Mutational Diversity in the Quinolone Resistance-Determining Regions of Type-II Topoisomerases of Salmonella Serovars
Abstract
1. Introduction
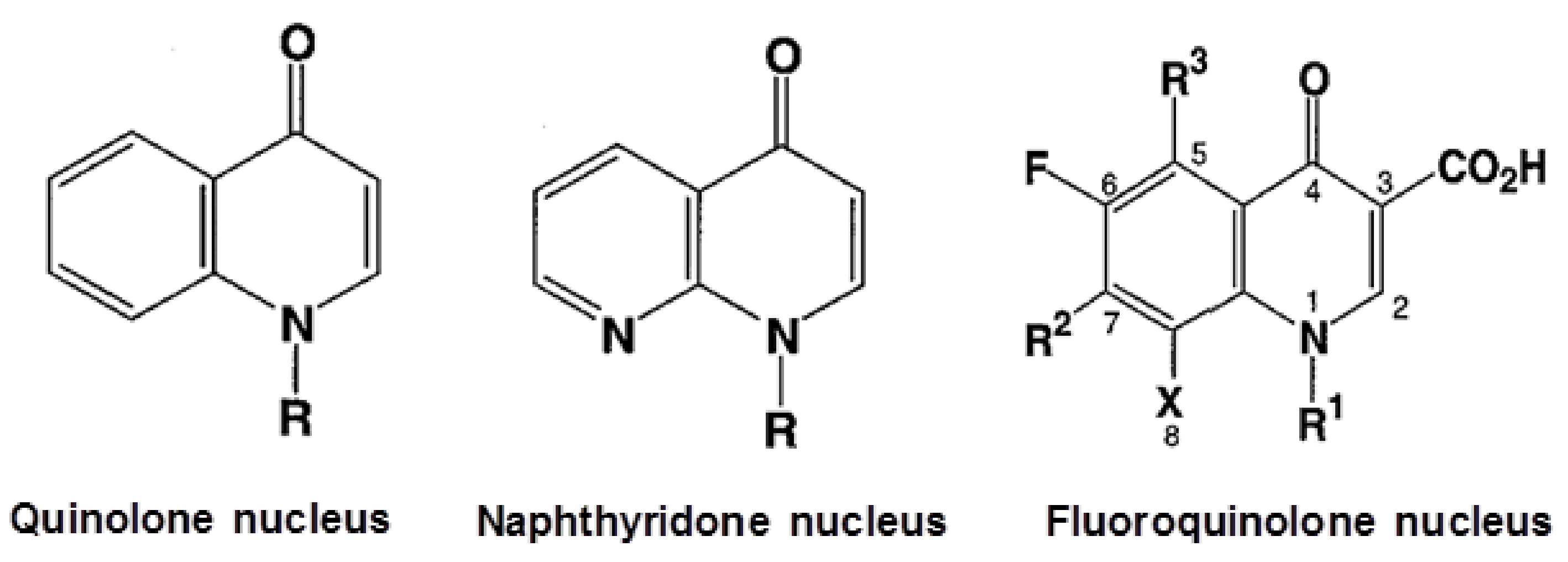
2. Mode of Action of Fluoroquinolones and QRDRs
3. Known Mechanisms of Quinolone Resistance
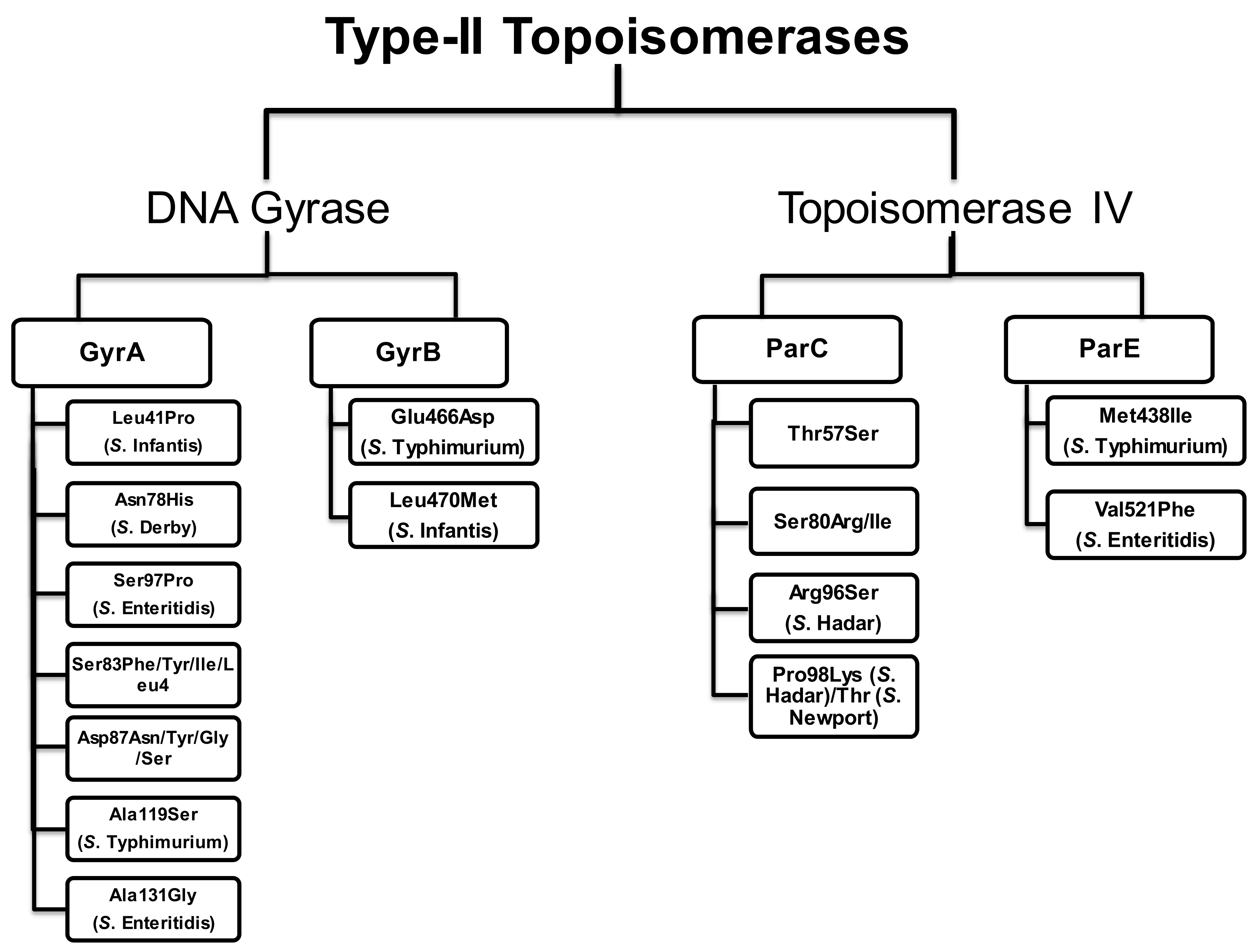
4. Mutations in the QRDRs of Salmonella Serovars
4.1. Mutations in the QRDRs of Typhoidal Salmonella
4.2. Mutations in the QRDRs of Non-Typhoidal Salmonella
4.2.1. Salmonella Enteritidis (S. Enteritidis)
4.2.2. Salmonella Typhimurium (S. Typhimurium)
4.2.3. Salmonella Hadar (S. Hadar)
4.2.4. Salmonella Kentucky (S. Kentucky)
4.2.5. Salmonella Indiana (S. Indiana)
4.2.6. Salmonella Infantis (S. Infantis)
4.2.7. Salmonella Derby (S. Derby)
4.2.8. Salmonella Newport (S. Newport)
4.2.9. Salmonella Virchow (S. Virchow)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone antibiotics. MedChemComm 2019, 10, 1719–1739. [Google Scholar] [CrossRef]
- Brighty, K.E.; Gootz, T.D. Chemistry and mechanism of action of the quinolone antibacterials. In The Quinolones, 3rd ed.; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Campoli-Richards, D.M.; Monk, J.P.; Price, A.; Benfield, P.; Todd, P.A.; Ward, A. Ciprofloxacin: A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 1988, 35, 373–447. [Google Scholar] [CrossRef]
- Monk, J.P.; Campoli-Richards, D.M. Ofloxacin: A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 1987, 33, 346–391. [Google Scholar] [CrossRef] [PubMed]
- Hamer, D.H.; Gorbach, S.L. Use of the quinolones for treatment and prophylaxis of bacterial gastrointestinal infections. In The Quinolones; Academic Press: Cambridge, MA, USA, 2000; p. 303. [Google Scholar]
- Crump, J.A.; Sjolund-Karlsson, M.; Gordon, M.A.; Parry, C.M. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin. Microbiol. Rev. 2015, 28, 901–937. [Google Scholar] [CrossRef] [PubMed]
- Kariuki, S.; Gordon, M.A.; Feasey, N.; Parry, C.M. Antimicrobial resistance and management of invasive Salmonella disease. Vaccine 2015, 33, C21–C29. [Google Scholar] [CrossRef]
- Gunn, J.S.; Marshall, J.M.; Baker, S.; Dongol, S. Salmonella chronic carriage: Epidemiology, diagnosis, and gallbladder persistence. Trends Microbiol. 2014, 22, 648–655. [Google Scholar] [CrossRef]
- Lodish, H.B.A.; Berk, A.; Zipursky, S.L.; Matsudaira, P.; Baltimore, D.; Darnell, J. The role of topoisomerases in DNA replication. In Molecular Cell Biology; WH Freeman: New York, NY, USA, 2000. [Google Scholar]
- Dax, S.L. Antibacterial Chemotherapeutic Agents; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Champoux, J.J. DNA topoisomerases: Structure, function, and mechanism. Annu. Rev. Biochem. 2001, 70, 369–413. [Google Scholar] [CrossRef]
- Wang, S.C.; Shapiro, L. The topoisomerase IV ParC subunit colocalizes with the Caulobacter replisome and is required for polar localization of replication origins. Proc. Natl. Acad. Sci. USA 2004, 101, 9251–9256. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C.; Jacoby, G.A. Mechanisms of drug resistance: Quinolone resistance. Ann. N. Y. Acad. Sci. 2015, 1345, 12–31. [Google Scholar] [CrossRef]
- Wang, J.C. DNA topoisomerases. Annu. Rev. Biochem. 1996, 65, 635–692. [Google Scholar] [CrossRef]
- Hegde, S.S.; Vetting, M.W.; Roderick, S.L.; Mitchenall, L.A.; Maxwell, A.; Takiff, H.E.; Blanchard, J.S. A fluoroquinolone resistance protein from Mycobacterium tuberculosis that mimics DNA. Science 2005, 308, 1480–1483. [Google Scholar] [CrossRef]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of quinolone action and resistance. Biochemistry 2014, 53, 1565–1574. [Google Scholar] [CrossRef]
- Hopkins, K.L.; Davies, R.H.; Threlfall, E.J. Mechanisms of quinolone resistance in Escherichia coli and Salmonella: Recent developments. Int. J. Antimicrob. Agents 2005, 25, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.S.; Fisher, L.M. DNA gyrase and topoisomerase IV are dual targets of clinafloxacin action in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 1998, 42, 2810–2816. [Google Scholar] [CrossRef]
- Park, H.S.; Jung, S.J.; Kwak, J.H.; Choi, D.R.; Choi, E.C. DNA gyrase and topoisomerase IV are dual targets of zabofloxacin in Streptococcus pneumoniae. Int. J. Antimicrob. Agents 2010, 36, 97–98. [Google Scholar] [CrossRef]
- Yoshida, H.; Bogaki, M.; Nakamura, M.; Nakamura, S. Quinolone resistance determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob. Agents Chemother. 1990, 34, 1271–1272. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Bogaki, M.; Nakamura, M.; Yamanaka, L.M. Quinolone resistance determining region in the DNA gyrase gyrB gene of Escherichia coli. Antimicrob. Agents Chemother. 1991, 35, 1647–1650. [Google Scholar] [CrossRef] [PubMed]
- Govinden, U.; Mocktar, C.; Moodley, P.; Sturm, A.; Essack, S. Detection of mutations in the gyrA of clinical Salmonella spp. Afr. J. Biotechnol. 2009, 8, 3911–3914. [Google Scholar]
- Procter, J.B.; Carstairs, G.M.; Soares, B.; Mourao, K. Alignment of Biological Sequences with Jalview. Methods Mol. Biol. 2021, 2231, 203–224. [Google Scholar] [PubMed]
- Afzal, A.; Sarwar, Y.; Ali, A.; Maqbool, A.; Salman, M.; Habeeb, M.A.; Haque, A. Molecular evaluation of drug resistance in clinical isolates of Salmonella enterica serovar Typhi from Pakistan. J. Infect. Dev. Ctries 2013, 7, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.M.; Lu, T.; Drlica, K. Mutation in the DNA gyrase A gene of Escherichia coli that expands the quinolone resistance determining region. Antimicrob. Agents Chemother. 2001, 45, 2378–2380. [Google Scholar] [CrossRef]
- Madurga, S.; Sanchez-Cespedes, J.; Belda, I.; Vila, J.; Giralt, E. Mechanism of binding of fluoroquinolones to the quinolone resistance-determining region of DNA gyrase: Towards an understanding of the molecular basis of quinolone resistance. ChemBioChem 2008, 9, 2081–2086. [Google Scholar] [CrossRef]
- An, R.; Alshalchi, S.; Breimhurst, P.; Munoz-Aguayo, J.; Flores-Figueroa, C.; Vidovic, S. Strong influence of livestock environments on the emergence and dissemination of distinct multidrug-resistant phenotypes among the population of non-typhoidal Salmonella. PLoS ONE 2017, 12, e0179005. [Google Scholar] [CrossRef] [PubMed]
