Phage-Encoded Endolysins
Abstract
1. Introduction
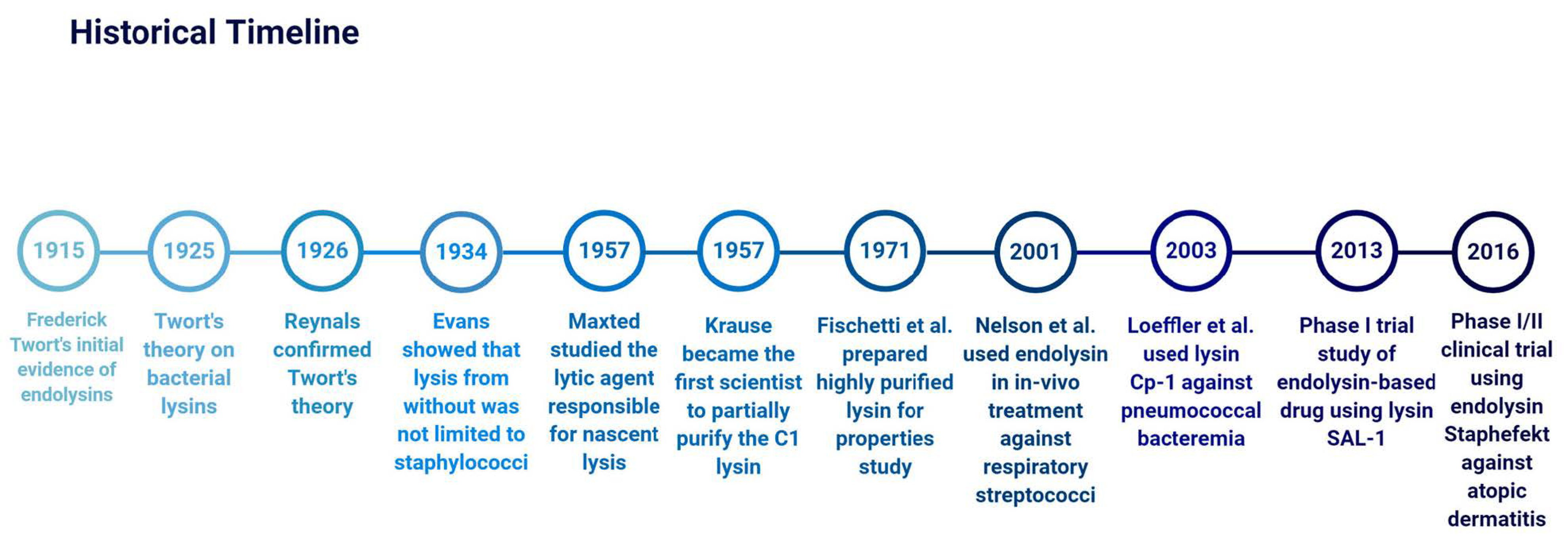
2. History of Endolysins
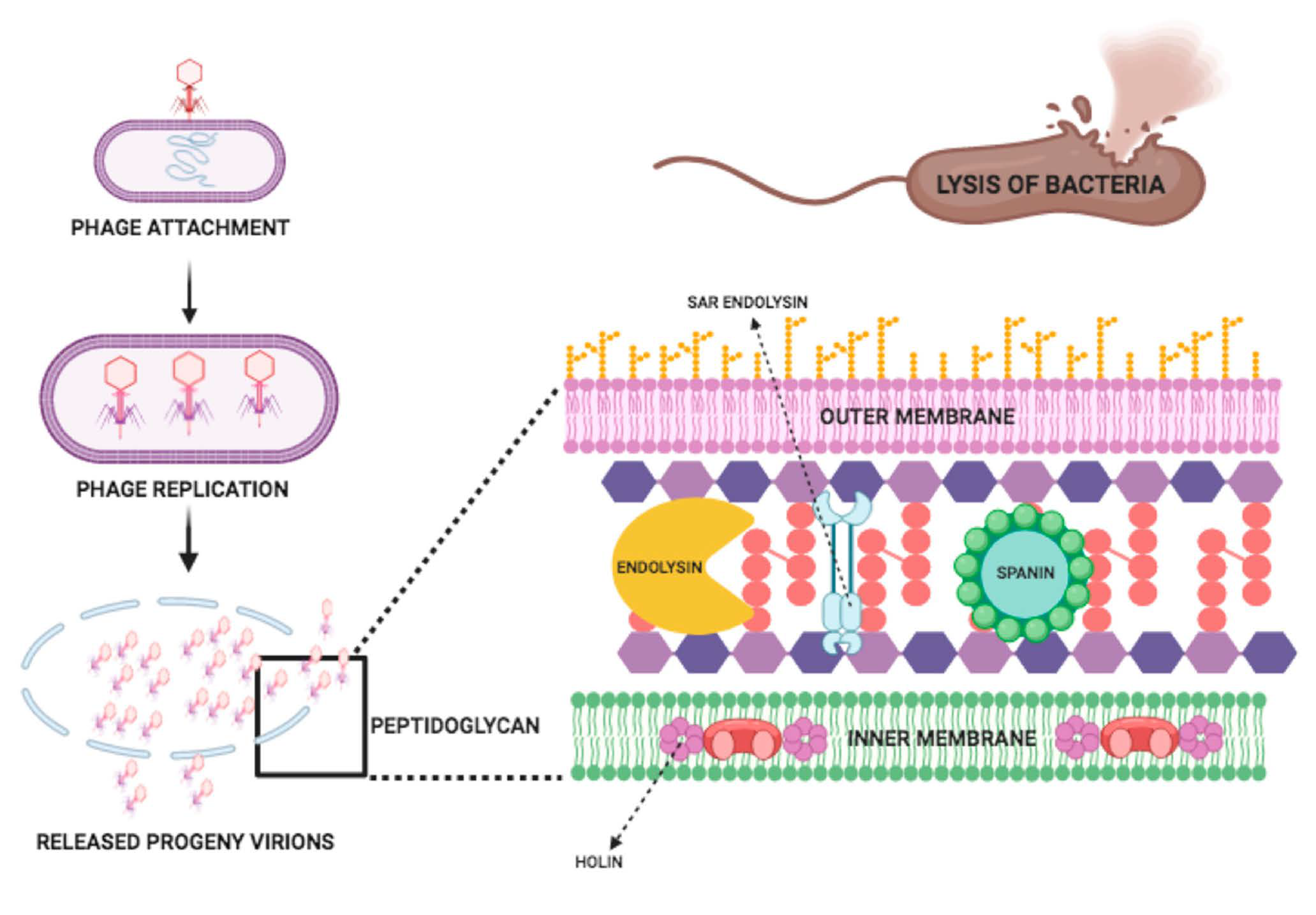
3. Endolysin Structure and Mode of Action
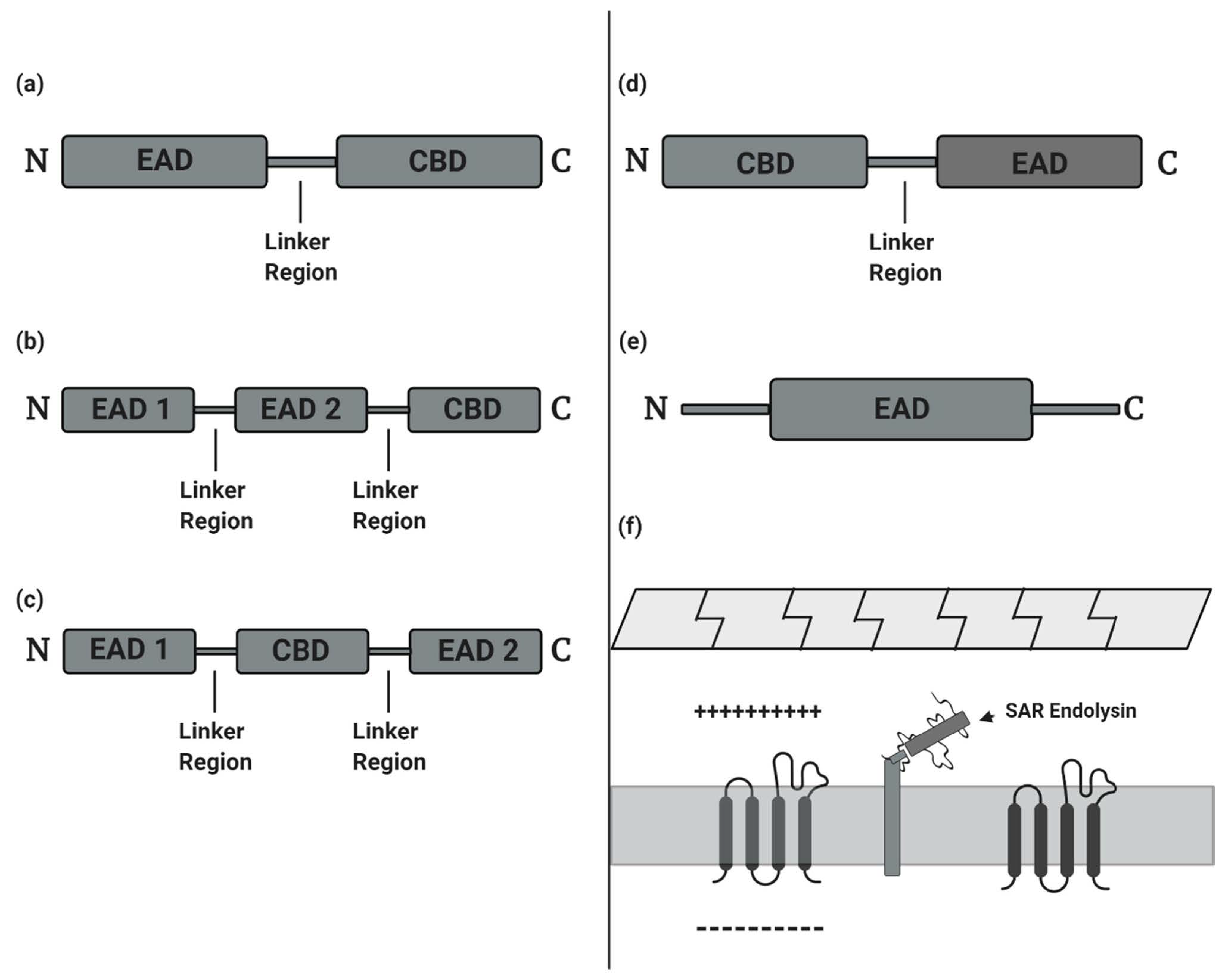
- (a)
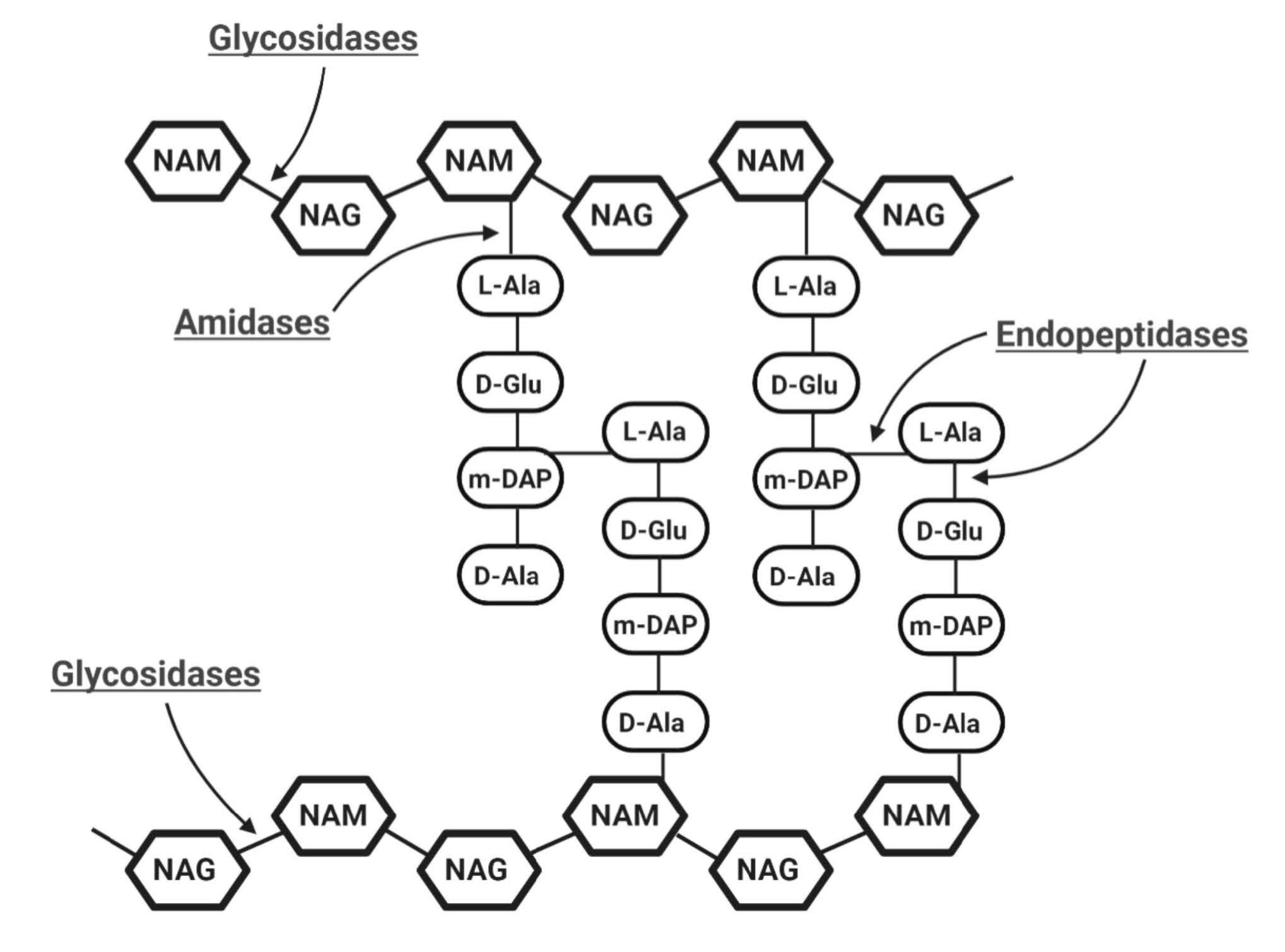
- Glycosidases generally cleave the β-1,4 glycosidic bonds linking alternating polymeric structures of N-acetylmuramic acids (MurNAc) and N-acetylglucosamines (GlcNAc) in the peptidoglycan layer. Subclasses of Glycosidases include N-acetyl-β-D-muramidases, which cleave bonds between MurNAc and GlcNAc; N-acetyl-β-D-glucosidases, which cleave bonds between GlcNAc and MurNAc residues; and lytic transglycosylases, which are not considered as true hydrolases as they do not require water molecules for their catalytic action [21]. Like the other two glycosidases, transglycosylases cleave β-1,4 bonds between MurNAc and GlcNAc, but also involve an intra-molecular reaction that results in the generation of a 1,6-anhydro ring at the MurNAc residue [26,27].
- (b)
- Amidases, as N-acetylmuramoyl-L-alanine amidase, catalyze the cleavage of amide bonds between the MurNAc and the first amino acid in the peptide stem moiety, L-alanine.
- (c)
- Endopeptidases cleave bonds between two amino acids of the stem peptide. Bond cleavage can either occur within interpeptide bridge or stem peptide–interpeptide bridge [27]. Examples include L-alanoyl-D-glutamate endopeptidase (VANY), c-D-glutamyl-m-diaminopimelic (DAP) acid peptidase, D-Ala-m-DAP endopeptidase, D-alanyl-glycyl endopeptidase (CHAP), etc. [28,29].
4. Regulation of Expression
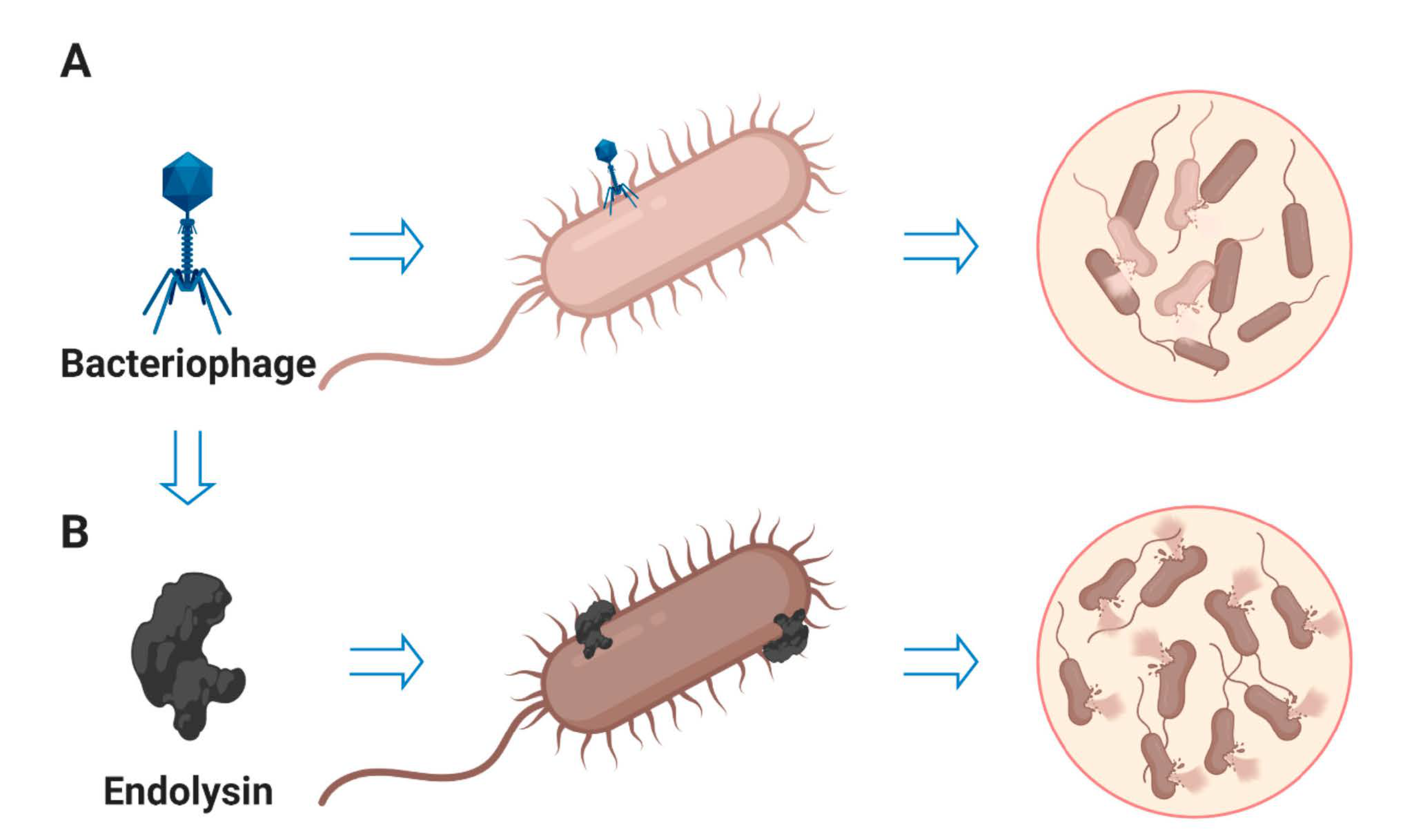
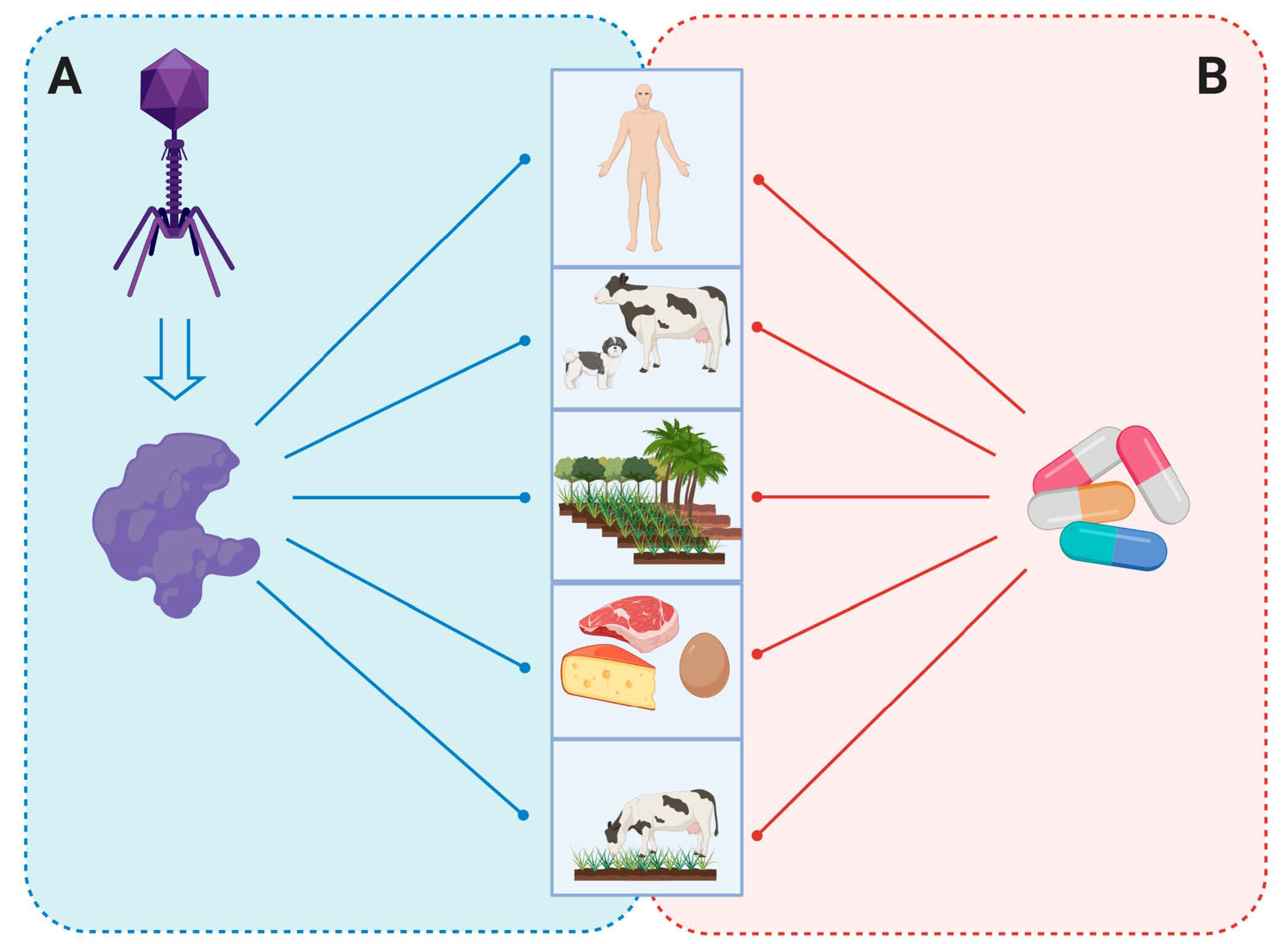
5. Endolysins as Antibacterial Agent
5.1. Human Medicine
5.2. Veterinary Sector
5.3. Agriculture and Plants
5.4. Food and Biotechnology
5.4.1. Food
5.4.2. Biotechnology
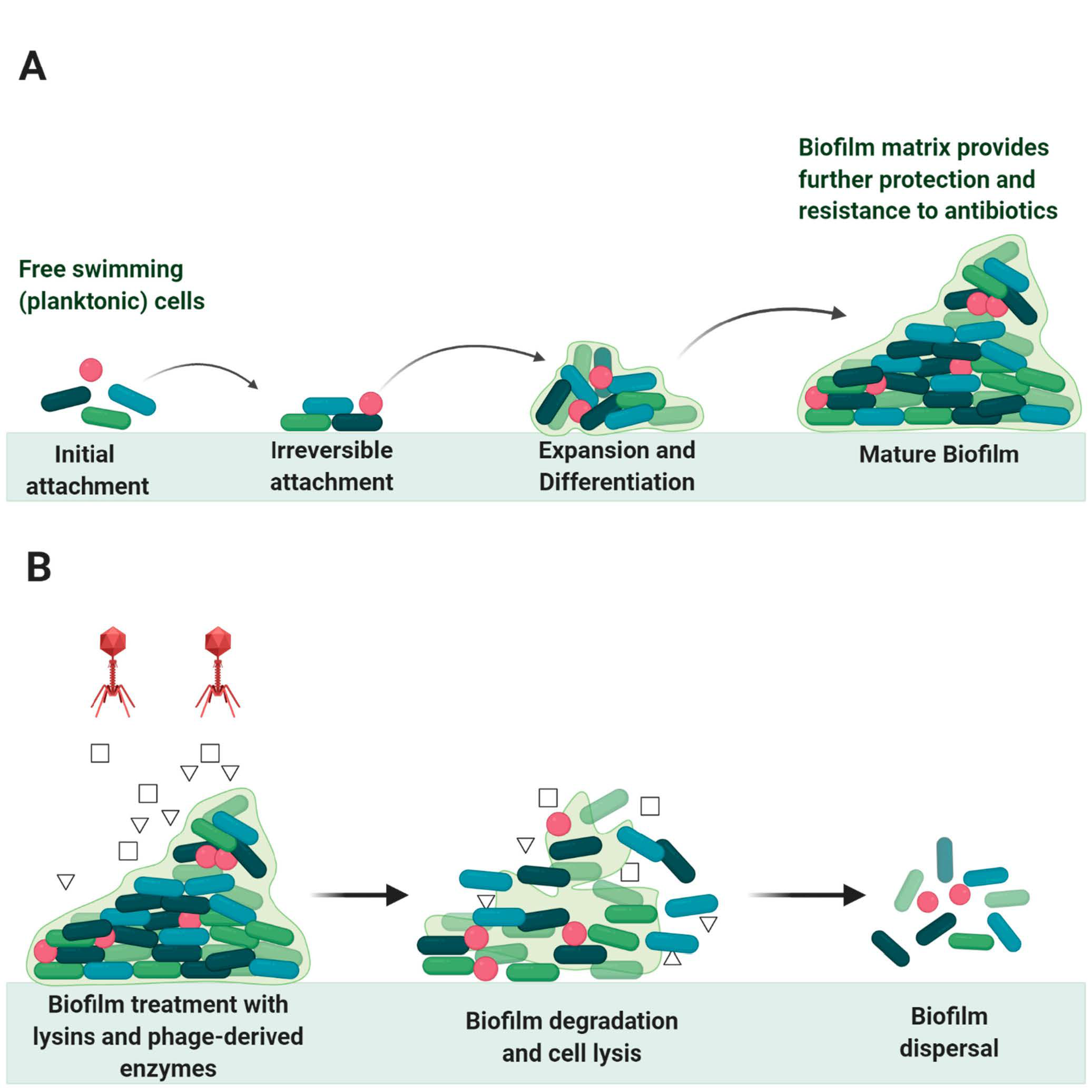
6. Endolysin in Biofilms Eradication
7. Immunogenicity, Safety, and Resistance
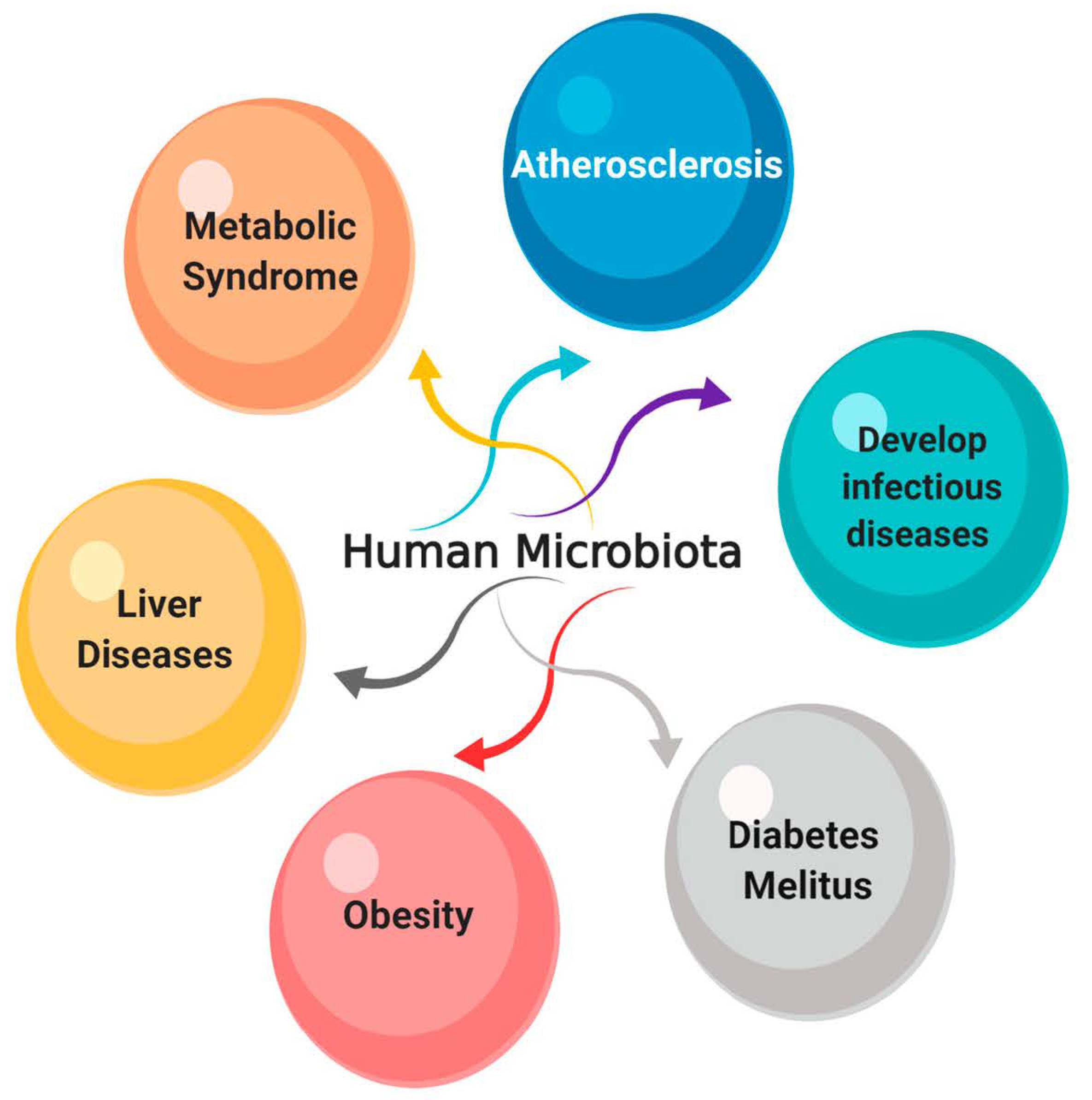
8. The Effect of Endolysins on the Normal Microbiota
9. Endolysins and Antibiotics
10. Formulation and Administration Routes
| Target Pathogen | Phage | Enzyme (Endolysin) | Activity (Mode of Action) | Administration Route | References |
|---|---|---|---|---|---|
| Streptococcus pneumoniae | Cp1 | Cpl-1 | Muramidase | Intravenous, nasal, oral, aerosols, and Intraperitoneal | [17,150,151,152,153,154] |
| Dp-1 | Pal | Amidase | Nasal and oral | [50,155] | |
| CP-7 | Cpl-7 | Muramidase | Immersion | [156,157] | |
| Streptococcus pyogenes | C1 | PlyC | Amidase | Oral, nasal | [16] |
| MGAS5005 prophage | PlyPy | Endopeptidase | Intraperitoneal | [158] | |
| Streptococcus agalactiae | NCTC11261 | PlyGBS | Endopeptidase and Muramidase | Intravaginal, oral and intranasal | [159] |
| SK1249 prophage | PlySK1249 | Amidase and endopeptidase | Intraperitoneal | [160] | |
| MRSA | GH15 | LysGH15 | Amidase and endopeptidase | Intravenous and Intraperitoneal | [123,161] |
| MR11 | MV-L | Amidase and endopeptidase | Intraperitoneal, nasal | [141] | |
| K | LysK | Amidase and endopeptidase | Intraperitoneal | [162] | |
| SAP-1 | SAL-1 | Amidase and endopeptidase | Intravenous | [18] | |
| phiSH2 prophage | phiSH2 | Amidase and endopeptidase | Intraperitoneal | [162] | |
| phiP68 | P68 | Amidase and endopeptidase | Intraperitoneal | [162] | |
| phiWMY | LysWMY | Amidase and endopeptidase | Intraperitoneal | [162] | |
| phi80α | 80αLyt2 | Amidase and endopeptidase | Intraperitoneal | [162] | |
| phi11 | phi11 | Amidase and endopeptidase | Intraperitoneal | [162] | |
| 2854 prophage | 2638A | Amidase and endopeptidase | Intraperitoneal | [162] | |
| Pseudomonas aeruginosa | phage PVP-SE1 | Artilysin® Engineered Endolysin-Based (PVP-SE1gp146) | Muramidase | Oral and topical | [79] |
| P. aeruginosa phage | PlyPa03 | Muramidase | Topical | [163] | |
| P. aeruginosa phage | PlyPa91 | Muramidase | Intranasal | [163] | |
| Acinetobacter baumannii | RL-2015 | PlyF307 | Muramidase | Intraperitoneal and Topical | [164] |
| SS3e | LysSS | Muramidase | Intraperitoneal | [165] | |
| Bacillus anthracis | γ-phage | PlyG | Amidase | Intraperitoneal | [93] |
| Enterococcus faecalis | E. faecalis phage IME-EF1 | LysIME-EF1 | Endopeptidase | Intraperitoneal | [166] |
| E. faecalis phage EF-P10 | LysEF-P10 | Endopeptidase | Intraperitoneal | [167] |
11. Insights into the Clinical Trials of Engineered Endolysins
12. Challenges in Clinical Trials
13. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gondil, V.S.; Harjai, K.; Chhibber, S. Endolysins as emerging alternative therapeutic agents to counter drug-resistant infections. Int. J. Antimicrob. Agents. 2020, 55, 105844. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rubio, L.; Gutiérrez, D.; Donovan, D.M.; Martínez, B.; Rodríguez, A.; García, P. Phage lytic proteins: Biotechnological applications beyond clinical antimicrobials. Crit. Rev. Biotechnol. 2016, 36, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; São-José, C.; Azeredo, J. Phage-Derived Peptidoglycan Degrading Enzymes: Challenges and Future Prospects for in-vivo Therapy. Viruses 2018, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.N.; Smith, D.L.; Young, R. 1 Holins: The Protein Clocks of Bacteriophage Infections. Annu. Rev. Microbiol. 2000, 54, 799–825. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.M.; García, P.; García, J.L. Construction of a Multifunctional Pneumococcal Murein Hydrolase by Module Assembly. Eur. J. Biochem. 1996, 235, 601–605. [Google Scholar] [CrossRef]
