular genetic distribution of metallo-β-lactamases producing Pseudomonas aeruginosa isolated from hospitals in Minia, Egypt. Infect. Drug Resist. 2019, 12, 2125–2133. [Google Scholar] [CrossRef]
- Helmy, O.M.; Kashef, M.T. Different phenotypic and molecular mechanisms associated with multidrug resistance in Gram-negative clinical isolates from Egypt. Infect. Drug Resist. 2017, 10, 479–498. [Google Scholar] [CrossRef]
- Dandachi, I.; Chaddad, A.; Hanna, J.; Matta, J.; Daoud, Z. Understanding the epidemiology of multi-drug resistant Gram-negative bacilli in the Middle East using a one health approach. Front. Microbiol. 2019, 10, 1941. [Google Scholar] [CrossRef]
- Ojdana, D.; Sacha, P.; Wieczorek, P.; Czaban, S.; Michalska-Falkowska, A.; Jaworowska, J.; Jurczak, A.; Poniatowski, B.; Tryniszewska, E. The occurrence of blaCTX-M, blaSHV, and blaTEM genes in extended-spectrum β-Lactamase-positive strains of Klebsiella pneumoniae, Escherichia coli, and Proteus mirabilis in Poland. J. Antibiot. 2014, 2014, 7. [Google Scholar] [CrossRef]
- Al-Agamy, M.H.M.; Ashour, M.S.E.-D.; Wiegand, I. First description of CTX-M β-lactamase-producing clinical Escherichia coli isolates from Egypt. Int. J. Antimicrob. Agents 2006, 27, 545–548. [Google Scholar]
- Ahmed, O.; Omar, A.; Asghar, A.; El hassan, M. Prevalence of TEM, SHV and CTX-M genes in Escherichia coli and Klebsiella spp urinary isolates from Sudan with confirmed ESBL phenotype. Life Sci. J. 2013, 10, 191–195. [Google Scholar]
- Elkhateeb, H.; Abdel-Mongy, M.; Othman, A. In vitro Detection of Antibacterial Activity of Glycyrrhizic Acid Nanoparticle against ESBL Producing Klebsiella pneumoniae Strains. Egypt. J. Microbiol. 2018, 53, 193–205. [Google Scholar] [CrossRef]
- Osman, A.E.M.A.E.; Hashim, S.O.; Musa, M.A.; Tahir, O.M. Detection of CTX-M, TEM and SHV Genes in Gram negative bacteria isolated from nosocomial patients at Port Sudan Teaching Hospital. Eur. J. Clin. Biomed. Sci. 2017, 3, 101. [Google Scholar] [CrossRef][Green Version]
- Altayb, H.N.; Siddig, M.; El Amin, N.M.; Maowia, A.I.H.; Mukhtar, M. Molecular Characterization of CTX-M ESBLs among Pathogenic Enterobacteriaceae isolated from different regions in Sudan. Glob. Adv. Res. J. Microbiol. 2018, 7, 40–47. [Google Scholar]
- El Hassan, M.; Ozbazk, H.A.; Hemeg, H.A.; Ahmed, A. Dissemination of CTX-M extended-spectrum β-lactamases (ESBLs) among Escherichia coli and Klebsiella pneumoniae in Al-Madenah Al-Monawwarah region, Saudi Arabia. Int. J. Clin. Exp. Med. 2016, 9, 11051–11057. [Google Scholar]
- Winn, W.C.; Allen, S.D.; Janda, W.M.; Koneman, E.W.; Procop, G.; Schreckenberger, P.; Woods, G.L. Color Atlas and Textbook of Diagnostic Microbiology, 6th ed.; Jones and Bartlett Learning: Burlington, MA, USA, 2005. [Google Scholar]
- Spector, W.S. Handbook of Toxicology; National Academy Of Sciences—National Research Council: Washington, DC, USA, 1955; Volume 1. [Google Scholar]