- Wasyl, D.; Hoszowski, A.; Zajac, M. Prevalence and characterisation of quinolone resistance mechanisms in Salmonella spp. Vet. Microbiol. 2014, 171, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.P.; Richardson, L.; Mahon, B.E.; Rothenberg, R.; Cole, D.J. The rise and decline in Salmonella enterica serovar Enteritidis outbreaks attributed to egg-containing foods in the United States, 1973–2009. Epidemiol. Infect. 2016, 144, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Eguale, T.; Birungi, J.; Asrat, D.; Njahira, M.N.; Njuguna, J.; Gebreyes, W.A.; Gunn, J.S.; Djikeng, A.; Engidawork, E. Genetic markers associated with resistance to beta-lactam and quinolone antimicrobials in non-typhoidal Salmonella isolates from humans and animals in central Ethiopia. Antimicrob. Resist. Infect. Control. 2017, 6, 13. [Google Scholar] [CrossRef]
- Chen, Z.; Bai, J.; Zhang, X.; Wang, S.; Chen, K.; Lin, Q.; Xu, C.; Qu, X.; Zhang, H.; Liao, M.; et al. Highly prevalent multidrug resistance and QRDR mutations in Salmonella isolated from chicken, pork and duck meat in Southern China, 2018–2019. Int. J. Food Microbiol. 2021, 340, 109055. [Google Scholar] [CrossRef] [PubMed]
- Casas, M.R.; Camargo, C.H.; Soares, F.B.; da Silveira, W.D.; Fernandes, S.A. Presence of plasmid mediated quinolone resistance determinants and mutations in gyrase and topoisomerase in Salmonella enterica isolates with resistance and reduced susceptibility to ciprofloxacin. Diagn. Microbiol. Infect. Dis. 2016, 85, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Cavaco, L.M.; Hasman, H.; Xia, S.; Aarestrup, F.M. qnrD, a novel gene conferring transferable quinolone resistance in Salmonella enterica serovar Kentucky and Bovismorbificans strains of human origin. Antimicrob. Agents Chemother. 2009, 53, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Ceyssens, P.J.; Mattheus, W.; Vanhoof, R.; Bertrand, S. Trends in serotype distribution and antimicrobial susceptibility in Salmonella enterica isolates from humans in Belgium, 2009 to 2013. Antimicrob. Agents Chemother. 2015, 59, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Gaind, R.; Paglietti, B.; Murgia, M.; Dawar, R.; Uzzau, S.; Cappuccinelli, P.; Deb, M.; Aggarwal, P.; Rubino, S. Molecular characterization of ciprofloxacin resistant Salmonella enterica serovar Typhi and Paratyphi A causing enteric fever in India. J. Antimicrob. Chemother. 2006, 58, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, T.; Dashti, A.A.; Albaksami, O.; Udo, E.E.; Jadaon, M.M.; Albert, M.J. Ciprofloxacin resistant Salmonella enterica serovar Typhi from Kuwait with novel mutations in gyrA and parC genes. J. Clin. Microbiol. 2009, 47, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Kogaluru Shivakumaraswamy, S.; Vijaya Kumar, D.; Moleyuru Nagarajappa, V.; Karunasagar, I.; Karunasagar, I. Multiple antimicrobial resistance and novel point mutation in fluoroquinolone-resistant Escherichia coli isolates from Mangalore, India. Microb. Drug Resist. 2017, 23, 994–1001. [Google Scholar]
- Correia, S.; Hebraud, M.; Chafsey, I.; Chambon, C.; Viala, D.; Torres, C.; de Toro, M.; Capelo, J.L.; Poeta, P.; Igrejas, G. Impacts of experimentally induced and clinically acquired quinolone resistance on the membrane and intracellular subproteomes of Salmonella typhimurium DT104B. J. Proteomics 2016, 145, 46–59. [Google Scholar] [CrossRef]
- Rushdy, A.A.; Mabrouk, M.I.; Abu-Sef, F.A.; Kheiralla, Z.H.; Mohamed Abdel-All, S.; Saleh, N.M. Contribution of different mechanisms to the resistance to fluoroquinolones in clinical isolates of Salmonella enterica. Braz. J. Infect. Dis. 2013, 17, 431–437. [Google Scholar] [CrossRef]
- Kim, K.Y.; Woo, G.J. Expression of acrB and ramA in fluoroquinolone resistant mutants from multi-drug resistant Salmonella enterica serovar Haardt. Lett. Appl. Microbiol. 2011, 52, 484–490. [Google Scholar] [CrossRef]
- Baucheron, S.; Le Hello, S.; Doublet, B.; Giraud, E.; Weill, F.X.; Cloeckaert, A. ramR mutations affecting fluoroquinolone susceptibility in epidemic multidrug-resistant Salmonella enterica serovar Kentucky ST198. Front. Microbiol. 2013, 4, 213. [Google Scholar] [CrossRef]
- Shaheen, A.; Tariq, A.; Shehzad, A.; Iqbal, M.; Mirza, O.; Maslov, D.A.; Rahman, M. Transcriptional regulation of drug resistance mechanisms in Salmonella: Where we stand and what we need to know. World J. Microbiol. Biotechnol. 2020, 36, 85. [Google Scholar] [CrossRef]
- Shaheen, A.; Ismat, F.; Iqbal, M.; Haque, A.; Ul-Haq, Z.; Mirza, O.; De Zorzi, R.; Walz, T.; Rahman, M. Characterization of the multidrug efflux transporter styMdtM from Salmonella enterica serovar Typhi. Proteins 2021, 89, 1193–1204. [Google Scholar] [CrossRef]
- Martinez-Martinez, L.; Pascual, A.; Jacoby, G.A. Quinolone resistance from a transferable plasmid. Lancet 1998, 351, 797–799. [Google Scholar] [CrossRef]
- Vetting, M.W.; Hegde, S.S.; Fajardo, J.E.; Fiser, A.; Roderick, S.L.; Takiff, H.E.; Blanchard, J.S. Pentapeptide repeat proteins. Biochemistry 2006, 45, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Heddle, J.G. Squaring up to DNA: Pentapeptide repeat proteins and DNA mimicry. Appl. Microbiol. Biotechnol. 2014, 98, 9545–9560. [Google Scholar] [CrossRef]
- Xiong, X.; Bromley, E.H.; Oelschlaeger, P.; Woolfson, D.N.; Spencer, J. Structural insights into quinolone antibiotic resistance mediated by pentapeptide repeat proteins: Conserved surface loops direct the activity of a Qnr protein from a gram-negative bacterium. Nucleic Acids Res. 2011, 39, 3917–3927. [Google Scholar] [CrossRef]
- Jacoby, G.A.; Walsh, K.; Mills, D.; Moreno, F. (Eds.) A new plasmid mediated gene for quinolone resistance. In Proceedings of the Forty-Fourth Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, USA, 30 October–2 November 2004. [Google Scholar]
