Antibiotics- and Heavy Metals-Based Titanium Alloy Surface Modifications for Local Prosthetic Joint Infections
Abstract
:1. Introduction
2. Etiopathology
- (1)
- Attachment. Microorganisms come into contact with the surface, a process that is at least partly stochastic, driven by physical and chemical forces [25,26,27]. Furthermore, host proteins rapidly coat the surface of medical devices, facilitating specific adhesion mediated by microbial surface components recognizing adhesive matrix molecules (MSCRAMMs), which are part of the surface of many bacteria, e.g., Staphylococcus spp. [28,29].
- (2)
- Maturation is characterized by intercellular aggregation coupled to a variety of molecules such as proteins or, usually, exopolysaccharides of a polysaccharide nature, and structuring forces that rearrange the biofilm into three-dimensional structures of variable morphology depending on the species and with microchannels within them [28]. During this stage, one of the most important processes is the production of the exopolysaccharide matrix, whose composition is characteristic of each species, and even of each strain [28,29,30,31]. At this stage, the relatively simple structure that the pre-biofilm acquired in irreversible adhesion takes on a much more structurally complex three-dimensional organization [32]. The nutritional gradient inside the biofilm gives rise to a variety of cells with metabolic differences, including starved cells, dormant cells, viable non-cultivable cells, “persister” cells, and dead cells [27,33].
- (3)
- Dispersal. This is the process by which mature biofilm cells disperse to adjacent areas passively or actively [23,27]. Through this stage, the infection spreads to adjacent niches in an environment or within the host once nutrients or space has been depleted [32], where it attaches again and restarts the cycle.
3. Conventional Prevention of Prosthetic Joint Infections
4. Local Preventive Antibiotic-Based Strategies
4.1. Active Titanium Surfaces Loaded with Antibiotics
4.2. Coating Loaded with Antibiotic for Titanium Alloys
4.3. The Antibiotic of Choice for Local Antibiotic-Based Therapy
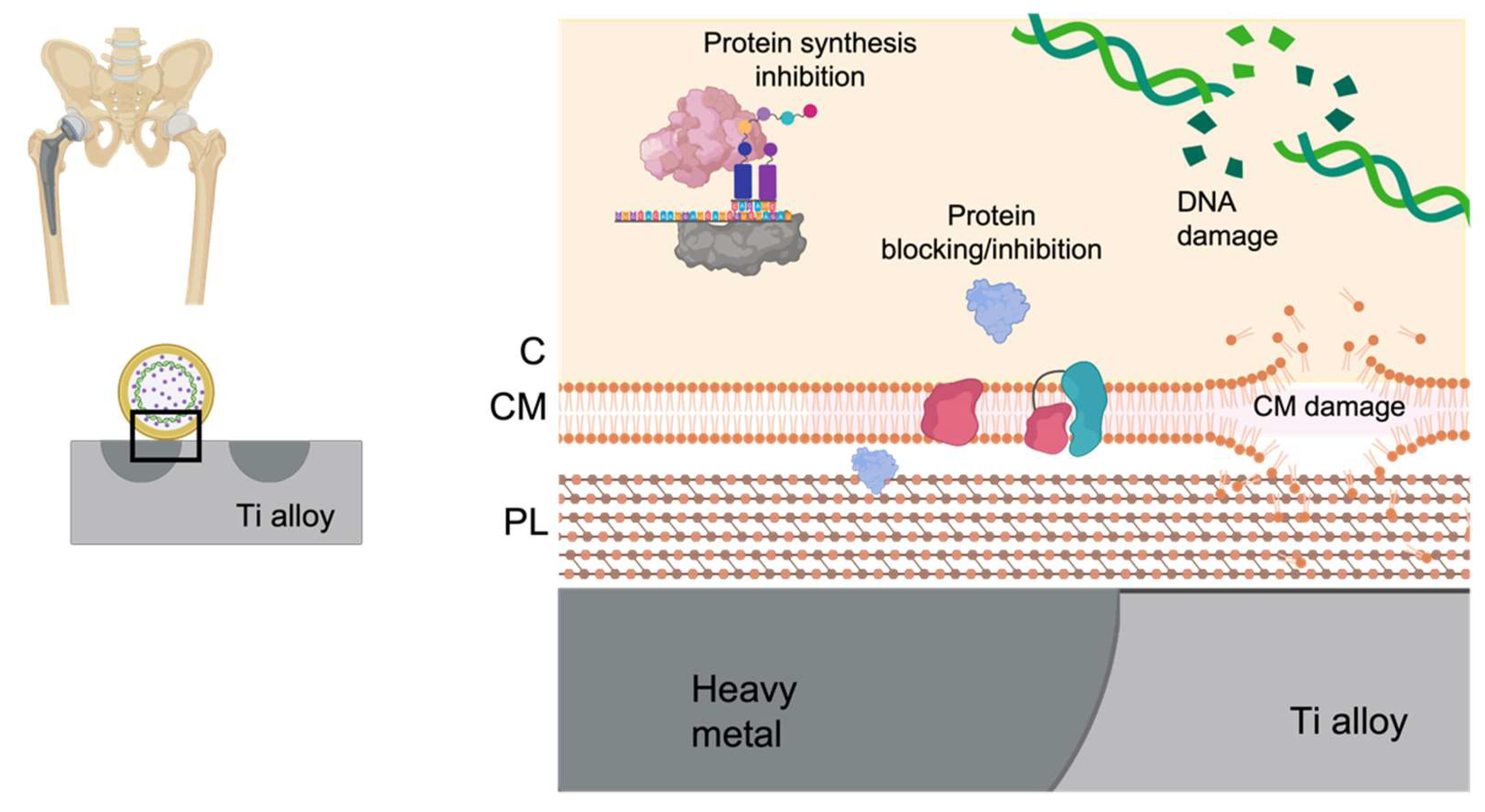
5. Local Preventive Heavy Metals-Based Strategies
6. Limitations Associated with Local PJI Prevention
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hawker, G.A.; Badley, E.M.; Borkhoff, C.M.; Croxford, R.; Davis, A.M.; Dunn, S.; Gignac, M.A.; Jaglal, S.B.; Kreder, H.J.; Sale, J.E.M. Which patients are most likely to benefit from total joint arthroplasty? Arthritis Rheum. 2013, 65, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Trampuz, A.; Zimmerli, W. Prosthetic joint infections: Update in diagnosis and treatment. Swiss Med. Wkly. 2005, 135, 243–251. [Google Scholar] [PubMed]
- Garrido-Gómez, J.; Arrabal-Polo, M.A.; Girón-Prieto, M.S.; Cabello-Salas, J.; Torres-Barroso, J.; Parra-Ruiz, J. Descriptive analysis of the economic costs of periprosthetic joint infection of the knee for the public health system of Andalusia. J. Arthroplasty 2013, 28, 1057–1060. [Google Scholar] [CrossRef] [PubMed]
- Dale, H.; Fenstad, A.M.; Hallan, G.; Havelin, L.I.; Furnes, O.; Overgaard, S.; Pedersen, A.B.; Kärrholm, J.; Garellick, G.; Pulkkinen, P.; et al. Increasing risk of prosthetic joint infection after total hip arthroplasty. Acta Orthop. 2012, 83, 449–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtz, S.M.; Lau, E.; Watson, H.; Schmier, J.K.; Parvizi, J. Economic burden of periprosthetic joint infection in the United States. J. Arthroplasty 2012, 27, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, B.P.; Weston, J.T.; Osmon, D.R.; Hanssen, A.D.; Berry, D.J.; Abdel, M.P. Prior hip or knee prosthetic joint infection in another joint increases risk three-fold of prosthetic joint infection after primary total knee arthroplasty: A matched control study. Bone Jt. J. 2019, 101, 91–97. [Google Scholar] [CrossRef]
- DeKeyser, G.J.; Anderson, M.B.; Meeks, H.D.; Pelt, C.E.; Peters, C.L.; Gililland, J.M. Socioeconomic status may not be a risk factor for periprosthetic joint infection. J. Arthroplasty 2020, 35, 1900–1905. [Google Scholar] [CrossRef]
- Benito, N.; Franco, M.; Ribera, A.; Soriano, A.; Rodriguez-Pardo, D.; Sorlí, L.; Fresco, G.; Fernández-Sampedro, M.; del Toro, D.M.; Guío, L.; et al. Time trends in the aetiology of prosthetic joint infections: A multicentre cohort study. Clin. Microbiol. Infect. 2016, 22, 732.e1–732.e8. [Google Scholar] [CrossRef] [Green Version]
- Benito, N.; Mur, I.; Ribera, A.; Soriano, A.; Rodríguez-Pardo, D.; Sorlí, L.; Cobo, J.; Fernández-Sampedro, M.; del Toro, M.D.; Guío, L.; et al. The different microbial etiology of prosthetic joint infections according to route of acquisition and time after prosthesis implantation, including the role of multidrug-resistant organisms. J. Clin. Med. 2019, 8, 673. [Google Scholar] [CrossRef] [Green Version]
- Villa, J.M.; Pannu, T.S.; Theeb, I.; Buttaro, M.A.; Oñativia, J.I.; Carbo, L.; Rienzi, D.H.; Fregeiro, J.I.; Kornilov, N.N.; Bozhkova, S.A.; et al. International organism profile of periprosthetic total hip and knee infections. J. Arthroplasty 2021, 36, 274–278. [Google Scholar] [CrossRef]