- Lopez, R.; Garcia, E.; Garcia, P.; Garcia, J.L. The Pneumococcal Cell Wall Degrading Enzymes: A Modular Design to Create New Lysins? Microb. Drug Resist. 1997, 3, 199–211. [Google Scholar] [CrossRef]
- Young, R. Bacteriophage Holins: Deadly Diversity. J. Mol. Microbiol. Biotechnol. 2002, 4, 21–36. [Google Scholar]
- Donovan, D.M. Bacteriophage and Peptidoglycan Degrading Enzymes with Antimicrobial Applications. Recent Pat. Biotechnol. 2007, 1, 113–122. [Google Scholar] [CrossRef]
- Twort, F.W. An investigation on the nature of ultra-microscopic viruses. Lancet 1915, 186, 1241–1243. [Google Scholar] [CrossRef]
- Yang, D.C.; Tan, K.; Joachimiak, A.; Bernhardt, T.G. A conformational switch controls cell wall-remodelling enzymes required for bacterial cell division. Mol. Microbiol. 2012, 85, 768–781. [Google Scholar] [CrossRef]
- Twort, F.; Twort, F.W. The transmissible bacterial lysin and its action on dead bacteria. Lancet 1925, 206, 642–644. [Google Scholar] [CrossRef]
- Reynals, F.D. Bacteriophage et microbes tues. Compt. Rend. Soc. Biol. 1926, 94, 242–243. [Google Scholar]
- Evans, A.C. Streptococcus bacteriophage: A study of four serological types. Public Health Rep. 1934, 49, 1386–1401. [Google Scholar] [CrossRef]
- Maxted, W.R. The Active Agent in Nascent Phage Lysis of Streptococci. J. Gen. Microbiol. 1957, 16, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Young, R. Bacteriophage lysis: Mechanism and regulation. Microbiol. Rev. 1992, 56, 430–481. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.; Loomis, L.; Fischetti, V.A. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc. Natl. Acad. Sci. USA 2001, 98, 4107–4112. [Google Scholar] [CrossRef]
- Loeffler, J.M.; Djurkovic, S.; Fischetti, V.A. Phage Lytic Enzyme Cpl-1 as a Novel Antimicrobial for Pneumococcal Bacteremia. Infect. Immun. 2003, 71, 6199–6204. [Google Scholar] [CrossRef]
- Jun, S.Y.; Jung, G.M.; Yoon, S.J.; Oh, M.-D.; Choi, Y.-J.; Lee, W.J.; Kong, J.-C.; Seol, J.G.; Kang, S.H. Antibacterial properties of a pre-formulated recombinant phage endolysin, SAL-1. Int. J. Antimicrob. Agents 2013, 41, 156–161. [Google Scholar] [CrossRef]
- Totté, J.E.E.; van Doorn, M.B.; Pasmans, S.G.M.A. Successful Treatment of Chronic Staphylococcus aureus-Related Dermatoses with the Topical Endolysin Staphefekt SA.100: A Report of 3 Cases. Case Rep. Derm. 2017. [Google Scholar] [CrossRef]
- Abdelkader, K.; Gerstmans, H.; Saafan, A.; Dishisha, T.; Briers, Y. The Preclinical and Clinical Progress of Bacteriophages and Their Lytic Enzymes: The Parts are Easier than the Whole. Viruses 2019, 11, 96. [Google Scholar] [CrossRef]
- Matamp, N.; Bhat, S. Phage Endolysins as Potential Antimicrobials against Multidrug Resistant Vibrio alginolyticus and Vibrio parahaemolyticus: Current Status of Research and Challenges Ahead. Microorganisms 2019, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Fischetti, V. Development of Phage Lysins as Novel Therapeutics: A Historical Perspective. Viruses 2018, 10, 310. [Google Scholar] [CrossRef] [PubMed]
- Briers, Y.; Volckaert, G.; Cornelissen, A.; Lagaert, S.; Michiels, C.W.; Hertveldt, K.; Lavigne, R. Muralytic activity and modular structure of the endolysins of Pseudomonas aeruginosa bacteriophages φKZ and EL. Mol. Microbiol. 2007, 65, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Struck, D.K.; Deaton, J.; Wang, I.N.; Young, R. A signal-arrest-release sequence mediates export and control of the phage P1 endolysin. Proc. Natl. Acad. Sci. USA 2004, 101, 6415–6420. [Google Scholar] [CrossRef] [PubMed]
- Kuty, G.F.; Xu, M.; Struck, D.K.; Summer, E.J.; Young, R. Regulation of a phage endolysin by disulfide caging. J. Bacteriol. 2010, 192, 5682–5687. [Google Scholar] [CrossRef]
- Höltje, J.V.; Mirelman, D.; Sharon, N.; Schwarz, U. Novel type of murein transglycosylase in Escherichia coli. J. Bacteriol. 1975, 124, 1067–1076. [Google Scholar] [CrossRef]
- Rodríguez-Rubio, L.; Gerstmans, H.; Thorpe, S.; Mesnage, S.; Lavigne, R.; Briers, Y. DUF3380 Domain from a Salmonella Phage Endolysin Shows Potent N -Acetylmuramidase Activity. Appl. Environ. Microbiol. 2016, 82, 4975–4981. [Google Scholar] [CrossRef]
- Payne, K.M.; Hatfull, G.F. Mycobacteriophage Endolysins: Diverse and Modular Enzymes with Multiple Catalytic Activities. PLoS ONE 2012, 7, e34052. [Google Scholar] [CrossRef]
- Oliveira, H.; Melo, L.D.R.; Santos, S.B.; Nobrega, F.L.; Ferreira, E.C.; Cerca, N.; Azeredo, J.; Kluskens, L.D. Molecular Aspects and Comparative Genomics of Bacteriophage Endolysins. J. Virol. 2013, 87, 4558–4570. [Google Scholar] [CrossRef]
- Schmelcher, M.; Donovan, D.M.; Loessner, M.J. Bacteriophage endolysins as novel antimicrobials. Future Microbiol. 2012, 7, 1147–1171. [Google Scholar] [CrossRef]
- Ganguly, J.; Low, L.Y.; Kamal, N.; Saile, E.; Forsberg, L.S.; Gutierrez-Sanchez, G.; Hoffmaster, A.R.; Liddington, R.; Quinn, C.P.; Carlson, R.W.; et al. The secondary cell wall polysaccharide of Bacillus anthracis provides the specific binding ligand for the C-terminal cell wall-binding domain of two phage endolysins, PlyL and PlyG. Glycobiology 2013, 23, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Borysowski, J.; Weber-Dąbrowska, B.; Górski, A. Bacteriophage endolysins as a novel class of antibacterial agents. Exp. Biol. Med. 2006, 231, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Thurber, R.V. Current insights into phage biodiversity and biogeography. Curr. Opin. Microbiol. 2009, 12, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Cahill, J.; Young, R. Phage Lysis: Multiple Genes for Multiple Barriers. In Advances in Virus Research; Academic Press: Cambridge, MA, USA, 2019; Volume 103, pp. 33–70. ISBN 9780128177228. [Google Scholar]
