Co-Application of Allicin and Chitosan Increases Resistance of Rosa roxburghii against Powdery Mildew and Enhances Its Yield and Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungicides
2.2. Field Site
2.3. In Vitro Toxicity Tests
2.4. Field Control Experiment of Powdery Mildew of R. roxburghii
2.5. Investigation of Control Effect of Powdery Mildew of R. roxburghii, and Determination of Its Resistance Parameters, Photosynthetic Rate and Chlorophyll
2.6. Determination of Yield and Quality of R. roxburghii
2.7. Statistical Analyses
3. Results
3.1. Toxicity of Allicin and Chitosan against Sphaerotheca sp.
3.2. Field Control Effect of Allicin and Chitosan against Powdery Mildew of Rosa roxburghii
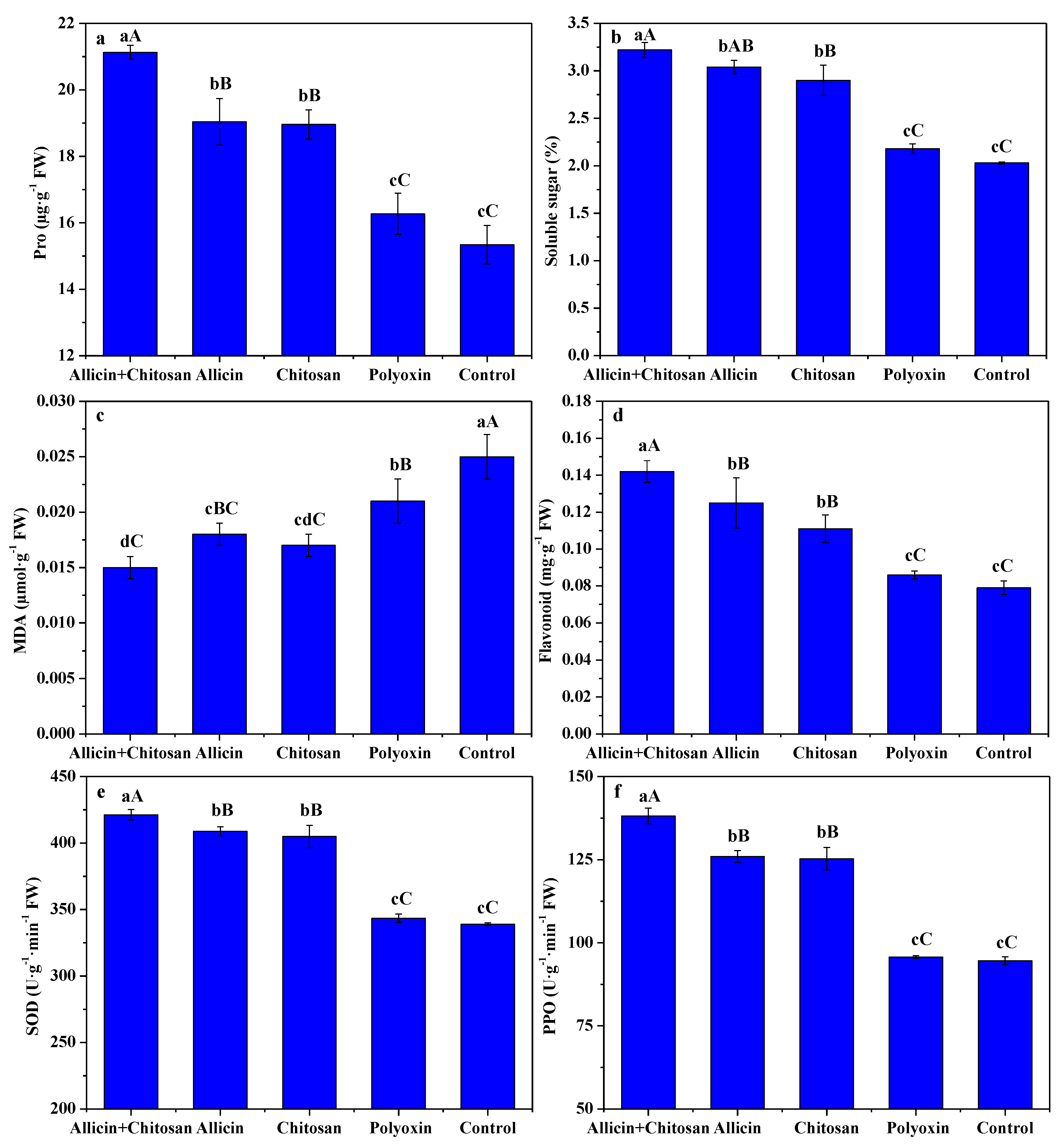
3.3. Effects of Allicin and Chitosan on Resistance Parameters of R. roxburghii Leaves
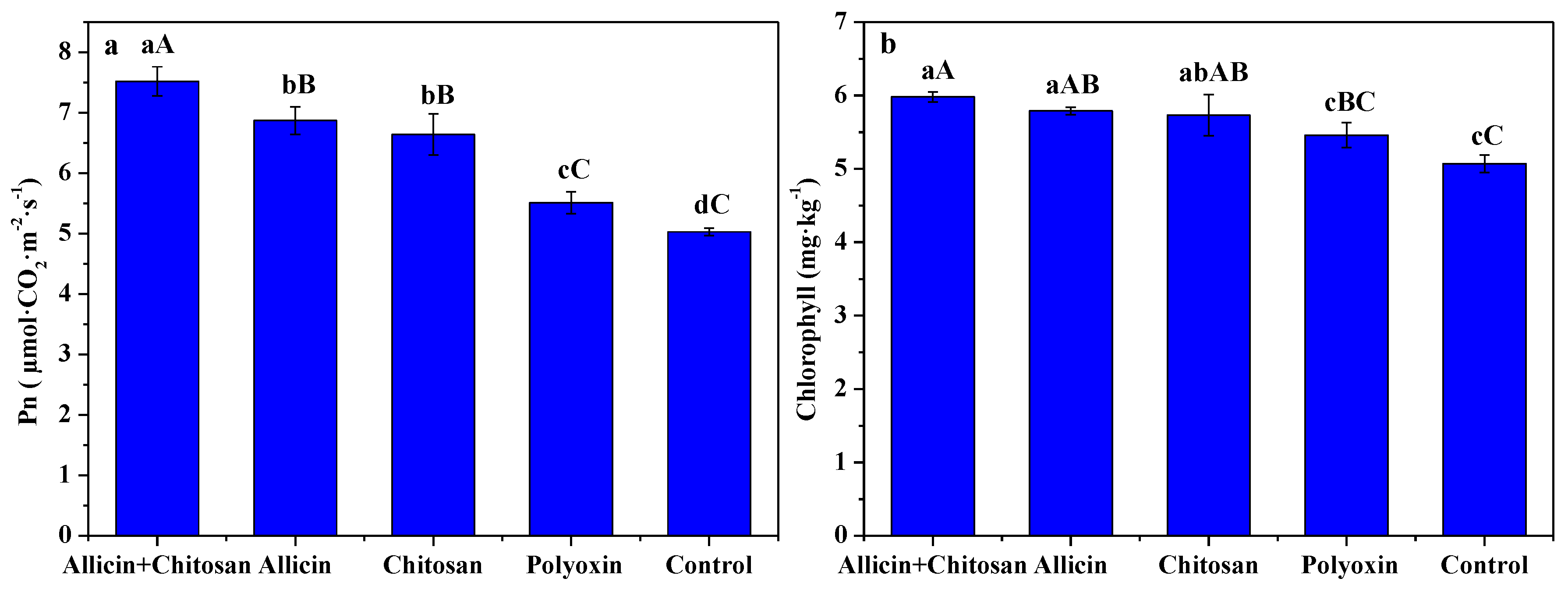
3.4. Effects of Allicin and Chitosan on Photosynthetic Rate and Chlorophyll Content of R. roxburghii Leaves
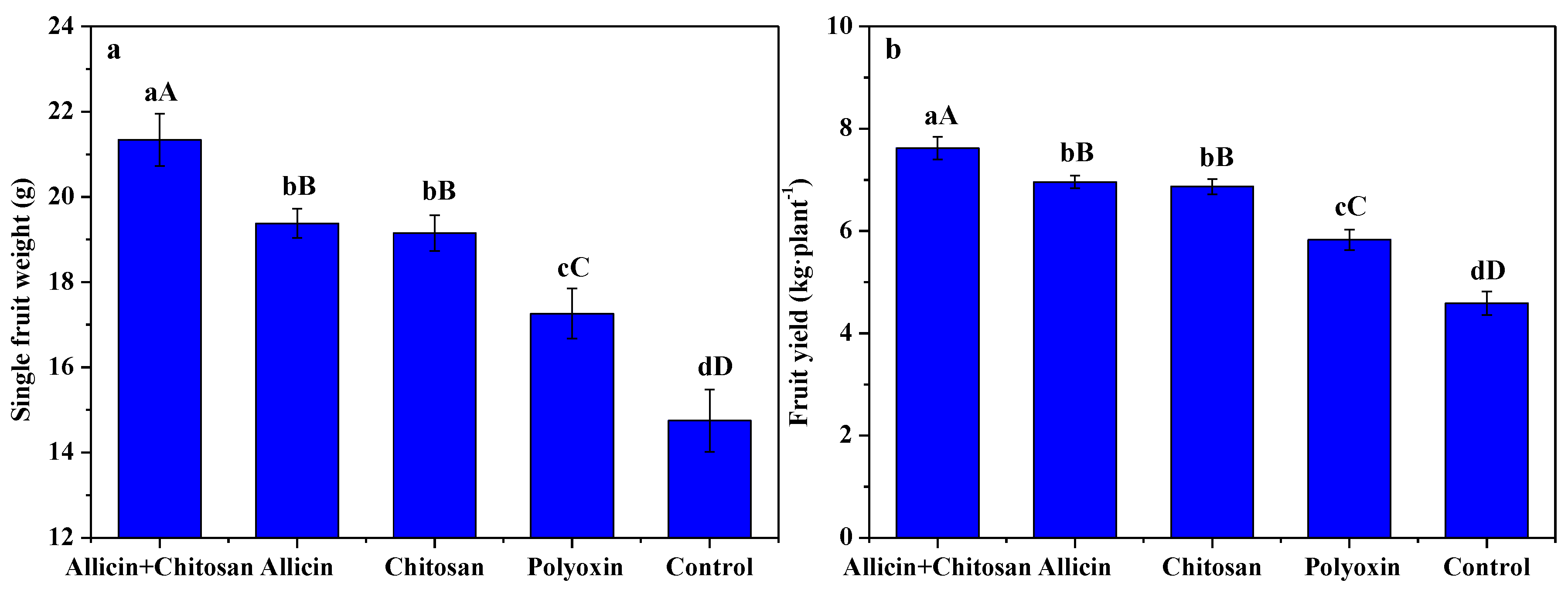
3.5. Effects of Allicin and Chitosan on Yield and Quality of R. roxburghii
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.-T.; Lv, M.-J.; An, J.-Y.; Fan, X.-H.; Dong, M.-Z.; Zhang, S.-D.; Wang, J.-D.; Wang, Y.-Q.; Cai, Z.-H.; Fu, Y.-J. Botanical characteristics, phytochemistry and related biological activities of Rosa roxburghii Tratt fruit, and its potential use in functional foods: A review. Food Funct. 2021, 12, 1432–1451. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.L.; Zhou, R.L. The Healthcare Function and Development Trend of Toxburgh Rose. Food Res. Dev. 2016, 37, 212–214. [Google Scholar]
- Wang, D.; Lu, M.; Ludlow, R.A.; Zeng, J.; Ma, W.; An, H. Comparative ultrastructure of trichomes on various organs of Rosa roxburghii. Microsc. Res. Tech. 2021, 84, 2095–2103. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Z.; Zhao, H.B.; Li, Y.F.; Yu, Z.H.; Liu, X.H.; Huang, M.Z. Identification and Oenological Properties Analysis of a Strain of Hanseniaspora uvarum from Rosa roxburghii. Food Ferment. Ind. 2020, 46, 97–104. [Google Scholar]