- Wang, M.; Guo, Q.; Xu, X.; Wang, X.; Ye, X.; Wu, S.; Hooper, D.C.; Wang, M. New plasmid mediated quinolone resistance gene, qnrC, found in a clinical isolate of Proteus mirabilis. Antimicrob. Agents Chemother. 2009, 53, 1892–1897. [Google Scholar] [CrossRef] [PubMed]
- Hata, M.; Suzuki, M.; Matsumoto, M.; Takahashi, M.; Sato, K.; Ibe, S.; Sakae, K. Cloning of a novel gene for quinolone resistance from a transferable plasmid in Shigella flexneri 2b. Antimicrob. Agents Chemother. 2005, 49, 801–803. [Google Scholar] [CrossRef]
- Pons, M.J.; Gomes, C.; Ruiz, J. QnrVC, a new transferable Qnr-like family. Enferm. Infecc. Microbiol. Clin. 2013, 31, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Robicsek, A.; Strahilevitz, J.; Jacoby, G.A.; Macielag, M.; Abbanat, D.; Park, C.H.; Bush, K.; Hooper, D.C. Fluoroquinolone modifying enzyme: A new adaptation of a common aminoglycoside acetyltransferase. Nat. Med. 2006, 12, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.H.; Johannesen, E.; Burmolle, M.; Sorensen, A.H.; Sorensen, S.J. Plasmid encoded multidrug efflux pump conferring resistance to olaquindox in Escherichia coli. Antimicrob. Agents Chemother. 2004, 48, 3332–3337. [Google Scholar] [CrossRef]
- Yamane, K.; Wachino, J.; Suzuki, S.; Arakawa, Y. Plasmid mediated qepA gene among Escherichia coli clinical isolates from Japan. Antimicrob. Agents Chemother. 2008, 52, 1564–1566. [Google Scholar] [CrossRef] [PubMed]
- Yamane, K.; Wachino, J.; Suzuki, S.; Kimura, K.; Shibata, N.; Kato, H.; Shibayama, K.; Konda, T.; Arakawa, Y. New plasmid mediated fluoroquinolone efflux pump, QepA, found in an Escherichia coli clinical isolate. Antimicrob. Agents Chemother. 2007, 51, 3354–3360. [Google Scholar] [CrossRef]
- Cattoir, V.; Poirel, L.; Nordmann, P. Plasmid-mediated quinolone resistance pump QepA2 in an Escherichia coli isolate from France. Antimicrob. Agents Chemother. 2008, 52, 3801–3804. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Huang, X.; Chen, J.; Mou, Y.; Li, H.; Yang, L. Characterization of genetic structures of the QepA3 gene in clinical isolates of Enterobacteriaceae. Front. Microbiol. 2015, 6, 1147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wayne, P.A. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-third informational supplement; Clinical and Laboratory Standards Institute: Annapolis Junction, MD, USA, 2010. [Google Scholar]
- Hirai, K.; Aoyama, H.; Suzue, S.; Irikura, T.; Iyobe, S.; Mitsuhashi, S. Isolation and characterization of norfloxacin-resistant mutants of Escherichia coli K-12. Antimicrob. Agents Chemother. 1986, 30, 248–253. [Google Scholar] [CrossRef]
- Piddock, L.J. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin. Microbiol. Rev. 2006, 19, 382–402. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, A.; Ismat, F.; Iqbal, M.; Haque, A.; De Zorzi, R.; Mirza, O.; Walz, T.; Rahman, M. Characterization of putative multidrug resistance transporters of the major facilitator-superfamily expressed in Salmonella typhi. J. Infect. Chemother. 2015, 21, 357–362. [Google Scholar] [CrossRef]
- Komp Lindgren, P.; Marcusson, L.L.; Sandvang, D.; Frimodt-Moller, N.; Hughes, D. Biological cost of single and multiple norfloxacin resistance mutations in Escherichia coli implicated in urinary tract infections. Antimicrob. Agents Chemother. 2005, 49, 2343–2351. [Google Scholar] [CrossRef]
- Piddock, L.J. Mechanisms of fluoroquinolone resistance: An update 1994–1998. Drugs 1999, 58, 11–18. [Google Scholar] [CrossRef]
- Tack, B.; Phoba, M.F.; Van Puyvelde, S.; Kalonji, L.M.; Hardy, L.; Barbe, B.; Van der Sande, M.A.B.; Monsieurs, E.; Deborggraeve, S.; Lunguya, O.; et al. Salmonella Typhi from blood cultures in the Democratic Republic of the Congo: A 10-year surveillance. Clin. Infect. Dis. 2019, 68, S130–S137. [Google Scholar] [CrossRef]
- Perichon, B.; Courvalin, P.; Galimand, M. Transferable resistance to aminoglycosides by methylation of G1405 in 16S rRNA and to hydrophilic fluoroquinolones by QepA-mediated efflux in Escherichia coli. Antimicrob. Agents Chemother. 2007, 51, 2464–2469. [Google Scholar] [CrossRef]
- Strahilevitz, J.; Jacoby, G.A.; Hooper, D.C.; Robicsek, A. Plasmid mediated quinolone resistance: A multifaceted threat. Clin. Microbiol. Rev. 2009, 22, 664–689. [Google Scholar] [CrossRef]
- Bugarel, M.; Tudor, A.; Loneragan, G.H.; Nightingale, K.K. Molecular detection assay of five Salmonella serotypes of public interest: Typhimurium, Enteritidis, Newport, Heidelberg, and Hadar. J. Microbiol. Methods. 2017, 134, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Singh, V. Salmonella serovars and their host specificity. J. Vet. Sci. Anim. Husb. 2013, 1, 301. [Google Scholar] [CrossRef]
- Gay, K.; Robicsek, A.; Strahilevitz, J.; Park, C.H.; Jacoby, G.; Barrett, T.J.; Medalla, F.; Chiller, T.M.; Hooper, D.C. Plasmid mediated quinolone resistance in non-Typhi serotypes of Salmonella enterica. Clin. Infect. Dis. 2006, 43, 297–304. [Google Scholar] [CrossRef]
- Siddiqui, T.R.; Bibi, S.; Mustufa, M.A.; Ayaz, S.M.; Khan, A. High prevalence of typhoidal Salmonella enterica serovars excreting food handlers in Karachi-Pakistan: A probable factor for regional typhoid endemicity. J. Health Popul. Nutr. 2015, 33, 27. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers, L.M.; Le Hello, S.; Fawal, N.; Fabre, L.; Tourdjman, M.; Dufour, M.; Sar, D.; Kham, C.; Phe, T.; Vlieghe, E.; et al. Genomic analysis of Salmonella enterica serotype Paratyphi A during an outbreak in Cambodia, 2013–2015. Microb. Genom. 2016, 2, e000092. [Google Scholar] [CrossRef]
- Accou-Demartin, M.; Gaborieau, V.; Song, Y.; Roumagnac, P.; Marchou, B.; Achtman, M.; Weill, F.X. Salmonella enterica serotype Typhi with nonclassical quinolone resistance phenotype. Emerg. Infect. Dis. 2011, 17, 1091–1094. [Google Scholar] [CrossRef]
- Commons, R.J.; McBryde, E.; Valcanis, M.; Powling, J.; Street, A.; Hogg, G. Twenty six years of enteric fever in Australia: An epidemiological analysis of antibiotic resistance. Med. J. Aust. 2012, 196, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Acharya, D.; Trakulsomboon, S.; Madhup, S.K.; Korbsrisate, S. Antibiotic susceptibility pattern and the indicator of decreased ciprofloxacin susceptibility of Salmonella enterica serovar Typhi isolated from Dhulikhel Hospital, Nepal. Jpn J. Infect. Dis. 2012, 65, 264–267. [Google Scholar] [CrossRef]