- Nelson, D.; Schuch, R.; Chahales, P.; Zhu, S.; Fischetti, V.A. PlyC: A multimeric bacteriophage lysin. Proc. Natl. Acad. Sci. USA 2006, 103, 10765–10770. [Google Scholar] [CrossRef] [PubMed]
- Wienhold, S.M.; Lienau, J.; Witzenrath, M. Towards inhaled phage therapy in Western Europe. Viruses 2019, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Linden, S.B.; Wang, J.; Yu, J.; Nelson, D.C.; Wei, H. A chimeolysin with extended-spectrum streptococcal host range found by an induced lysis-based rapid screening method. Sci. Rep. 2015, 5, 17257. [Google Scholar] [CrossRef]
- Gondil, V.S.; Dube, T.; Panda, J.J.; Yennamalli, R.M.; Harjai, K.; Chhibber, S. Comprehensive evaluation of chitosan nanoparticle based phage lysin delivery system; a novel approach to counter S. pneumoniae infections. Int. J. Pharm. 2020, 573. [Google Scholar] [CrossRef]
- Huang, L.; Luo, D.; Gondil, V.S.; Gong, Y.; Jia, M.; Yan, D.; He, J.; Hu, S.; Yang, H.; Wei, H. Construction and characterization of a chimeric lysin ClyV with improved bactericidal activity against Streptococcus agalactiae in vitro and in-vivo. Appl. Microbiol. Biotechnol. 2020, 104, 1609–1619. [Google Scholar] [CrossRef]
- Yang, H.; Luo, D.; Etobayeva, I.; Li, X.; Gong, Y.; Wang, S.; Li, Q.; Xu, P.; Yin, W.; He, J.; et al. Linker editing of pneumococcal lysin ClyJ conveys improved bactericidal activity. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Fischetti, V.A. Bacteriophage lytic enzymes: Novel anti-infectives. Trends Microbiol. 2005, 13, 491–496. [Google Scholar] [CrossRef]
- Rodríguez-Rubio, L.; Chang, W.L.; Gutiérrez, D.; Lavigne, R.; Martínez, B.; Rodríguez, A.; Govers, S.K.; Aertsen, A.; Hirl, C.; Biebl, M.; et al. “Artilysation” of endolysin λSa2lys strongly improves its enzymatic and antibacterial activity against streptococci. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Yang, H.; Wang, M.; Yu, J.; Wei, H. Antibacterial Activity of a Novel Peptide-Modified Lysin Against Acinetobacter baumannii and Pseudomonas aeruginosa. Front. Microbiol. 2015, 6, 1471. [Google Scholar] [CrossRef]
- Lai, W.C.B.; Chen, X.; Ho, M.K.Y.; Xia, J.; Leung, S.S.Y. Bacteriophage-derived endolysins to target gram-negative bacteria. Int. J. Pharm. 2020, 589, 119833. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.C.; Schmelcher, M.; Rodriguez-Rubio, L.; Klumpp, J.; Pritchard, D.G.; Dong, S.; Donovan, D.M. Endolysins as Antimicrobials, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 83, ISBN 9780123944382. [Google Scholar]
- Daniel, A.; Euler, C.; Collin, M.; Chahales, P.; Gorelick, K.J.; Fischetti, V.A. Synergism between a novel chimeric lysin and oxacillin protects against infection by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2010, 54, 1603–1612. [Google Scholar] [CrossRef] [PubMed]
- Kakikawa, M.; Yokoi, K.J.; Kimoto, H.; Nakano, M.; Kawasaki, K.I.; Taketo, A.; Kodaira, K.I. Molecular analysis of the lysis protein Lys encoded by Lactobacillus plantarum phage φg1e. Gene 2002, 299, 227–234. [Google Scholar] [CrossRef]
- Baker, J.R.; Liu, C.; Dong, S.; Pritchard, D.G. Endopeptidase and glycosidase activities of the bacteriophage B30 lysin. Appl. Environ. Microbiol. 2006, 72, 6825–6828. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Fischetti, V.A. Mutagenesis of a bacteriophage lytic enzyme PlyGBS significantly increases its antibacterial activity against group B streptococci. Appl. Microbiol. Biotechnol. 2007. [Google Scholar] [CrossRef]
- Loeffler, J.M.; Nelson, D.; Fischetti, V.A. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 2001, 294, 2170–2172. [Google Scholar] [CrossRef]
- Schmelcher, M.; Powell, A.M.; Becker, S.C.; Camp, M.J.; Donovan, D.M. Chimeric phage lysins act synergistically with lysostaphin to kill mastitis-causing Staphylococcus aureus in murine mammary glands. Appl. Environ. Microbiol. 2012, 78, 2297–2305. [Google Scholar] [CrossRef]
- Pohane, A.A.; Jain, V. Insights into the regulation of bacteriophage endolysin: Multiple means to the same end. Microbiology 2015, 161, 2269–2276. [Google Scholar] [CrossRef]
- Sanmukh, S.G.; Meshram, D.B.; Paunikar, W.N.; Swaminathan, S. Interaction of fishes with pathogenic micro-organisms and application of phages for their control: A review. Rev. Fish Biol. Fish. 2012, 22, 567–574. [Google Scholar] [CrossRef]
- Delbruck, M. The Growth of Bacteriophage and Lysis of the Host. J. Gen. Physiol. 1940, 23, 643–660. [Google Scholar] [CrossRef] [PubMed]
- Moak, M.; Molineux, I.J. Role of the Gp16 lytic transglycosylase motif in bacteriophage T7 virions at the initiation of infection. Mol. Microbiol. 2000, 37, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; Foley, S.; Bruttin, A.; Chennoufi, S.C.; Canchaya, C.; Brüssow, H. Transcription Mapping as a Tool in Phage Genomics: The Case of the Temperate Streptococcus thermophilus Phage Sfi21. Virology 2002, 296, 62–76. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luke, K.; Radek, A.; Liu, X.; Campbell, J.; Uzan, M.; Haselkorn, R.; Kogan, Y. Microarray Analysis of Gene Expression during Bacteriophage T4 Infection. Virology 2002, 299, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Duplessis, M.; Russell, W.M.; Romero, D.A.; Moineau, S. Global gene expression analysis of two Streptococcus thermophilus bacteriophages using DNA microarray. Virology 2005, 340, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Dedrick, R.M.; Marinelli, L.J.; Newton, G.L.; Pogliano, K.; Pogliano, J.; Hatfull, G.F. Functional requirements for bacteriophage growth: Gene essentiality and expression in mycobacteriophage Giles. Mol. Microbiol. 2013, 88, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.M.; Kang, C. Lysis Delay and Burst Shrinkage of Coliphage T7 by Deletion of Terminator Tφ Reversed by Deletion of Early Genes. J. Virol. 2014, 88, 2107–2115. [Google Scholar] [CrossRef]