- Huang, X.; Yan, H.; Zhai, L.; Yang, Z.; Yi, Y. Characterization of the Rosa roxburghii Tratt transcriptome and analysis of MYB genes. PLoS ONE 2019, 14, e0203014. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.G.; Pan, X.J.; Chen, H.; Yang, H.R.; Gong, F.F.; Guan, J.Y.; Wang, M.L.; Mu, R. Effects of Oxalic Acid on the Nutrient of Calcareous Cultivated Soil and Leaf, Fruit Yield and Quality of Rosa roxburghii Tratt. J. Fruit Sci. 2021, 38, 1113–1122. [Google Scholar] [CrossRef]
- Li, J.; Guo, Z.; Luo, Y.; Wu, X.; An, H. Chitosan Can Induce Rosa roxburghii Tratt. against Sphaerotheca sp. and Enhance Its Resistance, Photosynthesis, Yield, and Quality. Horticulturae 2021, 7, 289. [Google Scholar] [CrossRef]
- Yan, K.; Wang, J.L.; Zhou, Y.; Fu, D.P.; Huang, R.M. Efficacy of Five Fungicides in Rosa roxburghii Tratt against Sphaerotheca sp. Agrochemicals 2018, 57, 609–610. [Google Scholar]
- Xiang, J.; He, B. Toxicity Determination of Several Bio-fungicides to Powdery Mildew in Laboratory. Sci. Technol. Modern Agric. 2013, 19, 147. [Google Scholar]
- Meena, R.S.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Sharma, M.P.; Yadav, G.S.; Jhariya, M.K.; Jangir, C.K.; et al. Impact of Agrochemicals on Soil Microbiota and Management: A Review. Land 2020, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zhang, C.; Long, Y.; Wu, X.; Su, Y.; Lei, Y.; Ai, Q. Bioactivity and Control Efficacy of the Novel Antibiotic Tetramycin against Various Kiwifruit Diseases. Antibiotics 2021, 10, 289. [Google Scholar] [CrossRef]
- Massi, F.; Torriani, S.F.F.; Borghi, L.; Toffolatti, S.L. Fungicide Resistance Evolution and Detection in Plant Pathogens: Plasmopara viticola as a Case Study. Microorganisms 2021, 9, 119. [Google Scholar] [CrossRef]
- Slusarenko, A.J.; Patel, A.; Portz, D. Control of plant diseases by natural products: Allicin from garlic as a case study. Eur. J. Plant Pathol. 2008, 121, 313–322. [Google Scholar] [CrossRef]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.; Slusarenko, A.J. Allicin: Chemistry and Biological Properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [Green Version]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]