- Yan, M.; Li, X.; Liao, Q.; Li, F.; Zhang, J.; Kan, B. The emergence and outbreak of multidrug-resistant typhoid fever in China. Emerg. Microbes Infect. 2016, 5, e62. [Google Scholar] [CrossRef]
- Garcia-Fernandez, A.; Gallina, S.; Owczarek, S.; Dionisi, A.M.; Benedetti, I.; Decastelli, L.; Luzzi, I. Emergence of ciprofloxacin resistant Salmonella enterica serovar Typhi in Italy. PLoS ONE 2015, 10, e0132065. [Google Scholar]
- Chau, T.T.; Campbell, J.I.; Galindo, C.M.; Van Minh Hoang, N.; Diep, T.S.; Nga, T.T.; Van Vinh Chau, N.; Tuan, P.Q.; Page, A.L.; Ochiai, R.L.; et al. Antimicrobial drug resistance of Salmonella enterica serovar typhi in asia and molecular mechanism of reduced susceptibility to the fluoroquinolones. Antimicrob. Agents Chemother. 2007, 51, 4315–4323. [Google Scholar] [CrossRef]
- Veeraraghavan, B.; Anandan, S.; Muthuirulandi Sethuvel, D.P.; Puratchiveeran, N.; Walia, K.; Devanga Ragupathi, N.K. Molecular characterization of intermediate susceptible typhoidal Salmonella to ciprofloxacin, and its impact. Mol. Diagn. Ther. 2016, 20, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, Y.; Matten, J.; Rabsch, W. Salmonella enterica serovar Typhi with CTX-M beta-lactamase, Germany. Emerg. Infect. Dis. 2009, 15, 1533–1535. [Google Scholar] [CrossRef]
- Lee, C.J.; Su, L.H.; Huang, Y.C.; Chiu, C.H. First isolation of ciprofloxacin resistant Salmonella enterica serovar Typhi in Taiwan. J. Microbiol. Immunol. Infect. 2013, 46, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Unnikrishnan, M.; Turner, K.; Parija, S.C.; Churcher, C.; Wain, J.; Harish, N. Molecular analysis of fluoroquinolone-resistant Salmonella Paratyphi A isolate, India. Emerg. Infect. Dis. 2006, 12, 489–491. [Google Scholar] [CrossRef] [PubMed]
- Keddy, K.H.; Smith, A.M.; Sooka, A.; Ismail, H.; Oliver, S. Fluoroquinolone-resistant typhoid, South Africa. Emerg. Infect. Dis. 2010, 16, 879–880. [Google Scholar] [CrossRef] [PubMed]
- Pham Thanh, D.; Tran Vu Thieu, N.; Tran Thuy, C.; Loden, M.; Tuin, K.; Campbell, J.I.; Van Minh Hoang, N.; Voong Vinh, P.; Farrar, J.J.; Holt, K.E.; et al. Identification of Salmonella enterica serovar Typhi genotypes by use of rapid multiplex ligation dependent probe amplification. J. Clin. Microbiol. 2013, 51, 2950–2958. [Google Scholar] [CrossRef]
- Kariuki, S.; Revathi, G.; Kiiru, J.; Mengo, D.M.; Mwituria, J.; Muyodi, J.; Munyalo, A.; Teo, Y.Y.; Holt, K.E.; Kingsley, R.A. Typhoid in Kenya is associated with a dominant multidrug-resistant Salmonella enterica serovar Typhi haplotype that is also widespread in Southeast Asia. J. Clin. Microbiol. 2010, 48, 2171–2176. [Google Scholar] [CrossRef]
- Chiou, C.S.; Lauderdale, T.L.; Phung, D.C.; Watanabe, H.; Kuo, J.C.; Wang, P.J.; Liu, Y.Y.; Liang, S.Y.; Chen, P.C. Antimicrobial resistance in Salmonella enterica serovar Typhi isolates from Bangladesh, Indonesia, Taiwan, and Vietnam. Antimicrob. Agents Chemother. 2014, 58, 6501–6507. [Google Scholar] [CrossRef] [PubMed]
- Koirala, K.D.; Thanh, D.P.; Thapa, S.D.; Arjyal, A.; Karkey, A.; Dongol, S.; Shrestha, U.M.; Farrar, J.J.; Basnyat, B.; Baker, S. Highly resistant Salmonella enterica serovar Typhi with a novel gyrA mutation raises questions about the long-term efficacy of older fluoroquinolones for treating typhoid fever. Antimicrob. Agents Chemother. 2012, 56, 2761–2762. [Google Scholar] [CrossRef] [PubMed]
- Menezes, G.A.; Harish, B.N.; Khan, M.A.; Goessens, W.H.; Hays, J.P. Antimicrobial resistance trends in blood culture positive Salmonella Typhi isolates from Pondicherry, India, 2005–2009. Clin. Microbiol. Infect. 2012, 18, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Walther-Rasmussen, J.; Hoiby, N. Salmonella enterica serovar Typhi and S. Paratyphi A: Need to expand the QRDR region? Epidemiol. Infect. 2011, 139, 1281–1283. [Google Scholar] [CrossRef] [PubMed]
- Eibach, D.; Al-Emran, H.M.; Dekker, D.M.; Krumkamp, R.; Adu-Sarkodie, Y.; Cruz Espinoza, L.M.; Ehmen, C.; Boahen, K.; Heisig, P.; Im, J.; et al. The emergence of reduced ciprofloxacin susceptibility in Salmonella enterica causing bloodstream infections in rural Ghana. Clin. Infect. Dis. 2016, 62, S32–S36. [Google Scholar] [CrossRef] [PubMed]
- Vlieghe, E.R.; Phe, T.; De Smet, B.; Veng, C.H.; Kham, C.; Bertrand, S.; Vanhoof, R.; Lynen, L.; Peetermans, W.E.; Jacobs, J.A. Azithromycin and ciprofloxacin resistance in Salmonella bloodstream infections in Cambodian adults. PLoS Negl. Trop. Dis. 2012, 6, e1933. [Google Scholar] [CrossRef] [PubMed]
- Hamidian, M.; Tajbakhsh, M.; Tohidpour, A.; Rahbar, M.; Zali, M.R.; Walther-Rasmussen, J. Detection of novel gyrA mutations in nalidixic acid-resistant isolates of Salmonella enterica from patients with diarrhoea. Int. J. Antimicrob. Agents 2011, 37, 360–364. [Google Scholar] [CrossRef]
- Renuka, K.; Kapil, A.; Kabra, S.K.; Wig, N.; Das, B.K.; Prasad, V.V.; Chaudhry, R.; Seth, P. Reduced susceptibility to ciprofloxacin and gyrA gene mutation in North Indian strains of Salmonella enterica serotype Typhi and serotype Paratyphi A. Microb. Drug Resist. 2004, 10, 146–153. [Google Scholar] [CrossRef]
- Gupta, R.; Gaind, R.; Wain, J.; Deb, M.; Singh, L.C.; Basir, S.F. Characterization of non-classical quinolone resistance in Salmonella enterica serovar Typhi: Report of a novel mutation in gyrB gene and diagnostic challenges. Biomol. Detect. Quantif. 2014, 2, 30–34. [Google Scholar] [CrossRef]
- Eaves, D.J.; Randall, L.; Gray, D.T.; Buckley, A.; Woodward, M.J.; White, A.P.; Piddock, L.J. Prevalence of mutations within the quinolone resistance determining region of gyrA, gyrB, parC, and parE and association with antibiotic resistance in quinolone-resistant Salmonella enterica. Antimicrob. Agents Chemother. 2004, 48, 4012–4015. [Google Scholar] [CrossRef]