- Garcia, M.; Pimentel, M.; Moniz-Pereira, J. Expression of Mycobacteriophage Ms6 Lysis Genes Is Driven by Two σ70-Like Promoters and Is Dependent on a Transcription Termination Signal Present in the Leader RNA. J. Bacteriol. 2002, 184, 3034–3043. [Google Scholar] [CrossRef]
- Berdygulova, Z.; Westblade, L.F.; Florens, L.; Koonin, E.V.; Chait, B.T.; Ramanculov, E.; Washburn, M.P.; Darst, S.A.; Severinov, K.; Minakhin, L. Temporal Regulation of Gene Expression of the Thermus thermophilus Bacteriophage P23-45. J. Mol. Biol. 2011, 405, 125–142. [Google Scholar] [CrossRef]
- Schubert, R.A.; Dodd, I.B.; Egan, J.B.; Shearwin, K.E. Cro’s role in the CI Cro bistable switch is critical for {lambda}’s transition from lysogeny to lytic development. Genes Amp. Dev. 2007, 21, 2461–2472. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lewis, D.E.A.; Adhya, S. The Developmental Switch in Bacteriophage λ: A Critical Role of the Cro Protein. J. Mol. Biol. 2018, 430, 58–68. [Google Scholar] [CrossRef]
- Gutierrez, D.; Fernandez, L.; Rodriguez, A.; Garcia, P. Are phage lytic proteins the secret weapon to kill staphylococcus aureus? Mbio 2018, 9, e01923-17. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Feng, C.; Ren, J.; Zhuang, X.; Zhang, Y.; Zhu, Y.; Dong, K.; He, P.; Guo, X.; Qin, J. A Novel Antimicrobial Endolysin, LysPA26, against Pseudomonas aeruginosa. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.Y.; Jang, I.J.; Yoon, S.; Jang, K.; Yu, K.S.; Cho, J.Y.; Seong, M.W.; Jung, G.M.; Yoon, S.J.; Kang, S.H. Pharmacokinetics and Tolerance of the Phage endolysin-based candidate drug SAL200 after a single intravenous administration among healthy volunteers. Antimicrob. Agents Chemother. 2017. [Google Scholar] [CrossRef]
- Love, M.J.; Bhandari, D.; Dobson, R.C.J.; Billington, C. Potential for Bacteriophage Endolysins to Supplement or Replace Antibiotics in Food Production and Clinical Care. Antibiotics 2018, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Gerstmans, H.; Criel, B.; Briers, Y. Synthetic biology of modular endolysins. Biotechnol. Adv. 2018, 36, 624–640. [Google Scholar] [CrossRef]
- Hathaway, H.; Ajuebor, J.; Stephens, L.; Coffey, A.; Potter, U.; Sutton, J.M.; Jenkins, A.T.A. Thermally triggered release of the bacteriophage endolysin CHAPK and the bacteriocin lysostaphin for the control of methicillin resistant Staphylococcus aureus (MRSA). J. Control. Release 2017, 245, 108–115. [Google Scholar] [CrossRef]
- Dryden, M.S. Complicated skin and soft tissue infection. J. Antimicrob. Chemother. 2010. [Google Scholar] [CrossRef]
- Kashani, H.H.; Schmelcher, M.; Sabzalipoor, H.; Hosseini, E.S.; Moniri, R. Recombinant Endolysins as Potential Therapeutics against Antibiotic-Resistant Staphylococcus aureus: Current Status of Research and Novel Delivery Strategies. Clin. Microbiol. Rev. 2018, 31, 1–26. [Google Scholar] [CrossRef]
- Pastagia, M.; Schuch, R.; Fischetti, V.A.; Huang, D.B. Lysins: The arrival of pathogen-directed anti-infectives. J. Med. Microbiol. 2013, 62, 1506–1516. [Google Scholar] [CrossRef] [PubMed]
- Fenton, M.; Ross, P.; Mcauliffe, O.; O’Mahony, J.; Coffey, A. Recombinant bacteriophage lysins as antibacterials. Bioeng. Bugs 2010, 1, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Wertheim, H.F.; Melles, D.C.; Vos, M.C.; van Leeuwen, W.; van Belcum, A.; Verbrugh, H.A.; Nouwen, J.C. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 2005, 5, 751–762. [Google Scholar] [CrossRef]
- Vipra, A.A.; Desai, S.N.; Roy, P.; Patil, R.; Raj, J.M.; Narasimhaswamy, N.; Paul, V.D.; Chikkamadaiah, R.; Sriram, B. Antistaphylococcal activity of bacteriophage derived chimeric protein P128. BMC Microbiol. 2012. [Google Scholar] [CrossRef]
- Roach, D.R.; Donovan, D.M. Antimicrobial bacteriophage-derived proteins and therapeutic applications. Bacteriophage 2015. [Google Scholar] [CrossRef]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn wound infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef] [PubMed]
- Briers, Y.; Walmagh, M.; Puyenbroeck, V.V.; Cornelissen, A.; Cenens, W.; Aertsen, A.; Oliviera, H.; Azeredo, J.; Verween, G.; Pirnay, J.P.; et al. Engineered Endolysin-Based “Artilysins” To Combat Multidrug-Resistant Gram-Negative Pathogens. mBio 2014, 5, e01379-14. [Google Scholar] [CrossRef]
- Álvarez, A.; Fernández, L.; Gutiérrez, D.; Iglesias, B.; Rodríguez, A.; García, P. Methicillin-resistant staphylococcus aureus in hospitals: Latest trends and treatments based on bacteriophages. J. Clin. Microbiol. 2019. [Google Scholar] [CrossRef]
- McEwen, S.A. Antibiotic use in animal agriculture: What have we learned and where are we going? Anim. Biotechnol. 2006. [Google Scholar] [CrossRef]
- Silbergeld, E.K.; Graham, J.; Price, L.B. Industrial food animal production, antimicrobial resistance and human health. Annu. Rev. Public Health. 2008, 29, 151–169. [Google Scholar] [CrossRef]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Cantas, L.; Shah, S.Q.A.; Cavaco, L.M.; Manaia, C.M.; Walsh, F.; Popowska, M.; Garelick, H.; Bürgmann, H.; Sørum, H. A brief multi-disciplinary review on antimicrobial resistance in medicine and its linkage to the global environmental microbiota. Front. Microbiol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Woolhouse, M.E.; Ward, M.J. Microbiology. Sources of antimicrobial resistance. Science 2013, 341, 1460–1461. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.M.; Levy, S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef]