- Sandys, G.H. A new method of preventing swarming of Proteus sp. with a description of a new medium suitable for use in routine laboratory practice. J. Med. Lab. Technol. 1960, 17, 224–233. [Google Scholar]
- McLeod, J.W.; Wheatley, B.; Phelon, H.V. On Some of the Unexplained Difficulties met with in cultivating the Gonococcus: The part played by the amino-acids. Br. J. Exp. Pathol. 1927, 8, 25–37. [Google Scholar]
- MacConkey, A.T. Note on some Cases of Food-poisoning. In Epidemiology and Infection; Cambridge University Press: Cambridge, UK, 2009; Volume 6, pp. 570–573. [Google Scholar]
- Thayer, J.D.; Martin, J.E., Jr. A selective medium for the cultivation of N. gonorrhoeae and N. meningitidis. Public Health Rep. 1964, 79, 49–57. [Google Scholar] [CrossRef]
- Sutherland, C. District Laboratory Practice in Tropical Countries 2nd edition, Part 1. Monica Cheesbrough 454 pp Price £50 ISBN 0521676304 Cambridge: CUP, 2005. Trop. Dr. 2008, 38, 132. [Google Scholar] [CrossRef]
- Pincus, D.H.; Zbinden, R.; Bosshard, P.P.; Bottger, E.C. New Vitek 2 Colorimetric GN Card for Identification of Gram-Negative Nonfermentative Bacilli. J. Clin. Microbiol. 2007, 45, 4094–4095. [Google Scholar] [CrossRef][Green Version]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: 25th Informational Supplemen; CLSI Document M100–S25; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Miller, D.N.; Bryant, J.E.; Madsen, E.L.; Ghiorse, W.C. Evaluation and optimization of DNA extraction and purification procedures for soil and sediment samples. Appl. Environ. Microbiol. 1999, 65, 4715–4724. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.; Ling, C.L.; Ciesielczuk, H.L.; Lockwood, J.; Hopkins, S.; McHugh, T.D.; Gillespie, S.H.; Kibbler, C.C. Detection and identification of bacteria in clinical samples by 16S rRNA gene sequencing: Comparison of two different approaches in clinical practice. J. Med. Microbiol. 2012, 61, 483–488. [Google Scholar] [CrossRef]
- Sundsfjord, A.; Simonsen, G.S.; Haldorsen, B.C.; Haaheim, H.; Hjelmevoll, S.-O.; Littauer, P.; Dahl, K.H. Genetic methods for detection of antimicrobial resistance. Acta Pathol. Microbiol. Immunol. Scand. 2004, 112, 815–837. [Google Scholar] [CrossRef] [PubMed]
| Percentage of Resistance to Various Antibiotic Classes: | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Specimen | Penicillins | Cephalosporins 1st | Cephalosporins 2nd | Cephalosporins 3rd | Macrolides | Aminoglycosides | Tetracyclines | Quinolones | Sulfonamides | Nitrofurans | Carbapenems |
| Abscess | 81 | 70 | 82 | 81 | 70 | 57 | 85 | 24 | 67 | 57 | 6 |
| Blood | 82 | 46 | 50 | 54 | 90 | 59 | 55 | 37 | 69 | 0 | 64 |
| Ear Swab | 86 | 68 | 55 | 73 | 67 | 64 | 50 | 18 | 59 | 77 | 9 |
| Endotracheal tube | 100 | 63 | 63 | 57 | 0 | 38 | 33 | 38 | 57 | 100 | 38 |
| Nasal Swap | 100 | 100 | 100 | 67 | 50 | 33 | 100 | 67 | 50 | 50 | 67 |