- Qian, H.; Cheng, S.; Liu, G.; Tan, Z.; Dong, C.; Bao, J.; Hong, J.; Jin, D.; Bao, C.; Gu, B. Discovery of seven novel mutations of gyrB, parC and parE in Salmonella Typhi and Paratyphi strains from Jiangsu Province of China. Sci. Rep. 2020, 10, 7359. [Google Scholar] [CrossRef] [PubMed]
- Gopal, M.; Elumalai, S.; Arumugam, S.; Durairajpandian, V.; Kannan, M.A.; Selvam, E.; Seetharaman, S. GyrA ser83 and ParC trp106 mutations in Salmonella enterica serovar Typhi isolated from typhoid fever patients in tertiary care hospital. J. Clin. Diagn. Res. 2016, 10, DC14–DC18. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Yang, B.; Wang, Z.; Wang, S.; Zhang, X.; Zhou, Y.; Pang, B.; Diao, B.; Yang, R.; Wu, S.; et al. A large-scale community based outbreak of paratyphoid fever caused by hospital derived transmission in Southern China. PLoS Negl. Trop. Dis. 2015, 9, e0003859. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Morita, M.; Shimada, T.; Izumiya, H.; Kanayama, A.; Oishi, K.; Ohnishi, M.; Sunagawa, T. Increase in paratyphoid fever cases in Japanese travellers returning from Cambodia in 2013. Epidemiol. Infect. 2016, 144, 602–606. [Google Scholar] [CrossRef]
- Al-Emran, H.M.; Eibach, D.; Krumkamp, R.; Ali, M.; Baker, S.; Biggs, H.M.; Bjerregaard-Andersen, M.; Breiman, R.F.; Clemens, J.D.; Crump, J.A.; et al. A multicountry molecular analysis of Salmonella enterica serovar Typhi with reduced susceptibility to ciprofloxacin in Sub-Saharan Africa. Clin. Infect. Dis. 2016, 62, S42–S46. [Google Scholar] [CrossRef] [PubMed]
- Hassing, R.J.; Menezes, G.A.; van Pelt, W.; Petit, P.L.; van Genderen, P.J.; Goessens, W.H. Analysis of mechanisms involved in reduced susceptibility to ciprofloxacin in Salmonella enterica serotypes Typhi and Paratyphi A isolates from travellers to Southeast Asia. Int. J. Antimicrob. Agents 2011, 37, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Sagara, H.; Hirose, K.; Watanabe, H. Fluoroquinolone resistant Salmonella Paratyphi A. Emerg. Infect. Dis. 2005, 11, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Sjolund-Karlsson, M.; Howie, R.; Rickert, R.; Newton, A.; Gonzalez-Aviles, G.; Crump, J.A. Plasmid mediated quinolone resistance in isolates of Salmonella enterica serotype Typhi, USA. Int. J. Antimicrob. Agents 2015, 45, 88–90. [Google Scholar] [CrossRef]
- Yang, B.; Qiao, L.; Zhang, X.; Cui, Y.; Xia, X.; Cui, S.; Wang, X.; Meng, X.; Ge, W.; Shi, X. Serotyping, antimicrobial susceptibility, pulse field gel electrophoresis analysis of Salmonella isolates from retail foods in Henan Province, China. Food Cont. 2013, 32, 228–235. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2015. EFSA J. 2019, 17, e05598. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards. Scientific opinion on the public health risks of table eggs due to deterioration and development of pathogens. EFSA J. 2014, 12, 3782. [Google Scholar] [CrossRef]
- Lunn, A.D.; Fàbrega, A.; Sánchez-Céspedes, J.; Vila, J. Prevalence of mechanisms decreasing quinolone-susceptibility among Salmonella spp. clinical isolates. Int. Microbiol. 2010, 13, 15–20. [Google Scholar] [PubMed]
- Campos, M.J.; Palomo, G.; Hormeno, L.; Herrera-Leon, S.; Dominguez, L.; Vadillo, S.; Piriz, S.; Quesada, A. Prevalence of quinolone resistance determinants in non-typhoidal Salmonella isolates from human origin in Extremadura, Spain. Diagn. Microbiol. Infect. Dis. 2014, 79, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Thong, K.L.; Ngoi, S.T.; Chai, L.C.; Teh, C.S.J. Quinolone resistance mechanisms among Salmonella enterica in Malaysia. Microbial. Drug Resist. 2016, 22, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, R.; Tay, S.T.; Rahim, F.F.; Lim, B.B.; Puthucheary, S.D. Molecular analysis of ciprofloxacin resistance among non-typhoidal Salmonella with reduced susceptibility to ciprofloxacin isolated from patients at a tertiary care hospital in Kuala Lumpur, Malaysia. Jap. J. Infect. Dis. 2014, 67, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Park, J.H.; Kwak, H.S.; Woo, G.J. Characterization of the quinolone resistance mechanism in foodborne Salmonella isolates with high nalidixic acid resistance. Int. J. Food Microbiol. 2011, 146, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Bae, D.H.; Baek, H.J.; Jeong, S.J.; Lee, Y.J. Amino acid substitutions in gyrA and parC associated with quinolone resistance in nalidixic acid-resistant Salmonella isolates. Ir. Vet. J. 2013, 66, 23. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Galiana, A.; Cremades, R.; Rodriguez, J.C.; Magnani, M.; Tognim, M.C.; Oliveira, T.C.; Royo, G. Plasmid mediated quinolone resistance (PMQR) and mutations in the topoisomerase genes of Salmonella enterica strains from Brazil. Braz. J. Microbiol. 2013, 44, 651–656. [Google Scholar] [CrossRef]
- Campioni, F.; Souza, R.A.; Martins, V.V.; Stehling, E.G.; Bergamini, A.M.M.; Falcao, J.P. Prevalence of gyrA mutations in nalidixic acid-resistant strains of Salmonella Enteritidis isolated from humans, food, chickens, and the farm environment in Brazil. Microb. Drug Resist. 2017, 23, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Osawa, K.; Shigemura, K.; Shimizu, R.; Kato, A.; Kimura, M.; Katayama, Y.; Okuya, Y.; Yutaka, S.; Nishimoto, A.; Kishi, A.; et al. Antimicrobial resistance in Salmonella strains clinically isolated in Hyogo, Japan (2009–2012). Jpn. J. Infect. Dis. 2014, 67, 54–57. [Google Scholar] [CrossRef]