- Casewell, M.; Friis, C.; Marco, E.; McMullin, P.; Phillips, I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 2003. [Google Scholar] [CrossRef]
- Fernández, L.; Gutiérrez, D.; Rodríguez, A.; García, P. Application of Bacteriophages in the Agro-Food Sector: A Long Way Toward Approval. Front. Cell. Infect. Microbiol. 2018, 8, 1–5. [Google Scholar] [CrossRef]
- Timbermont, L.; Haesebrouck, F.; Ducatelle, R.; van Immerseel, F. Necrotic enteritis in broilers: An updated review on the pathogenesis. Avian Pathol. 2011, 40, 341–347. [Google Scholar] [CrossRef]
- Tamai, E.; Yoshida, H.; Sekiya, H.; Nariya, H.; Miyata, S.; Okabe, A.; Kuwahara, T.; Maki, J.; Kamitori, S. X-ray structure of a novel endolysin encoded by episomal phage phiSM101 of Clostridium perfringens. Mol. Microbiol. 2014. [Google Scholar] [CrossRef]
- Gervasi, T.; Lo Curto, R.; Minniti, E.; Narbad, A.; Mayer, M.J. Application of Lactobacillus johnsonii expressing phage endolysin for control of Clostridium perfringens. Lett. Appl. Microbiol. 2014. [Google Scholar] [CrossRef]
- Wernicki, A.; Nowaczek, A.; Urban-Chmiel, R. Bacteriophage therapy to combat bacterial infections in poultry. Virol. J. 2017. [Google Scholar] [CrossRef]
- Schuch, R.; Nelson, D.; Fischetti, V.A. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature 2002, 418, 884–889. [Google Scholar] [CrossRef] [PubMed]
- Filatova, L.Y.; Donovan, D.M.; Ishnazarova, N.T.; Foster-Frey, J.A.; Becker, S.C.; Pugachev, V.G.; Balabushevich, N.G.; Dmitrieva, N.F.; Klyachko, N.L. A Chimeric LysK-Lysostaphin Fusion Enzyme Lysing Staphylococcus aureus Cells: A Study of Both Kinetics of Inactivation and Specifics of Interaction with Anionic Polymers. Appl. Biochem. Biotechnol. 2016, 180, 544–557. [Google Scholar] [CrossRef]
- Hoopes, J.T.; Stark, C.J.; Kim, H.A.; Sussman, D.J.; Donovan, D.M.; Nelson, D.C. Use of a bacteriophage lysin, PlyC, as an enzyme disinfectant against Streptococcus equi. Appl. Environ. Microbiol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Junjappa, R.P.; Desai, S.N.; Roy, P.; Narasimhaswamy, N.; Raj, J.R.M.; Durgaiah, M.; Vipra, A.; Bhat, U.R.; Satyanarayana, S.K.; Shankara, N.; et al. Efficacy of anti-staphylococcal protein P128 for the treatment of canine pyoderma: Potential applications. Vet. Res. Commun. 2013. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.J.; Gonzalez, R.N.; Das, H.H. Bovine Mastitis Pathogens in New York and Pennsylvania: Prevalence and Effects on Somatic Cell Count and Milk Production. J. Dairy Sci. 1997. [Google Scholar] [CrossRef]
- Fan, J.; Zeng, Z.; Mai, K.; Yang, Y.; Feng, J.; Bai, Y.; Sun, B.; Xie, Q.; Tong, Y.; Ma, J. Preliminary treatment of bovine mastitis caused by Staphylococcus aureus, with trx-SA1, recombinant endolysin of S. aureus bacteriophage IME-SA1. Vet. Microbiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, J.H.; Lu, C.P. Purified recombinant phage lysin LySMP: An extensive spectrum of lytic activity for swine streptococci. Curr. Microbiol. 2009. [Google Scholar] [CrossRef]
- Hausbeck, M.K.; Bell, J.; Medina-Mora, C.; Podolsky, R.; Fulbright, D.W. Effect of bactericides on population sizes and spread of Clavibacter michiganensis subsp. michiganensis on Tomatoes in the greenhouse and on disease development and crop yield in the field. Phytopathology 2000. [Google Scholar] [CrossRef]
- Schmelcher, M.; Loessner, M.J. Bacteriophage endolysins: Applications for food safety. Curr. Opin. Biotechnol. 2016, 37, 76–87. [Google Scholar] [CrossRef]
- Yoong, P.; Schuch, R.; Nelson, D.; Fischetti, V.A. Identification of a broadly active phage lytic enzyme with lethal activity against antibiotic-resistant Enterococcus faecalis and Enterococcus faecium. J. Bacteriol. 2004. [Google Scholar] [CrossRef]
- Zhang, H.; Bao, H.; Billington, C.; Hudson, J.A.; Wang, R. Isolation and lytic activity of the Listeria bacteriophage endolysin LysZ5 against Listeria monocytogenes in soya milk. Food Microbiol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Van Nassau, T.J.; Lenz, C.A.; Scherzinger, A.S.; Vogel, R.F. Combination of endolysins and high pressure to inactivate Listeria monocytogenes. Food Microbiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Kim, M.; Ryu, S. Characterization of a novel endolysin LysSA11 and its utility as a potent biocontrol agent against Staphylococcus aureus on food and utensils. Food Microbiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Shi, Y.; Ji, W.; Meng, X.; Zhang, J.; Wang, H.; Lu, C.; Sun, J.; Yan, Y. Application of a bacteriophage lysin to disrupt biofilms formed by the animal pathogen Streptococcus suis. Appl. Environ. Microbiol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Son, J.S.; Lee, S.J.; Jun, S.Y.; Yoon, S.J.; Kang, S.H.; Paik, H.R.; Kang, J.O.; Choi, Y.J. Antibacterial and biofilm removal activity of a podoviridae Staphylococcus aureus bacteriophage SAP-2 and a derived recombinant cell-wall-degrading enzyme. Appl. Microbiol. Biotechnol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Ribelles, P.; Benbouziane, B.; Langella, P.; Suárez, J.E.; Bermúdez-Humarán, L.G.; Riazi, A. Protection against human papillomavirus type 16-induced tumors in mice using non-genetically modified lactic acid bacteria displaying E7 antigen at its surface. Appl. Microbiol. Biotechnol. 2013. [Google Scholar] [CrossRef]
- Abebe, G.M. The Role of Bacterial Biofilm in Antibiotic Resistance and Food Contamination. Int. J. Microbiol. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Ferriol-González, C.; Domingo-Calap, P. Phages for Biofilm Removal. Antibiotics 2020, 9, 268. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilm Formation: A Clinically Relevant Microbiological Process. Clin. Infect. Dis. 2001, 33, 1387–1392. [Google Scholar] [CrossRef]