- Iqbal, F.; Shafiq, B.; Zamir, M.; Noor, S.; Memon, N.; Memon, N.; Dina, T.K. Micro-organisms and risk factors associated with prosthetic joint infection following primary total knee replacement-our experience in Pakistan. Int. Orthop. 2020, 44, 283–289. [Google Scholar] [CrossRef]
- Yu, Y.; Kong, Y.; Ye, J.; Wang, A.; Si, W. Microbiological pattern of prosthetic hip and knee infections: A high-volume, single-centre experience in China. J. Med. Microbiol. 2021, 70. [Google Scholar] [CrossRef]
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, Y.; Chang, C.-H.; Lin, Y.-C.; Lee, S.-H.; Hsieh, P.-H.; Chang, Y. Different microbiological profiles between hip and knee prosthetic joint infections. J. Orthop. Surg. Hong Kong 2019, 27. [Google Scholar] [CrossRef] [Green Version]
- Paxton, E.S.; Green, A.; Krueger, V.S. Periprosthetic infections of the shoulder: Diagnosis and management. J. Am. Acad. Orthop. Surg. 2019, 27, e935–e944. [Google Scholar] [CrossRef]
- Bloom, D.E.; Cadarette, D. Infectious disease threats in the twenty-first century: Strengthening the global response. Front. Immunol. 2019, 10, 549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention (U.S.). Antibiotic Resistance Threats in the United States, 2019; Centers for Disease Control and Prevention (U.S.): Atlanta, GA, USA, 2019.
- Tacconelli, E. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organ. 2017, 27, 318–327. [Google Scholar]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Diggle, S.P. Microbial communication and virulence: Lessons from evolutionary theory. Microbiology 2010, 156, 3503–3512. [Google Scholar] [CrossRef] [Green Version]
- Nadell, C.D.; Xavier, J.B.; Foster, K.R. The sociobiology of biofilms. FEMS Microbiol. Rev. 2009, 33, 206–224. [Google Scholar] [CrossRef] [Green Version]
- West, S.A.; Griffin, A.S.; Gardner, A.; Diggle, S.P. Social evolution theory for microorganisms. Nat. Rev. Microbiol. 2006, 4, 597–607. [Google Scholar] [CrossRef]
- Monroe, D. Looking for chinks in the armor of bacterial biofilms. PLoS Biol. 2007, 5, e307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Høiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.; Moser, C.; Jensen, P.Ø.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The clinical impact of bacterial biofilms. Int. J. Oral Sci. 2011, 3, 55–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef]
- Kostakioti, M.; Hadjifrangiskou, M.; Hultgren, S.J. Bacterial biofilms: Development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb. Perspect. Med. 2013, 3, a010306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcal Biofilms. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcus Epidermidis—The “accidental” pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef] [Green Version]
- Laverty, G.; Gorman, S.P.; Gilmore, B.F. Biomolecular mechanisms of Pseudomonas aeruginosa and Escherichia coli biofilm formation. Pathog. Basel Switz. 2014, 3, 596–632. [Google Scholar] [CrossRef] [Green Version]
- Büttner, H.; Mack, D.; Rohde, H. Structural basis of staphylococcus epidermidis biofilm formation: Mechanisms and molecular interactions. Front. Cell. Infect. Microbiol. 2015, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- McConoughey, S.J.; Howlin, R.; Granger, J.F.; Manring, M.M.; Calhoun, J.H.; Shirtliff, M.; Kathju, S.; Stoodley, P. Biofilms in periprosthetic orthopedic infections. Future Microbiol. 2014, 9, 987–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Valour, F.; Trouillet-Assant, S.; Rasigade, J.-P.; Lustig, S.; Chanard, E.; Meugnier, H.; Tigaud, S.; Vandenesch, F.; Etienne, J.; Ferry, T.; et al. Staphylococcus epidermidis in orthopedic device infections: The role of bacterial internalization in human osteoblasts and biofilm formation. PLoS ONE 2013, 8, e67240. [Google Scholar] [CrossRef] [Green Version]
- Ierano, C.; Stewardson, A.J.; Peel, T. Prosthetic Joint Infections; Peel, T., Ed.; Springer International Publishing: Berlin, Germany, 2018; ISBN 978-3-319-65249-8. [Google Scholar]
- Berríos-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017, 152, 784–791. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Guidelines for the Prevention of Surgical Site Infection; WHO: Geneva, Switzerland, 2016; ISBN 978-92-4-154988-2. [Google Scholar]
- Levy, D.M.; Wetters, N.G.; Levine, B.R. Prevention of periprosthetic joint infections of the hip and knee. Am. J. Orthop. Belle Mead NJ 2016, 45, E299–E307. [Google Scholar]
- Alamanda, V.K.; Springer, B.D. The prevention of infection. Bone Jt. J. 2019, 101, 3–9. [Google Scholar] [CrossRef]
- Berbari, E.; Segreti, J.; Parvizi, J.; Berríos-Torres, S.I. Future research opportunities in peri-prosthetic joint infection prevention. Surg. Infect. 2017, 18, 409–412. [Google Scholar] [CrossRef]
- Goswami, K.; Stevenson, K.L.; Parvizi, J. Intraoperative and postoperative infection prevention. J. Arthroplasty 2020, 35, S2–S8. [Google Scholar] [CrossRef] [Green Version]
- General Assembly. Available online: https://icmphilly.com/general-assembly/ (accessed on 26 April 2021).