- Chakraborty, M.; Hasanuzzaman, M.; Rahman, M.; Khan, A.R.; Bhowmik, P.; Mahmud, N.U.; Tanveer, M.; Islam, T. Mechanism of Plant Growth Promotion and Disease Suppression by Chitosan Biopolymer. Agriculture 2020, 10, 624. [Google Scholar] [CrossRef]
- Torres-Rodriguez, J.A.; Reyes-Pérez, J.J.; Castellanos, T.; Angulo, C.; Quiñones-Aguilar, E.E.; Hernandez-Montiel, L.G. A biopolymer with antimicrobial properties and plant resistance inducer against phytopathogens: Chitosan. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12231. [Google Scholar] [CrossRef]
- Rahman, M.; Mukta, J.A.; Sabir, A.A.; Gupta, D.R.; Mohi-Ud-Din, M.; Hasanuzzaman, M.; Miah, M.G.; Rahman, M.; Islam, M.T. Chitosan biopolymer promotes yield and stimulates accumulation of antioxidants in strawberry fruit. PLoS ONE 2018, 13, e0203769. [Google Scholar] [CrossRef]
- Coutinho, T.C.; Ferreira, M.C.; Rosa, L.H.; de Oliveira, A.M.; Júnior, E.N.D.O. Penicillium citrinum and Penicillium mallochii: New phytopathogens of orange fruit and their control using chitosan. Carbohydr. Polym. 2020, 234, 115918. [Google Scholar] [CrossRef]
- El Amerany, F.; Meddich, A.; Wahbi, S.; Porzel, A.; Taourirte, M.; Rhazi, M.; Hause, B. Foliar Application of Chitosan Increases Tomato Growth and Influences Mycorrhization and Expression of Endochitinase-Encoding Genes. Int. J. Mol. Sci. 2020, 21, 535. [Google Scholar] [CrossRef] [Green Version]
- Cavallito, C.J.; Bailey, J.H. Allicin, the Antibacterial Principle of Allium sativum. I. Isolation, Physical Properties and Antibacterial Action. J. Am. Chem. Soc. 1944, 66, 1950–1951. [Google Scholar] [CrossRef]
- Auger, J.; Arnault, I.; Diwo-Allain, S.; Ravier, N.; Molia, F.; Pettiti, M. Insecticidal and fungicidal potential of Allium substances as biofumigants. Agroindustria 2004, 3, 5–8. [Google Scholar]