- Sharma, U.; Vipra, A.; Channabasappa, S. Phage-derived lysins as potential agents for eradicating biofilms and persisters. Drug Discov. Today 2018, 23, 848–856. [Google Scholar] [CrossRef]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New Technology for Rapid Determination of Antibiotic Susceptibilities of Bacterial Biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Talapko, J.; Škrlec, I. The Principles, Mechanisms, and Benefits of Unconventional Agents in the Treatment of Biofilm Infection. Pharmaceuticals 2020, 13, 299. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.C.; Roach, D.R.; Chauhan, V.S.; Shen, Y.; Foster-Frey, J.; Powell, A.M.; Bauchan, G.; Lease, R.A.; Mohammadi, H.; Harty, W.J.; et al. Triple-acting Lytic Enzyme Treatment of Drug-Resistant and Intracellular Staphylococcus aureus. Sci. Rep. 2016, 6, 25063. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Wiuff, C.; Zappala, R.M.; Regoes, R.R.; Garner, K.N.; Baquero, F.; Levin, B.R. Phenotypic Tolerance: Antibiotic Enrichment of Noninherited Resistance in Bacterial Populations. Antimicrob. Agents Chemother. 2005, 49, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Persister Cells. Annu. Rev. Microbiol. 2010, 64, 357–372. [Google Scholar] [CrossRef]
- Principi, N.; Silvestri, E.; Esposito, S. Advantages and Limitations of Bacteriophages for the Treatment of Bacterial Infections. Front. Pharm. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.; Goldsmith, D.; Schellekens, H. Immunogenicity of biopharmaceuticals. Nephrol. Dial. Transpl. 2006, 21, v9–v12. [Google Scholar] [CrossRef]
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G. Bacteriophage Therapy. Antimicrob. Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef]
- Fischetti, V.A. Bacteriophage endolysins: A novel anti-infective to control Gram-positive pathogens. Int. J. Med. Microbiol. 2010, 300, 357–362. [Google Scholar] [CrossRef]
- Zhang, L.; Li, D.; Li, X.; Hu, L.; Cheng, M.; Xia, F.; Gong, P.; Wang, B.; Ge, J.; Zhang, H.; et al. LysGH15 kills Staphylococcus aureus without being affected by the humoral immune response or inducing inflammation. Sci. Rep. 2016, 6, 29344. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.Y.; Jung, G.M.; Yoon, S.J.; Choi, Y.-J.; Koh, W.S.; Moon, K.S.; Kang, S.H. Preclinical Safety Evaluation of Intravenously Administered SAL200 Containing the Recombinant Phage Endolysin SAL-1 as a Pharmaceutical Ingredient. Antimicrob. Agents Chemother. 2014, 58, 2084–2088. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.Y.; Jung, G.M.; Yoon, S.J.; Youm, S.Y.; Han, H.-Y.; Lee, J.-H.; Kang, S.H. Pharmacokinetics of the phage endolysin-based candidate drug SAL200 in monkeys and its appropriate intravenous dosing period. Clin. Exp. Pharm. Physiol. 2016, 43, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Seed, K.D. Battling Phages: How Bacteria Defend against Viral Attack. PloS Pathog. 2015, 11, e1004847. [Google Scholar] [CrossRef] [PubMed]
- Pirnay, J.-P.; De Vos, D.; Verbeken, G.; Merabishvili, M.; Chanishvili, N.; Vaneechoutte, M.; Zizi, M.; Laire, G.; Lavigne, R.; Huys, I.; et al. The Phage Therapy Paradigm: Prêt-à-Porter or Sur-mesure? Pharm. Res. 2011, 28, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Keeney, K.M.; Yurist-Doutsch, S.; Arrieta, M.-C.; Finlay, B.B. Effects of Antibiotics on Human Microbiota and Subsequent Disease. Annu. Rev. Microbiol. 2014, 68, 217–235. [Google Scholar] [CrossRef]
- Ursell, T.S.; Nguyen, J.; Monds, R.D.; Colavin, A.; Billings, G.; Ouzounov, N.; Gitai, Z.; Shaevitz, J.W.; Huang, K.C. Rod-like bacterial shape is maintained by feedback between cell curvature and cytoskeletal localization. Proc. Natl. Acad. Sci. USA 2014, 111, E1025–E1034. [Google Scholar] [CrossRef]
- Cash, H.L.; Whitham, C.V.; Behrendt, C.L.; Hooper, L.V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 2006, 313, 1126–1130. [Google Scholar] [CrossRef]
- Wang, B.; Yao, M.; Lv, L.; Ling, Z.; Li, L. The Human Microbiota in Health and Disease. Engineering 2017, 3, 71–82. [Google Scholar] [CrossRef]
- Rahimzadeh, G.; Gill, P.; Rezai, M.S. Endolysins of Bacteriophages as an Anti-Methicillin Resistant Staphylococcus aureus Infection in Children: A Narrative Review. J. Pediatr. Rev. 2017, 6. [Google Scholar] [CrossRef]
- Fischetti, V.A. Bacteriophage lysins as effective antibacterials. Curr. Opin. Microbiol. 2008, 11, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Ghose, C.; Euler, C.W. Gram-Negative Bacterial Lysins. Antibiotics 2020, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Briers, Y.; Schmelcher, M.; Loessner, M.J.; Hendrix, J.; Engelborghs, Y.; Volckaert, G.; Lavigne, R. The high-affinity peptidoglycan binding domain of Pseudomonas phage endolysin KZ144. Biochem. Biophys. Res. Commun. 2009, 383, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, I.; Uchiyama, J.; Tsukui, T.; Hisatsune, J.; Ide, K.; Matsuzaki, S.; Sugai, M.; Nishifuji, K. Therapeutic Potential of an Endolysin Derived from Kayvirus S25-3 for Staphylococcal Impetigo. Viruses 2019, 11, 769. [Google Scholar] [CrossRef] [PubMed]
- Theuretzbacher, U.; Piddock, L.J.V. Non-traditional Antibacterial Therapeutic Options and Challenges. Cell Host Microbe 2019, 26, 61–72. [Google Scholar] [CrossRef]
- Beveridge, T.J. Structures of Gram-Negative Cell Walls and Their Derived Membrane Vesicles. J. Bacteriol. 1999, 181, 4725–4733. [Google Scholar] [CrossRef]