- Iannotti, F.; Prati, P.; Fidanza, A.; Iorio, R.; Ferretti, A.; Pèrez Prieto, D.; Kort, N.; Violante, B.; Pipino, G.; Schiavone Panni, A.; et al. Prevention of Periprosthetic joint infection (PJI): A clinical practice protocol in high-risk patients. Trop. Med. Infect. Dis. 2020, 5, 186. [Google Scholar] [CrossRef]
- Jämsen, E.; Furnes, O.; Engesaeter, L.B.; Konttinen, Y.T.; Odgaard, A.; Stefánsdóttir, A.; Lidgren, L. Prevention of deep infection in joint replacement surgery. Acta Orthop. 2010, 81, 660–666. [Google Scholar] [CrossRef]
- Siddiqi, A.; Forte, S.A.; Docter, S.; Bryant, D.; Sheth, N.P.; Chen, A.F. Perioperative antibiotic prophylaxis in total joint arthroplasty: A systematic review and meta-analysis. J. Bone Jt. Surg. Am. 2019, 101, 828–842. [Google Scholar] [CrossRef]
- Del Pozo, J.L.; Patel, R. Clinical practice. Infection associated with prosthetic joints. N. Engl. J. Med. 2009, 361, 787–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fillingham, Y.; Greenwald, A.S.; Greiner, J.; Oshkukov, S.; Parsa, A.; Porteous, A.; Squire, M.W. Hip and knee section, prevention, local antimicrobials: Proceedings of international consensus on orthopedic infections. J. Arthroplasty 2019, 34, S289–S292. [Google Scholar] [CrossRef]
- Baeza, J.; Cury, M.B.; Fleischman, A.; Ferrando, A.; Fuertes, M.; Goswami, K.; Lidgren, L.; Linke, P.; Manrique, J.; Makar, G.; et al. General assembly, prevention, local antimicrobials: Proceedings of international consensus on orthopedic infections. J. Arthroplasty 2019, 34, S75–S84. [Google Scholar] [CrossRef]
- Schiavone Panni, A.; Corona, K.; Giulianelli, M.; Mazzitelli, G.; del Regno, C.; Vasso, M. Antibiotic-loaded bone cement reduces risk of infections in primary total knee arthroplasty? A systematic review. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2016, 24, 3168–3174. [Google Scholar] [CrossRef]
- Schmitt, D.R.; Killen, C.; Murphy, M.; Perry, M.; Romano, J.; Brown, N. The impact of antibiotic-loaded bone cement on antibiotic resistance in periprosthetic knee infections. Clin. Orthop. Surg. 2020, 12, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Wall, V.; Nguyen, T.-H.; Nguyen, N.; Tran, P.A. Controlling antibiotic release from polymethylmethacrylate bone cement. Biomedicines 2021, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- García-Gareta, E.; Davidson, C.; Levin, A.; Coathup, M.J.; Blunn, G.W. Biofilm formation in total hip arthroplasty: Prevention and treatment. RSC Adv. 2016, 6, 80244–80261. [Google Scholar] [CrossRef] [Green Version]
- Rakow, A.; Perka, C.; Trampuz, A.; Renz, N. Origin and Characteristics of Haematogenous Periprosthetic Joint Infection. Clinical Microbiology and Infection 2019, 25, 845–850. [Google Scholar] [CrossRef]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-joint infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Speziale, P.; Arciola, C.R. Antibiotic-loaded biomaterials and the risks for the spread of antibiotic resistance following their prophylactic and therapeutic clinical use. Biomaterials 2010, 31, 6363–6377. [Google Scholar] [CrossRef]
- Romanò, C.L.; Petrosillo, N.; Argento, G.; Sconfienza, L.M.; Treglia, G.; Alavi, A.; Glaudemans, A.W.J.M.; Gheysens, O.; Maes, A.; Lauri, C.; et al. The role of imaging techniques to define a peri-prosthetic hip and knee joint infection: Multidisciplinary consensus statements. J. Clin. Med. 2020, 9, 2548. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Y.; Li, R.; Tang, X.; Guo, D.; Qing, Y.; Qin, Y. Enhanced antibacterial properties of orthopedic implants by titanium nanotube surface modification: A review of current techniques. Int. J. Nanomed. 2019, 14, 7217–7236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Mo, A. A review on the electrochemically self-organized titania nanotube arrays: Synthesis, modifications, and biomedical applications. Nanoscale Res. Lett. 2018, 13, 187. [Google Scholar] [CrossRef]
- López Zavala, M.Á.; Lozano Morales, S.A.; Ávila-Santos, M. Synthesis of stable TiO2 nanotubes: Effect of hydrothermal treatment, acid washing and annealing temperature. Heliyon 2017, 3, e00456. [Google Scholar] [CrossRef]
- Xie, Y. Photoelectrochemical application of nanotubular titania photoanode. Electrochim. Acta 2006, 51, 3399–3406. [Google Scholar] [CrossRef]
- Cheng, Y.; Feng, G.; Moraru, C.I. Micro- and nanotopography sensitive bacterial attachment mechanisms: A review. Front. Microbiol. 2019, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Puckett, S.D.; Taylor, E.; Raimondo, T.; Webster, T.J. The relationship between the nanostructure of titanium surfaces and bacterial attachment. Biomaterials 2010, 31, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Bartlet, K.; Movafaghi, S.; Dasi, L.P.; Kota, A.K.; Popat, K.C. Antibacterial activity on superhydrophobic titania nanotube arrays. Colloids Surf. B Biointerfaces 2018, 166, 179–186. [Google Scholar] [CrossRef]
- Arenas, M.A.; Pérez-Jorge, C.; Conde, A.; Matykina, E.; Hernández-López, J.M.; Pérez-Tanoira, R.; de Damborenea, J.J.; Gómez-Barrena, E.; Esteba, J. Doped TiO2 anodic layers of enhanced antibacterial properties. Colloids Surf. B Biointerfaces 2013, 105, 106–112. [Google Scholar] [CrossRef]
- Aguilera-Correa, J.-J.; Mediero, A.; Conesa-Buendía, F.-M.; Conde, A.; Arenas, M.-Á.; de-Damborenea, J.-J.; Esteban, J. Microbiological and cellular evaluation of a fluorine-phosphorus-doped titanium alloy, a novel antibacterial and osteostimulatory biomaterial with potential applications in orthopedic surgery. Appl. Environ. Microbiol. 2018, 85, e02271-18. [Google Scholar] [CrossRef] [Green Version]
- Perez-Jorge, C.; Arenas, M.-A.; Conde, A.; Hernández-Lopez, J.-M.; de Damborenea, J.-J.; Fisher, S.; Hunt, A.M.A.; Esteban, J.; James, G. Bacterial and fungal biofilm formation on anodized titanium alloys with fluorine. J. Mater. Sci. Mater. Med. 2017, 28, 8. [Google Scholar] [CrossRef]