- Khodavandi, A.; Alizadeh, F.; Harmal, N.S.; Sidik, S.M.; Othman, F.; Jahromi, M.A.F.; Sekawi, Z.; Ng, K.-P.; Chong, P.P. Comparison between efficacy of allicin and fluconazole against Candida albicans in vitro and in a systemic candidiasis mouse model. FEMS Microbiol. Lett. 2011, 315, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchese, A.; Barbieri, R.; Sanches-Silva, A.; Daglia, M.; Nabavi, S.F.; Jafari, N.J.; Izadi, M.; Ajami, M.; Nabavi, S.M. Antifungal and antibacterial activities of allicin: A review. Trends Food Sci. Technol. 2016, 52, 49–56. [Google Scholar] [CrossRef]
- Choo, S.; Chin, V.K.; Wong, E.H.; Madhavan, P.; Tay, S.T.; Yong, P.V.C.; Chong, P.P. Review: Antimicrobial properties of allicin used alone or in combination with other medications. Folia Microbiol. 2020, 65, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.; Al-Wabel, N. Organosulfur compounds and possible mechanism of garlic in cancer. Saudi Pharm. J. 2010, 18, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwivedi, V.P.; Bhattacharya, D.; Singh, M.; Bhaskar, A.; Kumar, S.; Fatima, S.; Sobia, P.; Van Kaer, L.; Das, G. Allicin enhances antimicrobial activity of macrophages during Mycobacterium tuberculosis infection. J. Ethnopharmacol. 2019, 243, 111634. [Google Scholar] [CrossRef]
- Buendía, A.S.A.; González, M.T.; Reyes, O.S.; Arroyo, F.E.G.; García, R.A.; Tapia, E.; Lozada, L.G.S.; Alonso, H.O. Immunomodulatory Effects of the Nutraceutical Garlic Derivative Allicin in the Progression of Diabetic Nephropathy. Int. J. Mol. Sci. 2018, 19, 3107. [Google Scholar] [CrossRef] [Green Version]
- Curtis, H.; Noll, U.; Störmann, J.; Slusarenko, A.J. Broad-spectrum activity of the volatile phytoanticipin allicin in extracts of garlic (Allium sativum L.) against plant pathogenic bacteria, fungi and Oomycetes. Physiol. Mol. Plant Pathol. 2004, 65, 79–89. [Google Scholar] [CrossRef]