- Utrarachkij, F.; Nakajima, C.; Changkwanyeun, R.; Siripanichgon, K.; Kongsoi, S.; Pornruangwong, S.; Changkaew, K.; Tsunoda, R.; Tamura, Y.; Suthienkul, O.; et al. Quinolone resistance determinants of clinical Salmonella Enteritidis in Thailand. Microb. Drug Resist. 2017, 23, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Kozoderovic, G.; Velhner, M.; Jelesic, Z.; Golic, N.; Lozo, J.; Kehrenberg, C. Prevalence of quinolone resistance and mutations in the topoisomerase genes in Salmonella enterica serotype Enteritidis isolates from Serbia. Int. J. Antimicrob. Agents 2012, 40, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, N.; Yun, S.; Hur, E.; Song, J.; Lee, H.; Kim, Y.; Ryu, S. Presence of plasmid-mediated quinolone resistance (PMQR) genes in non-typhoidal Salmonella strains with reduced susceptibility to fluoroquinolones isolated from human salmonellosis in Gyeonggi-do, South Korea from 2016 to 2019. Gut Pathog. 2021, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Koide, K.; San, L.L.; Pachanon, R.; Park, J.H.; Ouchi, Y.; Kongsoi, S.; Utrarachkij, F.; Nakajima, C.; Suzuki, Y. Amino acid substitution Ser83Ile in GyrA of DNA gyrases confers high-level quinolone resistance to nontyphoidal Salmonella without loss of supercoiling activity. Microb. Drug Resist. 2021, 27, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Sinwat, N.; Angkittitrakul, S.; Chuanchuen, R. Characterization of antimicrobial resistance in Salmonella enterica isolated from pork, chicken meat, and humans in Northeastern Thailand. Foodborne Pathog. Dis. 2015, 12, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Escribano, I.; Rodriguez, J.C.; Royo, G. Mutations in the gyrA gene in Salmonella enterica clinical isolates with decreased ciprofloxacin susceptibility. Int. J. Antimicrob. Agents 2004, 24, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Threlfall, E.; Wain, J.; Peters, T.; Lane, C.; De Pinna, E.; Little, C.; Wales, A.; Davies, R. Egg-borne infections of humans with Salmonella: Not only an S. Enteritidis problem. Worlds Poult. Sci. J. 2014, 70, 15–26. [Google Scholar] [CrossRef]
- Helms, M.; Ethelberg, S.; Molbak, K.; Group, D.T.S. International Salmonella Typhimurium DT104 infections, 1992–2001. Emerg. Infect. Dis. 2005, 11, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Threlfall, E.J.; Rowe, B.; Ward, L.R. A comparison of multiple drug resistance in salmonellas from humans and food animals in England and Wales, 1981 and 1990. Epidemiol. Infect. 1993, 111, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Xu, X.; Liang, B.; Wu, F.; Yang, X.; Ma, Q.; Yang, C.; Hu, X.; Liu, H.; et al. Antimicrobial resistance of Salmonella enterica serovar Typhimurium in Shanghai, China. Front. Microbiol. 2017, 8, 510. [Google Scholar] [CrossRef]
- Wong, M.H.; Yan, M.; Chan, E.W.; Biao, K.; Chen, S. Emergence of clinical Salmonella enterica serovar Typhimurium isolates with concurrent resistance to ciprofloxacin, ceftriaxone, and azithromycin. Antimicrob. Agents Chemother. 2014, 58, 3752–3756. [Google Scholar] [CrossRef]
- Al-Emran, H.M.; Heisig, A.; Dekker, D.; Adu-Sarkodie, Y.; Cruz Espinoza, L.M.; Panzner, U.; von Kalckreuth, V.; Marks, F.; Park, S.E.; Sarpong, N.; et al. Detection of a novel gyrB mutation associated with fluoroquinolone-nonsusceptible Salmonella enterica serovar Typhimurium isolated from a bloodstream infection in Ghana. Clin. Infect. Dis. 2016, 62, S47–S49. [Google Scholar] [CrossRef]
- Casin, I.; Breuil, J.; Darchis, J.P.; Guelpa, C.; Collatz, E. Fluoroquinolone resistance linked to GyrA, GyrB, and ParC mutations in Salmonella enterica Typhimurium isolates in humans. Emerg. Infect. Dis. 2003, 9, 1455–1457. [Google Scholar] [CrossRef]
- Zhang, W.H.; Zhang, C.Z.; Liu, Z.J.; Gu, X.X.; Li, W.; Yang, L.; Liu, Y.H.; Zeng, Z.L.; Jiang, H.X. In vitro development of ciprofloxacin resistance of Salmonella enterica serovars Typhimurium, Enteritidis, and Indiana isolates from food animals. Microb. Drug Resist. 2017, 23, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cui, S.; McDermott, P.F.; Zhao, S.; White, D.G.; Paulsen, I.; Meng, J. Contribution of target gene mutations and efflux to decreased susceptibility of Salmonella enterica serovar Typhimurium to fluoroquinolones and other antimicrobials. Antimicrob. Agents Chemother. 2007, 51, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Dai, M.; Hao, H.; Wang, Y.; Huang, L.; Almofti, Y.A.; Liu, Z.; Yuan, Z. The role of RamA on the development of ciprofloxacin resistance in Salmonella enterica serovar Typhimurium. PLoS ONE 2011, 6, e23471. [Google Scholar] [CrossRef] [PubMed]
- de Toro, M.; Rojo-Bezares, B.; Vinue, L.; Undabeitia, E.; Torres, C.; Saenz, Y. In vivo selection of aac(6′)-Ib-cr and mutations in the gyrA gene in a clinical qnrS1-positive Salmonella enterica serovar Typhimurium DT104B strain recovered after fluoroquinolone treatment. J. Antimicrob. Chemother. 2010, 65, 1945–1949. [Google Scholar] [CrossRef] [PubMed]
- Cernela, N.; Nuesch-Inderbinen, M.; Hachler, H.; Stephan, R. Antimicrobial resistance patterns and genotypes of Salmonella enterica serovar Hadar strains associated with human infections in Switzerland, 2005–2010. Epidemiol. Infect. 2014, 142, 84–89. [Google Scholar] [CrossRef]
- Abgottspon, H.; Zurfluh, K.; Nuesch-Inderbinen, M.; Hachler, H.; Stephan, R. Quinolone resistance mechanisms in Salmonella enterica serovars Hadar, Kentucky, Virchow, Schwarzengrund, and 4,5,12:i:-, isolated from humans in Switzerland, and identification of a novel qnrD variant, qnrD2, in S. Hadar. Antimicrob Agents Chemother. 2014, 58, 3560–3563. [Google Scholar] [CrossRef]
- Tamang, M.D.; Nam, H.-M.; Kim, A.; Lee, H.-S.; Kim, T.-S.; Kim, M.-J.; Jang, G.-C.; Jung, S.-C.; Lim, S.-K. Prevalence and mechanisms of quinolone resistance among selected nontyphoid Salmonella isolated from food animals and humans in Korea. Foodborne Pathog. Dis. 2011, 8, 1199–1206. [Google Scholar] [CrossRef]
- Murgia, M.; Bouchrif, B.; Timinouni, M.; Al-Qahtani, A.; Al-Ahdal, M.N.; Cappuccinelli, P.; Rubino, S.; Paglietti, B. Antibiotic resistance determinants and genetic analysis of Salmonella enterica isolated from food in Morocco. Int. J. Food Microbiol. 2015, 215, 31–39. [Google Scholar] [CrossRef]
- Lindstedt, B.; Aas, L.; Kapperud, G. Geographically dependent distribution of gyrA gene mutations at codons 83 and 87 in Salmonella Hadar, and a novel codon 81 Gly to His mutation in Salmonella Enteritidis. Apmis 2004, 112, 165–171. [Google Scholar] [CrossRef]
- Haugum, K.; Aas, L.; Lindstedt, B. Effect of quinolone antibiotics and chemicals on mutation types in Salmonella enterica serovars Enteritidis, Hadar and Virchow. J. Chin. Clin. Med. 2007, 2, 241–251. [Google Scholar]