- Lozano, D.; Hernández-López, J.M.; Esbrit, P.; Arenas, M.A.; Gómez-Barrena, E.; de Damborenea, J.; Esteban, J.; Pérez-Jorge, C.; Pérez-Tanoira, R.; Conde, A. Influence of the nanostructure of F-doped TiO2 films on osteoblast growth and function: Influence of the nanostructure of F-doped TiO2 films. J. Biomed. Mater. Res. A 2015, 103, 1985–1990. [Google Scholar] [CrossRef]
- Aguilera-Correa, J.-J.; Auñón, Á.; Boiza-Sánchez, M.; Mahillo-Fernández, I.; Mediero, A.; Eguibar-Blázquez, D.; Conde, A.; Arenas, M.-Á.; de-Damborenea, J.-J.; Cordero-Ampuero, J.; et al. Urine aluminum concentration as a possible implant biomarker of Pseudomonas aeruginosa Infection using a fluorine- and phosphorus-doped Ti-6Al-4V alloy with osseointegration capacity. ACS Omega 2019, 4, 11815–11823. [Google Scholar] [CrossRef] [Green Version]
- Ercan, B.; Taylor, E.; Alpaslan, E.; Webster, T.J. Diameter of titanium nanotubes influences anti-bacterial efficacy. Nanotechnology 2011, 22, 295102. [Google Scholar] [CrossRef]
- Lin, W.; Tan, H.; Duan, Z.; Yue, B.; Ma, R.; He, G.; Tang, T. Inhibited bacterial biofilm formation and improved osteogenic activity on gentamicin-loaded titania nanotubes with various diameters. Int. J. Nanomed. 2014, 9, 1215–1230. [Google Scholar] [CrossRef] [Green Version]
- Feng, W.; Geng, Z.; Li, Z.; Cui, Z.; Zhu, S.; Liang, Y.; Liu, Y.; Wang, R.; Yang, X. Controlled release behaviour and antibacterial effects of antibiotic-loaded titania nanotubes. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 105–112. [Google Scholar] [CrossRef]
- Liu, D.; He, C.; Liu, Z.; Xu, W. Gentamicin coating of nanotubular anodized titanium implant reduces implant-related osteomyelitis and enhances bone biocompatibility in rabbits. Int. J. Nanomed. 2017, 12, 5461–5471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Z.; Huang, S.; Lan, S.; Xiong, H.; Tao, B.; Ding, Y.; Liu, Y.; Liu, P.; Cai, K. Surface Engineering of titanium implants with enzyme-triggered antibacterial properties and enhanced osseointegration in vivo. J. Mater. Chem. B 2018, 6, 8090–8104. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, G.; Liu, P.; Tong, D.; Ding, C.; Zhang, Z.; Xie, Y.; Tang, H.; Ji, F. Vancomycin-loaded titanium coatings with an interconnected micro-patterned structure for prophylaxis of infections: An in vivo study. RSC Adv. 2018, 8, 9223–9231. [Google Scholar] [CrossRef] [Green Version]
- Auñón, Á.; Esteban, J.; Doadrio, A.L.; Boiza-Sánchez, M.; Mediero, A.; Eguibar-Blázquez, D.; Cordero-Ampuero, J.; Conde, A.; Arenas, M.; de-Damborenea, J.; et al. Staphylococcus aureus prosthetic joint infection is prevented by a fluorine- and phosphorus-doped nanostructured Ti–6Al–4V alloy loaded with gentamicin and vancomycin. J. Orthop. Res. 2020, 38, 588–597. [Google Scholar] [CrossRef]
- Aguilera-Correa, J.-J.; Doadrio, A.L.; Conde, A.; Arenas, M.-A.; de-Damborenea, J.-J.; Vallet-Regí, M.; Esteban, J. Antibiotic release from F-doped nanotubular oxide layer on TI6AL4V alloy to decrease bacterial viability. J. Mater. Sci. Mater. Med. 2018, 29, 118. [Google Scholar] [CrossRef]
- Xu, Q.A.; Trissel, L.A.; Saenz, C.A.; Ingram, D.S. Stability of gentamicin sulfate and tobramycin sulfate in autodose infusion system bags. Int. J. Pharm. Compd. 2002, 6, 152–154. [Google Scholar]
- Mullins, N.D.; Deadman, B.J.; Moynihan, H.A.; McCarthy, F.O.; Lawrence, S.E.; Thompson, J.; Maguire, A.R. The impact of storage conditions upon gentamicin coated antimicrobial implants. J. Pharm. Anal. 2016, 6, 374–381. [Google Scholar] [CrossRef] [Green Version]
- Melichercik, P.; Klapkova, E.; Landor, I.; Judl, T.; Sibek, M.; Jahoda, D. The effect of vancomycin degradation products in the topical treatment of osteomyelitis. Bratisl. Lek. Listy 2014, 115, 796–799. [Google Scholar] [CrossRef] [Green Version]
- Mousset, B.; Benoit, M.A.; Delloye, C.; Bouillet, R.; Gillard, J. Biodegradable implants for potential use in bone infection. An in vitro study of antibiotic-loaded calcium sulphate. Int. Orthop. 1995, 19, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Chen, C.-W.; Hsu, C.-Y.; Lai, S.-M.; Syu, W.-J.; Wang, T.-Y.; Lai, P.-S. Metal nanobullets for multidrug resistant bacteria and biofilms. Adv. Drug Deliv. Rev. 2014, 78, 88–104. [Google Scholar] [CrossRef]
- Badar, M.; Rahim, M.I.; Kieke, M.; Ebel, T.; Rohde, M.; Hauser, H.; Behrens, P.; Mueller, P.P. Controlled drug release from antibiotic-loaded layered double hydroxide coatings on porous titanium implants in a mouse model: Antibiotic-loaded layered double hydroxide coatinGS. J. Biomed. Mater. Res. A 2015, 103, 2141–2149. [Google Scholar] [CrossRef]
- Walter, M.S.; Frank, M.J.; Satué, M.; Monjo, M.; Rønold, H.J.; Lyngstadaas, S.P.; Haugen, H.J. Bioactive implant surface with electrochemically bound doxycycline promotes bone formation markers in vitro and in vivo. Dent. Mater. 2014, 30, 200–214. [Google Scholar] [CrossRef]
- Kucharíková, S.; Gerits, E.; de Brucker, K.; Braem, A.; Ceh, K.; Majdič, G.; Španič, T.; Pogorevc, E.; Verstraeten, N.; Tournu, H.; et al. Covalent immobilization of antimicrobial agents on titanium prevents Staphylococcus aureus and Candida albicans colonization and biofilm formation. J. Antimicrob. Chemother. 2016, 71, 936–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, B.; Long, T.; Ao, H.; Zhou, J.; Tang, T.; Yue, B. Covalent immobilization of enoxacin onto titanium implant surfaces for inhibiting multiple bacterial species infection and In Vivo methicillin-resistant staphylococcus aureus infection prophylaxis. Antimicrob. Agents Chemother. 2017, 61, e01766-16. [Google Scholar] [CrossRef] [Green Version]
- Nie, B.; Ao, H.; Long, T.; Zhou, J.; Tang, T.; Yue, B. Immobilizing bacitracin on titanium for prophylaxis of infections and for improving osteoinductivity: An in vivo study. Colloids Surf. B Biointerfaces 2017, 150, 183–191. [Google Scholar] [CrossRef]