- Thummeepak, R.; Kitti, T.; Kunthalert, D.; Sitthisak, S. Enhanced antibacterial activity of acinetobacter baumannii bacteriophage øABP-01 endolysin (LysABP-01) in combination with colistin. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K.; Saravolatz, L.D. Colistin: The Revival of Polymyxins for the Management of Multidrug-Resistant Gram-Negative Bacterial Infections. Clin. Infect. Dis. 2005, 40, 1333–1341. [Google Scholar] [CrossRef]
- Rashel, M.; Uchiyama, J.; Ujihara, T.; Uehara, Y.; Kuramoto, S.; Sugihara, S.; Yagyu, K.; Muraoka, A.; Sugai, M.; Hiramatsu, K.; et al. Efficient Elimination of Multidrug-Resistant Staphylococcus aureus by Cloned Lysin Derived from Bacteriophage φMR11. J. Infect. Dis. 2007, 196, 1237–1247. [Google Scholar] [CrossRef]
- Kim, N.H.; Park, W.B.; Cho, J.E.; Choi, Y.J.; Choi, S.J.; Jun, S.Y.; Kang, C.K.; Song, K.H.; Choe, P.G.; Bang, J.H.; et al. Effects of phage endolysin SAL200 combined with antibiotics on staphylococcus aureus infection. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef]
- Letrado, P.; Corsini, B.; Díez-Martínez, R.; Bustamante, N.; Yuste, J.E.; García, P. Bactericidal synergism between antibiotics and phage endolysin Cpl-711 to kill multidrug-resistant pneumococcus. Future Microbiol. 2018, 13, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.C.; Foster-frey, J.; Donovan, D.M. The phage K lytic enzyme LysK and lysostaphin act synergistically to kill MRSA. FEMS Microbiol. Lett. 2008. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, S.; Coffey, A.; Meaney, W.J.; Fitzgerald, G.F.; Ross, R.P. Inhibition of bacteriophage K proliferation on Staphylococcus aureus in raw bovine milk. Lett. Appl. Microbiol. 2005, 41, 274–279. [Google Scholar] [CrossRef]
- Dajcs, J.J.; Hume, E.B.H.; Moreau, J.M.; Caballero, A.R.; Cannon, B.M.; Callaghan, R.J.O. Lysostaphin Treatment of Methicillin-Resistant Staphylococcus aureus Keratitis in the Rabbit. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1432–1437. [Google Scholar] [CrossRef]
- Maciejewska, B.; Olszak, T.; Drulis-Kawa, Z. Applications of bacteriophages versus phage enzymes to combat and cure bacterial infections: An ambitious and also a realistic application? Appl. Microbiol. Biotechnol. 2018, 102, 2563–2581. [Google Scholar] [CrossRef]
- Chan, J.M.; Valencia, P.M.; Zhang, L.; Langer, R.; Farokhzad, O.C. Polymeric nanoparticles for drug delivery. Methods Mol. Biol. 2010, 624, 163–175. [Google Scholar] [CrossRef]
- Jun, S.; Jung, G.; Son, J.; Yoon, S.; Choi, Y.; Kang, S. Comparison of the antibacterial properties of phage endolysins SAL-1 and LysK. Antimicrob. Agents Chemother. 2011, 55, 1764–7176. [Google Scholar] [CrossRef]
- Entenza, J.M.; Loeffler, J.M.; Grandgirard, D.; Fischetti, V.A.; Moreillon, P. Therapeutic Effects of Bacteriophage Cpl-1 Lysin against Streptococcus pneumoniae Endocarditis in Rats. Antimicrob. Agents Chemother. 2005, 49, 4789–4792. [Google Scholar] [CrossRef]
- McCullers, J.A.; Karlström, Å.; Iverson, A.R.; Loeffler, J.M.; Fischetti, V.A. Novel Strategy to Prevent Otitis Media Caused by Colonizing Streptococcus pneumoniae. PLoS Pathog. 2007, 3, e28. [Google Scholar] [CrossRef]
- Grandgirard, D.; Loeffler, J.M.; Fischetti, V.A.; Leib, S.L. Phage Lytic Enzyme Cpl-1 for Antibacterial Therapy in Experimental Pneumococcal Meningitis. J. Infect. Dis. 2008, 197, 1519–1522. [Google Scholar] [CrossRef]
- Witzenrath, M.; Schmeck, B.; Doehn, J.M.; Tschernig, T.; Zahlten, J.; Loeffler, J.M.; Zemlin, M.; Müller, H.; Gutbier, B.; Schütte, H.; et al. Systemic use of the endolysin Cpl-1 rescues mice with fatal pneumococcal pneumonia*. Crit. Care Med. 2009, 37, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Doehn, J.M.; Fischer, K.; Reppe, K.; Gutbier, B.; Tschernig, T.; Hocke, A.C.; Fischetti, V.A.; Loffler, J.; Suttorp, N.; Hippenstiel, S.; et al. Delivery of the endolysin Cpl-1 by inhalation rescues mice with fatal pneumococcal pneumonia. J. Antimicrob. Chemother. 2013, 68, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, F. Resistance Development to Bacteriophages Occurring during Bacteriophage Therapy. Viruses 2018, 10, 351. [Google Scholar] [CrossRef]
- Bustamante, N.; Campillo, N.E.; García, E.; Gallego, C.; Pera, B.; Diakun, G.P.; Sáiz, J.L.; García, P.; Díaz, J.F.; Menéndez, M. Cpl-7, a Lysozyme Encoded by a Pneumococcal Bacteriophage with a Novel Cell Wall-binding Motif. J. Biol. Chem. 2010, 285, 33184–33196. [Google Scholar] [CrossRef] [PubMed]
- Díez-Martínez, R.; de Paz, H.; Bustamante, N.; García, E.; Menéndez, M.; García, P. Improving the Lethal Effect of Cpl-7, a Pneumococcal Phage Lysozyme with Broad Bactericidal Activity, by Inverting the Net Charge of Its Cell Wall-Binding Module. Antimicrob. Agents Chemother. 2013, 57, 5355–5365. [Google Scholar] [CrossRef] [PubMed]
- Lood, R.; Raz, A.; Molina, H.; Euler, C.W.; Fischetti, V.A. A Highly Active and Negatively Charged Streptococcus pyogenes Lysin with a Rare d-Alanyl-l-Alanine Endopeptidase Activity Protects Mice against Streptococcal Bacteremia. Antimicrob. Agents Chemother. 2014, 58, 3073–3084. [Google Scholar] [CrossRef]
- Cheng, Q.; Nelson, D.; Zhu, S.; Fischetti, V.A. Removal of Group B Streptococci Colonizing the Vagina and Oropharynx of Mice with a Bacteriophage Lytic Enzyme. Antimicrob. Agents Chemother. 2005, 49, 111–117. [Google Scholar] [CrossRef]
- Oechslin, F.; Daraspe, J.; Giddey, M.; Moreillon, P.; Resch, G. In Vitro Characterization of PlySK1249, a Novel Phage Lysin, and Assessment of Its Antibacterial Activity in a Mouse Model of Streptococcus agalactiae Bacteremia. Antimicrob. Agents Chemother. 2013, 57, 6276–6283. [Google Scholar] [CrossRef]
- Gu, J.; Zuo, J.; Lei, L.; Zhao, H.; Sun, C.; Feng, X.; Du, C.; Li, X.; Yang, Y.; Han, W. LysGH15 reduces the inflammation caused by lethal methicillin-resistant Staphylococcus aureus infection in mice. Bioeng. Bugs 2011, 2, 96–99. [Google Scholar] [CrossRef][Green Version]