- Zhang, C.; Long, Y.-H.; Wang, Q.-P.; Li, J.-H.; Wu, X.-M.; Li, M. The effect of preharvest 28.6% chitosan composite film sprays for controlling the soft rot on kiwifruit. Hortic. Sci. 2019, 46, 180–194. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Long, Y.; Li, J.; Li, M.; Xing, D.; An, H.; Wu, X.; Wu, Y. A Chitosan Composite Film Sprayed before Pathogen Infection Effectively Controls Postharvest Soft Rot in Kiwifruit. Agronomy 2020, 10, 265. [Google Scholar] [CrossRef] [Green Version]
- Xing, K.; Zhu, X.; Peng, X.; Qin, S. Chitosan antimicrobial and eliciting properties for pest control in agriculture: A review. Agron. Sustain. Dev. 2015, 35, 569–588. [Google Scholar] [CrossRef] [Green Version]
- Berger, L.R.R.; Stamford, N.P.; Willadino, L.G.; Laranjeira, D.; de Lima, M.A.B.; Malheiros, S.M.M.; de Oliveira, W.J.; Stamford, T.C.M. Cowpea resistance induced against Fusarium oxysporum f. sp. tracheiphilum by crustaceous chitosan and by biomass and chitosan obtained from Cunninghamella elegans. Biol. Control. 2016, 92, 45–54. [Google Scholar] [CrossRef]
- Obianom, C.; Romanazzi, G.; Sivakumar, D. Effects of chitosan treatment on avocado postharvest diseases and expression of phenylalanine ammonia-lyase, chitinase and lipoxygenase genes. Postharvest Biol. Technol. 2019, 147, 214–221. [Google Scholar] [CrossRef]
- El-Mohamedya, R.S.R.; Abd El-Aziz, M.E.; Kamel, S. Antifungal Activity of Chitosan Nanoparticles against Some Plant Pathogenic Fungi In Vitro. Agric. Eng. Int. CIGR J. 2019, 21, 201–209. [Google Scholar]
- Yan, J.; Cao, J.; Jiang, W.; Zhao, Y. Effects of preharvest oligochitosan sprays on postharvest fungal diseases, storage quality, and defense responses in jujube (Zizyphus jujuba Mill. cv. Dongzao) fruit. Sci. Hortic. 2012, 142, 196–204. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, L.; Yan, H.; Kennedy, J.F.; Meng, X. Chitosan and oligochitosan enhance the resistance of peach fruit to brown rot. Carbohydr. Polym. 2013, 94, 272–277. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, C.; Wu, X.; Long, Y.; Su, Y. Chitosan Augments Tetramycin against Soft Rot in Kiwifruit and Enhances Its Improvement for Kiwifruit Growth, Quality and Aroma. Biomolecules 2021, 11, 1257. [Google Scholar] [CrossRef]