- Gerits, E.; Kucharíková, S.; van Dijck, P.; Erdtmann, M.; Krona, A.; Lövenklev, M.; Fröhlich, M.; Dovgan, B.; Impellizzeri, F.; Braem, A.; et al. Antibacterial activity of a new broad-spectrum antibiotic covalently bound to titanium surfaces. J. Orthop. Res. 2016, 34, 2191–2198. [Google Scholar] [CrossRef]
- Chen, C.-P.; Wickstrom, E. Self-protecting bactericidal titanium alloy surface formed by covalent bonding of daptomycin bisphosphonates. Bioconjug. Chem. 2010, 21, 1978–1986. [Google Scholar] [CrossRef] [Green Version]
- Lawson, M.C.; Hoth, K.C.; Deforest, C.A.; Bowman, C.N.; Anseth, K.S. Inhibition of Staphylococcus epidermidis biofilms using polymerizable vancomycin derivatives. Clin. Orthop. 2010, 468, 2081–2091. [Google Scholar] [CrossRef] [Green Version]
- Mohorcič, M.; Jerman, I.; Zorko, M.; Butinar, L.; Orel, B.; Jerala, R.; Friedrich, J. Surface with antimicrobial activity obtained through silane coating with covalently bound polymyxin B. J. Mater. Sci. Mater. Med. 2010, 21, 2775–2782. [Google Scholar] [CrossRef]
- Moskowitz, J.S.; Blaisse, M.R.; Samuel, R.E.; Hsu, H.-P.; Harris, M.B.; Martin, S.D.; Lee, J.C.; Spector, M.; Hammond, P.T. The effectiveness of the controlled release of gentamicin from polyelectrolyte multilayers in the treatment of Staphylococcus aureus infection in a rabbit bone model. Biomaterials 2010, 31, 6019–6030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alt, V.; Kirchhof, K.; Seim, F.; Hrubesch, I.; Lips, K.S.; Mannel, H.; Domann, E.; Schnettler, R. Rifampicin–fosfomycin coating for cementless endoprostheses: Antimicrobial effects against methicillin-sensitive Staphylococcus aureus (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA). Acta Biomater. 2014, 10, 4518–4524. [Google Scholar] [CrossRef] [PubMed]
- Mattioli-Belmonte, M.; Cometa, S.; Ferretti, C.; Iatta, R.; Trapani, A.; Ceci, E.; Falconi, M.; de Giglio, E. Characterization and cytocompatibility of an antibiotic/chitosan/cyclodextrins nanocoating on titanium implants. Carbohydr. Polym. 2014, 110, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Ordikhani, F.; Tamjid, E.; Simchi, A. Characterization and antibacterial performance of electrodeposited chitosan–vancomycin composite coatings for prevention of implant-associated infections. Mater. Sci. Eng. C 2014, 41, 240–248. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, J.; Yin, Z.; Tang, C.; Guo, Y.; Li, D.; Wei, B.; Gu, Q.; Xu, Y.; Wang, L. Electrospun vancomycin-loaded coating on titanium implants for the prevention of implant-associated infections. Int. J. Nanomed. 2014, 3027. [Google Scholar] [CrossRef] [Green Version]
- Metsemakers, W.-J.; Emanuel, N.; Cohen, O.; Reichart, M.; Potapova, I.; Schmid, T.; Segal, D.; Riool, M.; Kwakman, P.H.S.; de Boer, L.; et al. A doxycycline-loaded polymer-lipid encapsulation matrix coating for the prevention of implant-related osteomyelitis due to doxycycline-resistant methicillin-resistant Staphylococcus Aureus. J. Control. Release 2015, 209, 47–56. [Google Scholar] [CrossRef]
- Neut, D.; Dijkstra, R.; Thompson, J.; Kavanagh, C.; van der Mei, H.; Busscher, H. A biodegradable gentamicin-hydroxyapatite-coating for infection prophylaxis in cementless hip prostheses. Eur. Cell. Mater. 2015, 29, 42–56. [Google Scholar] [CrossRef]
- Diefenbeck, M.; Schrader, C.; Gras, F.; Mückley, T.; Schmidt, J.; Zankovych, S.; Bossert, J.; Jandt, K.D.; Völpel, A.; Sigusch, B.W.; et al. Gentamicin coating of plasma chemical oxidized titanium alloy prevents implant-related osteomyelitis in rats. Biomaterials 2016, 101, 156–164. [Google Scholar] [CrossRef]
- Jennings, J.A.; Beenken, K.E.; Skinner, R.A.; Meeker, D.G.; Smeltzer, M.S.; Haggard, W.O.; Troxel, K.S. Antibiotic-loaded phosphatidylcholine inhibits staphylococcal bone infection. World J. Orthop. 2016, 7, 467. [Google Scholar] [CrossRef]
- Ma, K.; Cai, X.; Zhou, Y.; Wang, Y.; Jiang, T. In vitro and in vivo evaluation of tetracycline loaded chitosan-gelatin nanosphere coatings for titanium surface functionalization. Macromol. Biosci. 2017, 17, 1600130. [Google Scholar] [CrossRef]
- Song, W.; Seta, J.; Chen, L.; Bergum, C.; Zhou, Z.; Kanneganti, P.; Kast, R.E.; Auner, G.W.; Shen, M.; Markel, D.C.; et al. Doxycycline-loaded coaxial nanofiber coating of titanium implants enhances osseointegration and inhibits Staphylococcus Aureus infection. Biomed. Mater. 2017, 12, 045008. [Google Scholar] [CrossRef]
- Cheng, T.; Qu, H.; Zhang, G.; Zhang, X. Osteogenic and antibacterial properties of vancomycin-laden mesoporous bioglass/PLGA composite scaffolds for bone regeneration in infected bone defects. Artif. Cells Nanomed. Biotechnol. 2017, 46, 1935–1947. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Weng, W.; Chen, B.; Feng, W.; Wang, W.; Nie, W.; Chen, L.; Mo, X.; Su, J.; He, C. Mesoporous silica nanoparticles/gelatin porous composite scaffolds with localized and sustained release of vancomycin for treatment of infected bone defects. J. Mater. Chem. B 2018, 6, 740–752. [Google Scholar] [CrossRef]
- Grohmann, S.; Menne, M.; Hesse, D.; Bischoff, S.; Schiffner, R.; Diefenbeck, M.; Liefeith, K. Biomimetic multilayer coatings deliver gentamicin and reduce implant-related osteomyelitis in rats. Biomed. Eng. Biomed. Tech. 2019, 64, 383–395. [Google Scholar] [CrossRef]
- Janson, O.; Sörensen, J.H.; Strømme, M.; Engqvist, H.; Procter, P.; Welch, K. Evaluation of an alkali-treated and hydroxyapatite-coated orthopedic implant loaded with tobramycin. J. Biomater. Appl. 2019, 34, 699–720. [Google Scholar] [CrossRef] [PubMed]