- Schmelcher, M.; Shen, Y.; Nelson, D.C.; Eugster, M.R.; Eichenseher, F.; Hanke, D.C.; Loessner, M.J.; Dong, S.; Pritchard, D.G.; Lee, J.C.; et al. Evolutionarily distinct bacteriophage endolysins featuring conserved peptidoglycan cleavage sites protect mice from MRSA infection. J. Antimicrob. Chemother. 2015, 70, 1453–1465. [Google Scholar] [CrossRef]
- Raz, A.; Serrano, A.; Hernandez, A.; Euler, C.W.; Fischetti, V.A. Isolation of Phage Lysins That Effectively Kill Pseudomonas aeruginosa in Mouse Models of Lung and Skin Infection. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Lood, R.; Winer, B.Y.; Pelzek, A.J.; Diez-Martinez, R.; Thandar, M.; Euler, C.W.; Schuch, R.; Fischetti, V.A. Novel Phage Lysin Capable of Killing the Multidrug-Resistant Gram-Negative Bacterium Acinetobacter baumannii in a Mouse Bacteremia Model. Antimicrob. Agents Chemother. 2015, 59, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, D.-W.; Jin, J.-S.; Kim, J. Antimicrobial activity of LysSS, a novel phage endolysin, against Acinetobacter baumannii and Pseudomonas aeruginosa. J. Glob. Antimicrob. Resist. 2020, 22, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhen, X.; Zhou, H.; Zhao, F.; Fan, C.; Perčulija, V.; Tong, Y.; Mi, Z.; Ouyang, S. Structural and functional insights into a novel two-component endolysin encoded by a single gene in Enterococcus faecalis phage. PLoS Pathog. 2020, 16, e1008394. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Zhang, Y.; Li, X.; Liang, J.; Hu, L.; Gong, P.; Zhang, L.; Cai, R.; Zhang, H.; Ge, J.; et al. Endolysin LysEF-P10 shows potential as an alternative treatment strategy for multidrug-resistant Enterococcus faecalis infections. Sci. Rep. 2017, 7, 10164. [Google Scholar] [CrossRef]
- Schmitz, J.E.; Schuch, R.; Fischetti, V.A. Identifying Active Phage Lysins through Functional Viral Metagenomics. Appl. Environ. Microbiol. 2010, 76, 7181–7187. [Google Scholar] [CrossRef] [PubMed]
- Farrag, H.A.; Abdallah, N.; Shehata, M.M.K.; Awad, E.M. Natural outer membrane permeabilizers boost antibiotic action against irradiated resistant bacteria. J. Biomed. Sci. 2019, 26, 69. [Google Scholar] [CrossRef]
- Reuter, M.; Kruger, D.H. Approaches to optimize therapeutic bacteriophage and bacteriophage-derived products to combat bacterial infections. Virus Genes 2020, 56, 136–149. [Google Scholar] [CrossRef]
- Mayer, M.J.; Garefalaki, V.; Spoerl, R.; Narbad, A.; Meijers, R. Structure-Based Modification of a Clostridium difficile-Targeting Endolysin Affects Activity and Host Range. J. Bacteriol. 2011, 193, 5477–5486. [Google Scholar] [CrossRef]
- O’Flaherty, S.; Coffey, A.; Meaney, W.; Fitzgerald, G.F.; Ross, R.P. The Recombinant Phage Lysin LysK Has a Broad Spectrum of Lytic Activity against Clinically Relevant Staphylococci, Including Methicillin-Resistant Staphylococcus aureus. J. Bacteriol. 2005, 187, 7161–7164. [Google Scholar] [CrossRef]
- Pineda, C.; Castaneda Hernandez, G.; Jacobs, I.A.; Alvarez, D.F.; Carini, C. Assessing the immunogenicity of biopharmaceuticals. BioDrugs 2016, 30, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M. The Concept of an Ideal Antibiotic: Implications for Drug Design. Molecules 2019, 24, 892. [Google Scholar] [CrossRef] [PubMed]
- Jado, I.; Lopez, R.; Garcia, E.; Fenoll, A.; Casal, J.; Garcia, P.; The Spanish Pneumococcal Infection Study Network. Phage lytic enzymes as therapy for antibiotic-resistant Streptococcus pneumoniae infection in a murine sepsis model. J. Antimicrob. Chemother. 2003, 52, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Nau, R.; Eiffert, H. Modulation of release of proinflammatory bacterial compounds by antibacterials: Potential impact on course of inflammation and outcome in sepsis and meningitis. Clin. Microbiol. Rev. 2002, 15, 95–110. [Google Scholar] [CrossRef]
| Endolysin | Study | Phase | Clinical Trials Identifier | Status |
|---|---|---|---|---|
| CF-301 | Safety, Efficacy, and Pharmacokinetics of CF-301 vs. Placebo in Addition to Antibacterial Therapy for Treatment of S. aureus Bacteremia | III | NCT04160468 | Ongoing trial |
| N-Rephasin® SAL200 (Intron Biotechnology, Inc., South Korea) | To evaluate safety and to explore the efficacy of a single intravenous dose of N-Rephasin® SAL200 (3 mg/kg) | IIa | NCT03089697 | Ongoing trial |
| P-128 | Safety & Efficacy of an Antibacterial Protein Molecule Applied Topically to the Nostrils of Volunteers and Patients | II | NCT01746654 | Completed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelrahman, F.; Easwaran, M.; Daramola, O.I.; Ragab, S.; Lynch, S.; Oduselu, T.J.; Khan, F.M.; Ayobami, A.; Adnan, F.; Torrents, E.; et al. Phage-Encoded Endolysins. Antibiotics 2021, 10, 124. https://doi.org/10.3390/antibiotics10020124
Abdelrahman F, Easwaran M, Daramola OI, Ragab S, Lynch S, Oduselu TJ, Khan FM, Ayobami A, Adnan F, Torrents E, et al. Phage-Encoded Endolysins. Antibiotics. 2021; 10(2):124. https://doi.org/10.3390/antibiotics10020124
Chicago/Turabian StyleAbdelrahman, Fatma, Maheswaran Easwaran, Oluwasegun I. Daramola, Samar Ragab, Stephanie Lynch, Tolulope J. Oduselu, Fazal Mehmood Khan, Akomolafe Ayobami, Fazal Adnan, Eduard Torrents, and et al. 2021. "Phage-Encoded Endolysins" Antibiotics 10, no. 2: 124. https://doi.org/10.3390/antibiotics10020124
APA StyleAbdelrahman, F., Easwaran, M., Daramola, O. I., Ragab, S., Lynch, S., Oduselu, T. J., Khan, F. M., Ayobami, A., Adnan, F., Torrents, E., Sanmukh, S., & El-Shibiny, A. (2021). Phage-Encoded Endolysins. Antibiotics, 10(2), 124. https://doi.org/10.3390/antibiotics10020124