- Vlot, A.C.; Sales, J.H.; Lenk, M.; Bauer, K.; Brambilla, A.; Sommer, A.; Chen, Y.; Wenig, M.; Nayem, S. Systemic propagation of immunity in plants. New Phytol. 2021, 229, 1234–1250. [Google Scholar] [CrossRef]
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular Mechanisms of Chitosan Interactions with Fungi and Plants. Int. J. Mol. Sci. 2019, 20, 332. [Google Scholar] [CrossRef] [Green Version]
- Dzung, N.A.; Khanh, V.T.P.; Dzung, T.T. Research on impact of chitosan oligomers on biophysical characteristics, growth, development and drought resistance of coffee. Carbohydr. Polym. 2011, 84, 751–755. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Parameters | Content | Parameters | Content |
|---|---|---|---|
| Organic matter | 13.17 g·kg−1 | Exchangeable calcium | 18.32 cmol·kg−1 |
| Total nitrogen | 1.37 g kg−1 | Exchangeable magnesium | 305.37 mg·kg−1 |
| Total phosphorus | 1.72 g kg−1 | Available zinc | 0.63 mg·kg−1 |
| Total potassium | 1.11 g kg−1 | Available iron | 6.42 mg·kg−1 |
| Available nitrogen | 57.43 mg·kg−1 | Available manganese | 15.33 mg·kg−1 |
| Available phosphorus | 4.21 mg·kg−1 | Available boron | 0.14 mg·kg−1 |
| Available potassium | 26.75 mg·kg−1 | pH | 6.89 |
| Treatments | Regression Equation | Determination Coefficient (R2) | EC50 (mg kg−1) |
|---|---|---|---|
| 5% Allicin ME | y = 2.3339 + 1.2274 x | 0.9626 | 148.65 |
| Chitosan | y = 2.5343 + 0.9413 x | 0.9748 | 416.21 |
| 3% Polyoxin WP | y = 2.9799 + 0.8922 x | 0.9937 | 183.68 |
| 6% Kasugamycin WP | y = 2.4254 + 0.8542 x | 0.9406 | 1032.88 |
| Treatments | Incidence Rate (%) | Disease Index | Control Effect (%) |
|---|---|---|---|
| Allicin + Chitosan | 11.00 ± 1.00 cC | 2.14 ± 0.18 dD | 85.97 ± 1.16 aA |
| Allicin | 14.33 ± 1.53 cC | 3.53 ± 0.22 cC | 76.70 ± 1.10 bB |
| Chitosan | 16.00 ± 3.61 cC | 4.42 ± 0.10 cC | 70.93 ± 2.12 cB |
| Polyoxin | 26.67 ± 1.53 bB | 6.04 ± 0.19 bB | 60.23 ± 4.17 dC |
| Control | 45.67 ± 4.51 aA | 15.26 ± 1.12 aA |