- Stavrakis, A.I.; Zhu, S.; Loftin, A.H.; Weixian, X.; Niska, J.; Hegde, V.; Segura, T.; Bernthal, N.M. Controlled release of vancomycin and tigecycline from an orthopaedic implant coating prevents Staphylococcus Aureus infection in an open fracture animal model. BioMed Res. Int. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Thompson, K.; Petkov, S.; Zeiter, S.; Sprecher, C.M.; Richards, R.G.; Moriarty, T.F.; Eijer, H. Intraoperative loading of calcium phosphate-coated implants with gentamicin prevents experimental Staphylococcus Aureus Infection in Vivo. PLoS ONE 2019, 14, e0210402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Braun, B.M.; Skelly, J.D.; Ayers, D.C.; Song, J. Significant suppression of Staphylococcus aureus colonization on intramedullary Ti6Al4V implants surface-grafted with vancomycin-bearing polymer brushes. ACS Appl. Mater. Interfaces 2019, 11, 28641–28647. [Google Scholar] [CrossRef]
- Paris, J.L.; Vallet-Regí, M. Mesoporous silica nanoparticles for co-delivery of drugs and nucleic acids in oncology: A review. Pharmaceutics 2020, 12, 526. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Correa, J.J.; Garcia-Casas, A.; Mediero, A.; Romera, D.; Mulero, F.; Cuevas-López, I.; Jiménez-Morales, A.; Esteban, J. A new antibiotic-loaded sol-gel can prevent bacterial prosthetic joint infection: From in vitro studies to an in vivo model. Front. Microbiol. 2019, 10, 2935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramalhete, R.; Brown, R.; Blunn, G.; Skinner, J.; Coathup, M.; Graney, I.; Sanghani-Kerai, A. A novel antimicrobial coating to prevent periprosthetic joint infection. Bone Jt. Res. 2020, 9, 848–856. [Google Scholar] [CrossRef]
- Romera, D.; Toirac, B.; Aguilera-Correa, J.-J.; García-Casas, A.; Mediero, A.; Jiménez-Morales, A.; Esteban, J. A biodegradable antifungal-loaded sol–gel coating for the prevention and local treatment of yeast prosthetic-joint infections. Materials 2020, 13, 3144. [Google Scholar] [CrossRef]
- Garlito-Díaz, H.; Esteban, J.; Mediero, A.; Carias-Cálix, R.A.; Toirac, B.; Mulero, F.; Faus-Rodrigo, V.; Jiménez-Morales, A.; Calvo, E.; Aguilera-Correa, J.J. A new antifungal-loaded sol-gel can prevent candida albicans prosthetic joint infection. Antibiotics 2021, 10, 711. [Google Scholar] [CrossRef]
- Ujcic, A.; Krejcikova, S.; Nevoralova, M.; Zhigunov, A.; Dybal, J.; Krulis, Z.; Fulin, P.; Nyc, O.; Slouf, M. Thermoplastic starch composites with titanium dioxide and vancomycin antibiotic: Preparation, morphology, thermomechanical properties, and antimicrobial susceptibility testing. Front. Mater. 2020, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Souza, J.G.S.; Bertolini, M.M.; Costa, R.C.; Nagay, B.E.; Dongari-Bagtzoglou, A.; Barão, V.A.R. Targeting implant-associated infections: Titanium surface loaded with antimicrobial. iScience 2021, 24, 102008. [Google Scholar] [CrossRef]
- Aguilera-Correa, J.J.; Vidal-Laso, R.; Carias-Cálix, R.A.; Toirac, B.; García-Casas, A.; Velasco-Rodríguez, D.; Llamas-Sillero, P.; Jiménez-Morales, A.; Esteban, J. A new antibiotic-loaded sol-gel can prevent bacterial intravenous catheter-related infections. Materials 2020, 13, 2946. [Google Scholar] [CrossRef]
- da Silva, A.C.; Córdoba de Torresi, S.I. Advances in conducting, biodegradable and biocompatible copolymers for biomedical applications. Front. Mater. 2019, 6, 98. [Google Scholar] [CrossRef] [Green Version]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and safety of PLA and its copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef]
- Garcia-Casas, A.; Aguilera-Correa, J.J.; Mediero, A.; Esteban, J.; Jimenez-Morales, A. Functionalization of sol-gel coatings with organophosphorus compounds for prosthetic devices. Colloids Surf. B Biointerfaces 2019, 181, 973–980. [Google Scholar] [CrossRef]
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020, 148, 104501. [Google Scholar] [CrossRef]
- Paris, J.L.; Lafuente-Gómez, N.; Cabañas, M.V.; Román, J.; Peña, J.; Vallet-Regí, M. Fabrication of a nanoparticle-containing 3D porous bone scaffold with proangiogenic and antibacterial properties. Acta Biomater. 2019, 86, 441–449. [Google Scholar] [CrossRef]
- Aebli, N.; Krebs, J.; Schwenke, D.; Stich, H.; Schawalder, P.; Theis, J.C. Degradation of hydroxyapatite coating on a well-functioning femoral component. J. Bone Jt. Surg. Br. 2003, 85, 499–503. [Google Scholar] [CrossRef] [Green Version]
- Schmidmaier, G.; Wildemann, B.; Stemberger, A.; Haas, N.P.; Raschke, M. Biodegradable poly(D,L-lactide) coating of implants for continuous release of growth factors. J. Biomed. Mater. Res. 2001, 58, 449–455. [Google Scholar] [CrossRef]
- Fuchs, T.; Stange, R.; Schmidmaier, G.; Raschke, M.J. The use of gentamicin-coated nails in the tibia: Preliminary results of a prospective study. Arch. Orthop. Trauma Surg. 2011, 131, 1419–1425. [Google Scholar] [CrossRef] [Green Version]
- Schmidmaier, G.; Kerstan, M.; Schwabe, P.; Südkamp, N.; Raschke, M. Clinical experiences in the use of a gentamicin-coated titanium nail in tibia fractures. Injury 2017, 48, 2235–2241. [Google Scholar] [CrossRef]
- Drago, L.; Boot, W.; Dimas, K.; Malizos, K.; Hänsch, G.M.; Stuyck, J.; Gawlitta, D.; Romanò, C.L. Does implant coating with antibacterial-loaded hydrogel reduce bacterial colonization and biofilm formation in vitro? Clin. Orthop. 2014, 472, 3311–3323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romanò, C.L.; de Vecchi, E.; Bortolin, M.; Morelli, I.; Drago, L. Hyaluronic acid and its composites as a local antimicrobial/antiadhesive barrier. J. Bone Jt. Infect. 2017, 2, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Ardizzoni, A.; Neglia, R.G.; Baschieri, M.C.; Cermelli, C.; Caratozzolo, M.; Righi, E.; Palmieri, B.; Blasi, E. Influence of hyaluronic acid on bacterial and fungal species, including clinically relevant opportunistic pathogens. J. Mater. Sci. Mater. Med. 2011, 22, 2329–2338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giavaresi, G.; Meani, E.; Sartori, M.; Ferrari, A.; Bellini, D.; Sacchetta, A.C.; Meraner, J.; Sambri, A.; Vocale, C.; Sambri, V.; et al. Efficacy of antibacterial-loaded coating in an in vivo model of acutely highly contaminated implant. Int. Orthop. 2014, 38, 1505–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boot, W.; Vogely, H.C.; Jiao, C.; Nikkels, P.G.; Pouran, B.; van Rijen, M.H.; Ekkelenkamp, M.B.; Hänsch, G.M.; Dhert, W.J.; Gawlitta, D. Prophylaxis of implant-related infections by local release of vancomycin from a hydrogel in rabbits. Eur. Cell. Mater. 2020, 39, 108–120. [Google Scholar] [CrossRef]