| Sputum | 88 | 86 | 88 | 75 | - | 63 | 67 | 75 | 63 | - | 50 |
| Throat Swap | 100 | 100 | 100 | 67 | 100 | 33 | 50 | 0 | 100 | 100 | 0 |
| Urethral | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Urine | 88 | 81 | 61 | 54 | 91 | 45 | 71 | 43 | 59 | 29 | 4 |
| Vaginal Swap | 79 | 80 | 47 | 49 | 84 | 32 | 70 | 35 | 49 | 31 | 0 |
| Resistance to: | E. coli Strains (%) | Klebsiella pneumoniae Strains (%) | Pseudomonas aeruginosa Strains (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Egypt | Sudan | Saudi Arabia | Egypt | Sudan | Saudi Arabia | Egypt | Sudan | Saudi Arabia | |
| Penicillins | 87.9 | 87.5 | 90.7 | 81.3 | 100.0 | 83.3 | 100.0 | 94.1 | 90.5 |
| Cephalosporins 1st | 75.0 | 80.0 | 92.5 | 80.0 | 100.0 | 70.8 | 61.5 | 58.9 | 95.1 |
| Cephalosporins 2nd | 72.7 | 62.5 | 51.9 | 81.3 | 100.0 | 41.7 | 79.2 | 100.0 | 66.8 |
| Cephalosporins 3rd | 75.8 | 56.3 | 44.4 | 75.0 | 100.0 | 50.0 | 66.7 | 70.6 | 42.9 |
| Macrolides | 90.9 | 100.0 | 98.1 | 100.0 | 100.0 | 100.0 | 100.0 | 35.3 | 90.5 |
| Aminoglycosides | 41.9 | 21.4 | 48.1 | 62.5 | 71.4 | 50.0 | 41.7 | 12.5 | 50.0 |
| Tetracyclines | 66.7 | 80.0 | 82.4 | 33.3 | 28.6 | 73.9 | 83.3 | 29.4 | 92.7 |
| Quinolones | 57.6 | 50.0 | 35.2 | 81.3 | 100.0 | 33.3 | 47.8 | 11.8 | 16.7 |
| Sulfonamide | 48.5 | 66.7 | 61.5 | 56.3 | 28.6 | 54.2 | 78.3 | 58.8 | 66.0 |
| Nitrofuran | 4.5 | 6.3 | 11.1 | 85.7 | 85.7 | 42.1 | 100.0 | 18.8 | 54.8 |
| Carbapenem | 0 | 0 | 2.3 | 56.3 | 0 | 0.0 | 25.0 | 0 | 4.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azab, K.S.M.; Abdel-Rahman, M.A.; El-Sheikh, H.H.; Azab, E.; Gobouri, A.A.; Farag, M.M.S. Distribution of Extended-Spectrum β-Lactamase (ESBL)-Encoding Genes among Multidrug-Resistant Gram-Negative Pathogens Collected from Three Different Countries. Antibiotics 2021, 10, 247. https://doi.org/10.3390/antibiotics10030247
Azab KSM, Abdel-Rahman MA, El-Sheikh HH, Azab E, Gobouri AA, Farag MMS. Distribution of Extended-Spectrum β-Lactamase (ESBL)-Encoding Genes among Multidrug-Resistant Gram-Negative Pathogens Collected from Three Different Countries. Antibiotics. 2021; 10(3):247. https://doi.org/10.3390/antibiotics10030247
Chicago/Turabian StyleAzab, Khaled S. M., Mohamed Ali Abdel-Rahman, Hussien H. El-Sheikh, Ehab Azab, Adil A. Gobouri, and Mohamed M. S. Farag. 2021. "Distribution of Extended-Spectrum β-Lactamase (ESBL)-Encoding Genes among Multidrug-Resistant Gram-Negative Pathogens Collected from Three Different Countries" Antibiotics 10, no. 3: 247. https://doi.org/10.3390/antibiotics10030247
APA StyleAzab, K. S. M., Abdel-Rahman, M. A., El-Sheikh, H. H., Azab, E., Gobouri, A. A., & Farag, M. M. S. (2021). Distribution of Extended-Spectrum β-Lactamase (ESBL)-Encoding Genes among Multidrug-Resistant Gram-Negative Pathogens Collected from Three Different Countries. Antibiotics, 10(3), 247. https://doi.org/10.3390/antibiotics10030247