| Treatments | Vitamin C (mg·g−1) | Soluble Solid (%) | Soluble Sugar(%) | Total Acidity (%) | Soluble Protein(%) | Flavonoid ggx(mg·g−1) | SOD Activity ggx(U·g−1 FW) |
|---|---|---|---|---|---|---|---|
| Allicin + Chitosan | 23.85 ± 0.16 a | 12.65 ± 0.08 a | 4.21 ± 0.10 a | 3.94 ± 0.06 a | 15.63 ± 0.47 a | 0.127 ± 0.006 a | 454.89 ± 2.05 a |
| Allicin | 22.78 ± 0.66 b | 12.18 ± 0.15 b | 3.92 ± 0.04 b | 3.62 ± 0.15 b | 14.87 ± 0.72 a | 0.119 ± 0.005 a | 444.45 ± 4.89 b |
| Chitosan | 22.56 ± 0.59 b | 12.12 ± 0.11 b | 3.87 ± 0.10 b | 3.53 ± 0.14 b | 14.59 ± 0.59 a | 0.117 ± 0.004 a | 441.12 ± 9.72 b |
| Polyoxin | 19.64 ± 0.52 c | 11.17 ± 0.13 c | 3.26 ± 0.03 c | 2.86 ± 0.09 c | 13.42 ± 0.61 b | 0.108 ± 0.008 b | 407.62 ± 5.04 c |
| Control | 17.88 ± 0.61 d | 10.35 ± 0.22 d | 3.14 ± 0.07 c | 2.51 ± 0.14 d | 12.65 ± 0.55 b | 0.096 ± 0.003 c | 376.95 ± 1.49 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, R.; Zhang, C.; Guo, Z.; Wu, X.; An, H. Co-Application of Allicin and Chitosan Increases Resistance of Rosa roxburghii against Powdery Mildew and Enhances Its Yield and Quality. Antibiotics 2021, 10, 1449. https://doi.org/10.3390/antibiotics10121449
Li J, Li R, Zhang C, Guo Z, Wu X, An H. Co-Application of Allicin and Chitosan Increases Resistance of Rosa roxburghii against Powdery Mildew and Enhances Its Yield and Quality. Antibiotics. 2021; 10(12):1449. https://doi.org/10.3390/antibiotics10121449
Chicago/Turabian StyleLi, Jiaohong, Rongyu Li, Cheng Zhang, Zhenxiang Guo, Xiaomao Wu, and Huaming An. 2021. "Co-Application of Allicin and Chitosan Increases Resistance of Rosa roxburghii against Powdery Mildew and Enhances Its Yield and Quality" Antibiotics 10, no. 12: 1449. https://doi.org/10.3390/antibiotics10121449
APA StyleLi, J., Li, R., Zhang, C., Guo, Z., Wu, X., & An, H. (2021). Co-Application of Allicin and Chitosan Increases Resistance of Rosa roxburghii against Powdery Mildew and Enhances Its Yield and Quality. Antibiotics, 10(12), 1449. https://doi.org/10.3390/antibiotics10121449