- Boot, W.; Gawlitta, D.; Nikkels, P.G.J.; Pouran, B.; van Rijen, M.H.P.; Dhert, W.J.A.; Vogely, H.C. Hyaluronic acid-based hydrogel coating does not affect bone apposition at the implant surface in a rabbit model. Clin. Orthop. 2017, 475, 1911–1919. [Google Scholar] [CrossRef] [Green Version]
- Romanò, C.L.; Malizos, K.; Capuano, N.; Mezzoprete, R.; D’Arienzo, M.; van der Straeten, C.; Scarponi, S.; Drago, L. Does an antibiotic-loaded hydrogel coating reduce early post-surgical infection after joint arthroplasty? J. Bone Jt. Infect. 2016, 1, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Malizos, K.; Blauth, M.; Danita, A.; Capuano, N.; Mezzoprete, R.; Logoluso, N.; Drago, L.; Romanò, C.L. Fast-resorbable antibiotic-loaded hydrogel coating to reduce post-surgical infection after internal osteosynthesis: A multicenter randomized controlled trial. J. Orthop. Traumatol. Off. J. Ital. Soc. Orthop. Traumatol. 2017, 18, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Trentinaglia, M.T.; van der Straeten, C.; Morelli, I.; Logoluso, N.; Drago, L.; Romanò, C.L. Economic evaluation of antibacterial coatings on healthcare costs in first year following total joint arthroplasty. J. Arthroplasty 2018, 33, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Romanò, C.L.; Tsuchiya, H.; Morelli, I.; Battaglia, A.G.; Drago, L. Antibacterial coating of implants: Are we missing something? Bone Jt. Res. 2019, 8, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Michael, C.A.; Dominey-Howes, D.; Labbate, M. The antimicrobial resistance crisis: Causes, consequences, and management. Front. Public Health 2014, 2, 145. [Google Scholar] [CrossRef] [PubMed]
- Raymond, B. Five rules for resistance management in the antibiotic apocalypse, a road map for integrated microbial management. Evol. Appl. 2019, 12, 1079–1091. [Google Scholar] [CrossRef]
- Bell, G.; MacLean, C. The search for ‘evolution-proof’ antibiotics. Trends Microbiol. 2018, 26, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Shanks, R.M.Q.; Davis, C.M.; Craft, D.W.; Wood, T.K.; Hamlin, B.R.; Urish, K.L. Viable bacteria persist on antibiotic spacers following two-stage revision for periprosthetic joint infection. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2018, 36, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmolders, J.; Hischebeth, G.T.; Friedrich, M.J.; Randau, T.M.; Wimmer, M.D.; Kohlhof, H.; Molitor, E.; Gravius, S. Evidence of MRSE on a gentamicin and vancomycin impregnated polymethyl-methacrylate (PMMA) bone cement spacer after two-stage exchange arthroplasty due to periprosthetic joint infection of the knee. BMC Infect. Dis. 2014, 14, 144. [Google Scholar] [CrossRef]
- Aguilera-Correa, J.-J.; Conde, A.; Arenas, M.-A.; de-Damborenea, J.-J.; Marin, M.; Doadrio, A.L.; Esteban, J. Bactericidal activity of the Ti–13Nb–13Zr alloy against different species of bacteria related with implant infection. Biomed. Mater. 2017, 12, 045022. [Google Scholar] [CrossRef]
- da Silva, R.B.; Salles, M.J. Outcomes and risk factors in prosthetic joint infections by multidrug-resistant gram-negative bacteria: A retrospective cohort study. Antibiotics 2021, 10, 340. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.W. History of the medical use of silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djurišić, A.B.; Leung, Y.H.; Ng, A.M.C.; Xu, X.Y.; Lee, P.K.H.; Degger, N.; Wu, R.S.S. Toxicity of metal oxide nanoparticles: Mechanisms, characterization, and avoiding experimental artefacts. Small 2015, 11, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Tsuchiya, H.; Shimizu, T.; Ohtani, K.; Zen, Y.; Tomita, K. Prevention of Pin tract infection with titanium-copper alloys. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91B, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Tanoira, R.; Pérez-Jorge, C.; Endrino, J.L.; Gómez-Barrena, E.; Horwat, D.; Pierson, J.F.; Esteban, J. Antibacterial properties of biomedical surfaces containing micrometric silver islands. J. Phys. Conf. Ser. 2010, 252, 012015. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X.; Meng, F.; Chu, P.K. Biological actions of silver nanoparticles embedded in titanium controlled by micro-galvanic effects. Biomaterials 2011, 32, 693–705. [Google Scholar] [CrossRef]
- Secinti, K.D.; Özalp, H.; Attar, A.; Sargon, M.F. Nanoparticle silver ion coatings inhibit biofilm formation on titanium implants. J. Clin. Neurosci. 2011, 18, 391–395. [Google Scholar] [CrossRef]
- Ionita, D.; Grecu, M.; Ungureanu, C.; Demetrescu, I. Antimicrobial activity of the surface coatings on TiAlZr implant biomaterial. J. Biosci. Bioeng. 2011, 112, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Li, F.; Wang, H.; Liu, J.; Wang, C.; Li, M.; Yang, K. A new antibacterial titanium–copper sintered alloy: Preparation and antibacterial property. Mater. Sci. Eng. C 2013, 33, 4280–4287. [Google Scholar] [CrossRef]
- Zhao, C.; Feng, B.; Li, Y.; Tan, J.; Lu, X.; Weng, J. Preparation and antibacterial activity of titanium nanotubes loaded with Ag nanoparticles in the dark and under the UV light. Appl. Surf. Sci. 2013, 280, 8–14. [Google Scholar] [CrossRef]
- Saidin, S.; Chevallier, P.; Abdul Kadir, M.R.; Hermawan, H.; Mantovani, D. Polydopamine as an intermediate layer for silver and hydroxyapatite immobilisation on metallic biomaterials surface. Mater. Sci. Eng. C 2013, 33, 4715–4724. [Google Scholar] [CrossRef] [PubMed]