- Fiegen, U.; Klein, G.; de Jong, A.; Kehrenberg, C. Detection of a novel qnrB19-carrying plasmid variant mediating decreased fluoroquinolone susceptibility in Salmonella enterica serovar Hadar. Microb. Drug Resist. 2017, 23, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Le Hello, S.; Hendriksen, R.S.; Doublet, B.; Fisher, I.; Nielsen, E.M.; Whichard, J.M.; Bouchrif, B.; Fashae, K.; Granier, S.A.; Jourdan-Da Silva, N.; et al. International spread of an epidemic population of Salmonella enterica serotype Kentucky ST198 resistant to ciprofloxacin. J. Infect. Dis. 2011, 204, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Le Hello, S.; Bekhit, A.; Granier, S.A.; Barua, H.; Beutlich, J.; Zajac, M.; Munch, S.; Sintchenko, V.; Bouchrif, B.; Fashae, K.; et al. The global establishment of a highly-fluoroquinolone resistant Salmonella enterica serotype Kentucky ST198 strain. Front. Microbiol. 2013, 4, 395. [Google Scholar] [CrossRef] [PubMed]
- Westrell, T.; Monnet, D.L.; Gossner, C.; Heuer, O.; Takkinen, J. Drug resistant Salmonella enterica serotype Kentucky in Europe. Lancet Infect. Dis. 2014, 14, 270–271. [Google Scholar] [CrossRef]
- Haley, B.J.; Kim, S.W.; Pettengill, J.; Luo, Y.; Karns, J.S.; Van Kessel, J.A. Genomic and evolutionary analysis of two Salmonella enterica serovar Kentucky sequence types isolated from bovine and poultry sources in North America. PLoS ONE 2016, 11, e0161225. [Google Scholar] [CrossRef]
- Mulvey, M.R.; Boyd, D.A.; Finley, R.; Fakharuddin, K.; Langner, S.; Allen, V.; Ang, L.; Bekal, S.; El Bailey, S.; Haldane, D.; et al. Ciprofloxacin resistant Salmonella enterica serovar Kentucky in Canada. Emerg. Infect. Dis. 2013, 19, 999–1001. [Google Scholar] [CrossRef]
- Seiffert, S.N.; Perreten, V.; Johannes, S.; Droz, S.; Bodmer, T.; Endimiani, A. OXA-48 carbapenemase-producing Salmonella enterica serovar Kentucky isolate of sequence type 198 in a patient transferred from Libya to Switzerland. Antimicrob. Agents Chemother. 2014, 58, 2446–2449. [Google Scholar] [CrossRef]
- Jeong, H.S.; Kim, J.A.; Shin, J.H.; Chang, C.L.; Jeong, J.; Cho, J.H.; Kim, M.N.; Kim, S.; Kim, Y.R.; Lee, C.H.; et al. Prevalence of plasmid mediated quinolone resistance and mutations in the gyrase and topoisomerase IV genes in Salmonella isolated from 12 tertiary-care hospitals in Korea. Microb. Drug Resist. 2011, 17, 551–557. [Google Scholar] [CrossRef]
- Weill, F.X.; Bertrand, S.; Guesnier, F.; Baucheron, S.; Cloeckaert, A.; Grimont, P.A. Ciprofloxacin resistant Salmonella Kentucky in travelers. Emerg. Infect. Dis. 2006, 12, 1611–1612. [Google Scholar] [CrossRef]
- Palomo, G.; Campos, M.J.; Ugarte, M.; Porrero, M.C.; Alonso, J.M.; Borge, C.; Vadillo, S.; Domínguez, L.; Quesada, A.; Píriz, S. Dissemination of antimicrobial-resistant clones of Salmonella enterica among domestic animals, wild animals, and humans. Foodborne Pathog. Dis. 2013, 10, 171–176. [Google Scholar] [CrossRef]
- Wasyl, D.; Kern-Zdanowicz, I.; Domanska-Blicharz, K.; Zajac, M.; Hoszowski, A. High-level fluoroquinolone resistant Salmonella enterica serovar Kentucky ST198 epidemic clone with IncA/C conjugative plasmid carrying bla(CTX-M-25) gene. Vet. Microbiol. 2015, 175, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.X.; Song, L.; Liu, J.; Zhang, X.H.; Ren, Y.N.; Zhang, W.H.; Zhang, J.Y.; Liu, Y.H.; Webber, M.A.; Ogbolu, D.O.; et al. Multiple transmissible genes encoding fluoroquinolone and third-generation cephalosporin resistance co-located in non-typhoidal Salmonella isolated from food-producing animals in China. Int. J. Antimicrob. Agents 2014, 43, 242–247. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, H.; Liu, Y.; Zhou, X.; Wang, J.; Liu, T.; Beier, R.C.; Hou, X. Characterization of quinolone resistance in Salmonella enterica serovar Indiana from chickens in China. Poult. Sci. 2015, 94, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhao, J.; Gan, X.; Wang, J.; Zhang, X.; Cui, S.; Xia, S.; Hu, Y.; Yan, S.; Wang, J.; et al. Emergence and diversity of Salmonella enterica serovar Indiana isolates with concurrent resistance to ciprofloxacin and cefotaxime from patients and food-producing animals in China. Antimicrob. Agents Chemother. 2016, 60, 3365–3371. [Google Scholar] [CrossRef]
- Bai, L.; Lan, R.; Zhang, X.; Cui, S.; Xu, J.; Guo, Y.; Li, F.; Zhang, D. Prevalence of Salmonella isolates from chicken and pig slaughterhouses and emergence of ciprofloxacin and cefotaxime co-resistant S. enterica serovar Indiana in Henan, China. PLoS ONE 2015, 10, e0144532. [Google Scholar] [CrossRef]
- Lin, D.; Chen, K.; Wai-Chi Chan, E.; Chen, S. Increasing prevalence of ciprofloxacin-resistant foodborne Salmonella strains harboring multiple PMQR elements but not target gene mutations. Sci. Rep. 2015, 5, 14754. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Kelly, P.; Wang, C. Prevalence and antimicrobial resistance of Salmonella enterica serovar Indiana in China (1984–2016). Zoonoses Public Health 2017, 64, 239–251. [Google Scholar] [CrossRef]
- Velhner, M.; Kozoderovic, G.; Grego, E.; Galic, N.; Stojanov, I.; Jelesic, Z.; Kehrenberg, C. Clonal spread of Salmonella enterica serovar Infantis in Serbia: Acquisition of mutations in the topoisomerase genes gyrA and parC leads to increased resistance to fluoroquinolones. Zoonoses Public Health 2014, 61, 364–370. [Google Scholar] [CrossRef]
- Rahmani, M.; Peighambari, S.M.; Svendsen, C.A.; Cavaco, L.M.; Agerso, Y.; Hendriksen, R.S. Molecular clonality and antimicrobial resistance in Salmonella enterica serovars Enteritidis and Infantis from broilers in three Northern regions of Iran. BMC Vet. Res. 2013, 9, 66. [Google Scholar] [CrossRef]
- Hauser, E.; Hebner, F.; Tietze, E.; Helmuth, R.; Junker, E.; Prager, R.; Schroeter, A.; Rabsch, W.; Fruth, A.; Malorny, B. Diversity of Salmonella enterica serovar Derby isolated from pig, pork and humans in Germany. Int. J. Food Microbiol. 2011, 151, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Anjum, M.F.; Choudhary, S.; Morrison, V.; Snow, L.C.; Mafura, M.; Slickers, P.; Ehricht, R.; Woodward, M.J. Identifying antimicrobial resistance genes of human clinical relevance within Salmonella isolated from food animals in Great Britain. J. Antimicrob. Chemother. 2011, 66, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Bonardi, S.; Bassi, L.; Brindani, F.; D'Incau, M.; Barco, L.; Carra, E.; Pongolini, S. Prevalence, characterization and antimicrobial susceptibility of Salmonella enterica and Yersinia enterocolitica in pigs at slaughter in Italy. Int. J. Food Microbiol. 2013, 163, 248–257. [Google Scholar] [CrossRef]
- Wong, M.H.; Chen, S. First detection of oqxAB in Salmonella spp. isolated from food. Antimicrob. Agents Chemother. 2013, 57, 658–660. [Google Scholar] [CrossRef]
- Deekshit, V.K.; Kumar, B.K.; Rai, P.; Karunasagar, I.; Karunasagar, I. Differential expression of virulence genes and role of gyrA mutations in quinolone resistant and susceptible strains of Salmonella Weltevreden and Newport isolated from seafood. J. Appl. Microbiol. 2015, 119, 970–980. [Google Scholar] [CrossRef]
- Cavaco, L.M.; Korsgaard, H.; Sorensen, G.; Aarestrup, F.M. Plasmid mediated quinolone resistance due to qnrB5 and qnrS1 genes in Salmonella enterica serovars Newport, Hadar and Saintpaul isolated from turkey meat in Denmark. J. Antimicrob. Chemother. 2008, 62, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.; Tagg, K.; Bicknese, A.; McCullough, A.; Chen, J.; Karp, B.E.; Folster, J.P. Identification and Characterization of Salmonella enterica Serotype Newport Isolates with Decreased Susceptibility to Ciprofloxacin in the United States. Antimicrob. Agents Chemother. 2018, 62, e00653-18. [Google Scholar] [CrossRef] [PubMed]
- Bonalli, M.; Stephan, R.; Kappeli, U.; Cernela, N.; Adank, L.; Hachler, H. Salmonella enterica serotype Virchow associated with human infections in Switzerland: 2004–2009. BMC Infect. Dis. 2011, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Clemente, L.; Correia, I.; Themudo, P.; Neto, I.; Canica, M.; Bernardo, F. Antimicrobial susceptibility of Salmonella enterica isolates from healthy breeder and broiler flocks in Portugal. Vet. J. 2014, 200, 276–281. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, J.K.; Kim, K.S. Prevalence and characterization of plasmid mediated quinolone resistance genes in Salmonella isolated from poultry in Korea. Avian Pathol. 2013, 42, 221–229. [Google Scholar] [CrossRef]
- Morgan-Linnell, S.K.; Becnel Boyd, L.; Steffen, D.; Zechiedrich, L. Mechanisms accounting for fluoroquinolone resistance in Escherichia coli clinical isolates. Antimicrob. Agents Chemother. 2009, 53, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Hooda, Y.; Sajib, M.S.I.; Rahman, H.; Luby, S.P.; Bondy-Denomy, J.; Santosham, M.; Andrews, J.R.; Saha, S.K.; Saha, S. Molecular mechanism of azithromycin resistance among typhoidal Salmonella strains in Bangladesh identified through passive pediatric surveillance. PLoS Negl. Trop. Dis. 2019, 13, e0007868. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kumari, B.; Dahiya, S.; Kulsum, U.; Kumar, S.; Manral, N.; Pandey, S.; Kaur, P.; Sood, S.; Das, B.K.; et al. Azithromycin resistance mechanisms in typhoidal salmonellae in India: A 25 years analysis. Indian J. Med. Res. 2019, 149, 404–411. [Google Scholar]
- Fu, Y.; Xu, X.; Zhang, L.; Xiong, Z.; Ma, Y.; Wei, Y.; Chen, Z.; Bai, J.; Liao, M.; Zhang, J. Fourth Generation Cephalosporin Resistance Among Salmonella enterica Serovar Enteritidis Isolates in Shanghai, China Conferred by bla CTX-M-55 Harboring Plasmids. Front. Microbiol. 2020, 11, 910. [Google Scholar] [CrossRef] [PubMed]
- Feger, G.; Angelov, B.; Angelova, A. Prediction of Amphiphilic Cell-Penetrating Peptide Building Blocks from Protein-Derived Amino Acid Sequences for Engineering of Drug Delivery Nanoassemblies. J. Phys. Chem. B 2020, 124, 4069–4078. [Google Scholar] [CrossRef] [PubMed]
- Tariq, A.; Sana, M.; Shaheen, A.; Ismat, F.; Mahboob, S.; Rauf, W.; Mirza, O.; Iqbal, M.; Rahman, M. Restraining the multidrug efflux transporter STY4874 of Salmonella Typhi by reserpine and plant extracts. Lett. Appl. Microbiol. 2019, 69, 161–167. [Google Scholar] [CrossRef] [PubMed]





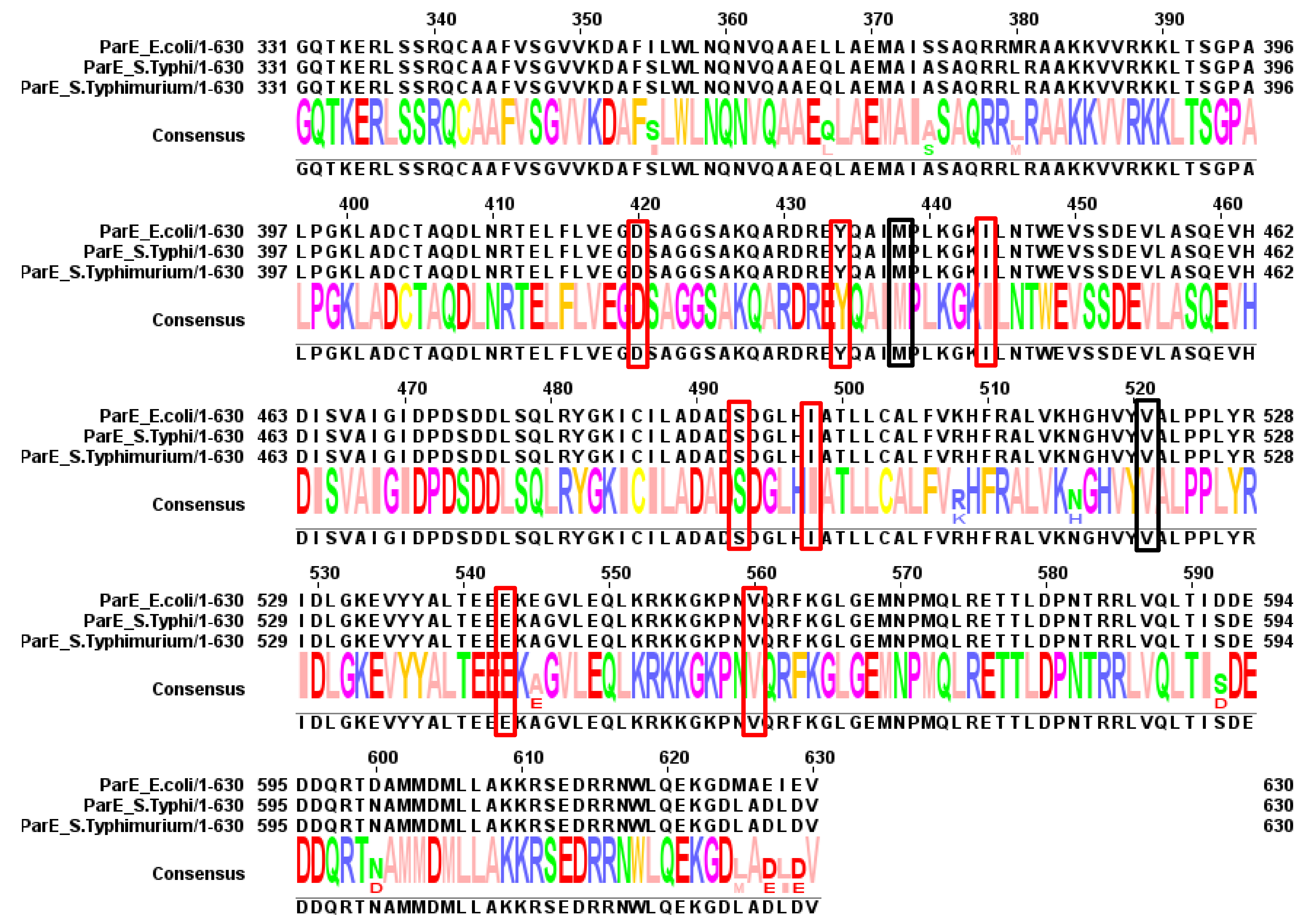
| Bacteria | GyrA | GyrB | ParC | ParE |
|---|---|---|---|---|
| Genbank Accession Numbers | ||||
| E. coli O157:H7 str. EDL933 | AAG57360.1 | AAG58896.1 | AAG58155.1 | AAG58169.1 |
| S. Typhi str. Ty2 | AAO68297.1 | AAO71180.1 | AAO70639.1 | AAO70645.1 |
| S. Typhimurium str. LT2 | AAL21173.1 | AAL22694.1 | AAL22048.1 | AAL22055.1 |
| Salmonella enterica subsp. enterica | Discussed In | |
|---|---|---|
| Typhoidal Salmonella | Typhi | Section 4.1 |
| Paratyphi A, B and C | Section 4.1 | |
| Non-typhoidal Salmonella (NTS) | Enteritidis | Section 4.2.1 |
| Typhimurium | Section 4.2.2 | |
| Hadar | Section 4.2.3 | |
| Kentucky | Section 4.2.4 | |
| Indiana | Section 4.2.5 | |
| Infantis | Section 4.2.6 | |
| Derby | Section 4.2.7 | |
| Newport | Section 4.2.8 | |
| Virchow | Section 4.2.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaheen, A.; Tariq, A.; Iqbal, M.; Mirza, O.; Haque, A.; Walz, T.; Rahman, M. Mutational Diversity in the Quinolone Resistance-Determining Regions of Type-II Topoisomerases of Salmonella Serovars. Antibiotics 2021, 10, 1455. https://doi.org/10.3390/antibiotics10121455
Shaheen A, Tariq A, Iqbal M, Mirza O, Haque A, Walz T, Rahman M. Mutational Diversity in the Quinolone Resistance-Determining Regions of Type-II Topoisomerases of Salmonella Serovars. Antibiotics. 2021; 10(12):1455. https://doi.org/10.3390/antibiotics10121455
Chicago/Turabian StyleShaheen, Aqsa, Anam Tariq, Mazhar Iqbal, Osman Mirza, Abdul Haque, Thomas Walz, and Moazur Rahman. 2021. "Mutational Diversity in the Quinolone Resistance-Determining Regions of Type-II Topoisomerases of Salmonella Serovars" Antibiotics 10, no. 12: 1455. https://doi.org/10.3390/antibiotics10121455
APA StyleShaheen, A., Tariq, A., Iqbal, M., Mirza, O., Haque, A., Walz, T., & Rahman, M. (2021). Mutational Diversity in the Quinolone Resistance-Determining Regions of Type-II Topoisomerases of Salmonella Serovars. Antibiotics, 10(12), 1455. https://doi.org/10.3390/antibiotics10121455