- De Giglio, E.; Cafagna, D.; Cometa, S.; Allegretta, A.; Pedico, A.; Giannossa, L.C.; Sabbatini, L.; Mattioli-Belmonte, M.; Iatta, R. An innovative, easily fabricated, silver nanoparticle-based titanium implant coating: Development and analytical characterization. Anal. Bioanal. Chem. 2013, 405, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, F.; Liu, C.; Wang, H.; Ren, B.; Yang, K.; Zhang, E. Effect of Cu Content on the antibacterial activity of titanium–copper sintered alloys. Mater. Sci. Eng. C 2014, 35, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Ma, Z.; Li, M.; Zhang, Y.; Liu, W.; Liao, Z.; Yang, K. Antibacterial properties of Ti–6Al–4V–XCu alloys. J. Mater. Sci. Technol. 2014, 30, 699–705. [Google Scholar] [CrossRef]
- Xie, C.-M.; Lu, X.; Wang, K.-F.; Meng, F.-Z.; Jiang, O.; Zhang, H.-P.; Zhi, W.; Fang, L.-M. Silver nanoparticles and growth factors incorporated hydroxyapatite coatings on metallic implant surfaces for enhancement of osteoinductivity and antibacterial properties. ACS Appl. Mater. Interfaces 2014, 6, 8580–8589. [Google Scholar] [CrossRef]
- Rodríguez-Cano, A.; Pacha-Olivenza, M.-Á.; Babiano, R.; Cintas, P.; González-Martín, M.-L. Non-covalent derivatization of aminosilanized titanium alloy implants. Surf. Coat. Technol. 2014, 245, 66–73. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, E.; Zhang, L. Microstructure, mechanical properties, bio-corrosion properties and antibacterial properties of Ti–Ag sintered alloys. Mater. Sci. Eng. C 2016, 62, 350–360. [Google Scholar] [CrossRef]
- Chen, M.; Yang, L.; Zhang, L.; Han, Y.; Lu, Z.; Qin, G.; Zhang, E. Effect of nano/micro-ag compound particles on the bio-corrosion, antibacterial properties and cell biocompatibility of Ti-Ag alloys. Mater. Sci. Eng. C 2017, 75, 906–917. [Google Scholar] [CrossRef]
- Yamanoglu, R.; Efendi, E.; Kolayli, F.; Uzuner, H.; Daoud, I. Production and mechanical properties of Ti–5Al–2.5Fe– x Cu alloys for biomedical applications. Biomed. Mater. 2018, 13, 025013. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Zhang, H.; Zhang, E.; You, J.; Ma, X.; Bai, X. Antibacterial activities and biocompatibilities of Ti-Ag alloys prepared by spark plasma sintering and acid etching. Mater. Sci. Eng. C 2018, 92, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhang, S.; Sun, Z.; Ren, L.; Yang, K. Effect of annealing temperature on mechanical and antibacterial properties of Cu-bearing titanium alloy and its preliminary study of antibacterial mechanism. Mater. Sci. Eng. C 2018, 93, 495–504. [Google Scholar] [CrossRef]
- Wang, X.; Dong, H.; Liu, J.; Qin, G.; Chen, D.; Zhang, E. In vivo antibacterial property of Ti-Cu sintered alloy implant. Mater. Sci. Eng. C 2019, 100, 38–47. [Google Scholar] [CrossRef]
- Cochis, A.; Azzimonti, B.; Chiesa, R.; Rimondini, L.; Gasik, M. Metallurgical gallium additions to titanium alloys demonstrate a strong time-increasing antibacterial activity without any cellular toxicity. ACS Biomater. Sci. Eng. 2019, 5, 2815–2820. [Google Scholar] [CrossRef] [Green Version]
- Tao, S.C.; Xu, J.L.; Yuan, L.; Luo, J.M.; Zheng, Y.F. Microstructure, mechanical properties and antibacterial properties of the microwave sintered porous Ti–3Cu alloys. J. Alloys Compd. 2020, 812, 152142. [Google Scholar] [CrossRef]
- Bolzoni, L.; Alqattan, M.; Peters, L.; Alshammari, Y.; Yang, F. Ternary Ti alloys functionalised with antibacterial activity. Sci. Rep. 2020, 10, 22201. [Google Scholar] [CrossRef]
- Shi, A.; Zhu, C.; Fu, S.; Wang, R.; Qin, G.; Chen, D.; Zhang, E. What controls the antibacterial activity of Ti-Ag alloy, Ag ion or Ti2Ag particles? Mater. Sci. Eng. C 2020, 109, 110548. [Google Scholar] [CrossRef]
- Yang, J.; Qin, H.; Chai, Y.; Zhang, P.; Chen, Y.; Yang, K.; Qin, M.; Zhang, Y.; Xia, H.; Ren, L.; et al. Molecular mechanisms of osteogenesis and antibacterial activity of Cu-bearing Ti alloy in a bone defect model with infection in vivo. J. Orthop. Transl. 2021, 27, 77–89. [Google Scholar] [CrossRef]
- Zhuang, Y.; Ren, L.; Zhang, S.; Wei, X.; Yang, K.; Dai, K. Antibacterial effect of a copper-containing titanium alloy against implant-associated infection induced by methicillin-resistant Staphylococcus aureus. Acta Biomater. 2021, 119, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Shao, H.; Li, H.; Zhou, Y. Is surface modification effective to prevent periprosthetic joint infection? A systematic review of preclinical and clinical studies. Orthop. Traumatol. Surg. Res. 2019, 105, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Hardes, J.; von Eiff, C.; Streitbuerger, A.; Balke, M.; Budny, T.; Henrichs, M.P.; Hauschild, G.; Ahrens, H. Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma: Silver-coated prostheses in sarcoma patients. J. Surg. Oncol. 2010, 101, 389–395. [Google Scholar] [CrossRef]
- Hussmann, B.; Johann, I.; Kauther, M.D.; Landgraeber, S.; Jäger, M.; Lendemans, S. Measurement of the silver ion concentration in wound fluids after implantation of silver-coated megaprostheses: Correlation with the clinical outcome. BioMed. Res. Int. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wafa, H.; Grimer, R.J.; Reddy, K.; Jeys, L.; Abudu, A.; Carter, S.R.; Tillman, R.M. Retrospective Evaluation of the Incidence of Early Periprosthetic Infection with Silver-Treated Endoprostheses in High-Risk Patients: Case-Control Study. Bone Jt. J. 2015, 97, 252–257. [Google Scholar] [CrossRef]
- Schmolders, J.; Koob, S.; Schepers, P.; Pennekamp, P.H.; Gravius, S.; Wirtz, D.C.; Placzek, R.; Strauss, A.C. Lower limb reconstruction in tumor patients using modular silver-coated megaprostheses with regard to perimegaprosthetic joint infection: A case series, including 100 patients and review of the literature. Arch. Orthop. Trauma Surg. 2017, 137, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Zajonz, D.; Birke, U.; Ghanem, M.; Prietzel, T.; Josten, C.; Roth, A.; Fakler, J.K.M. Silver-coated modular megaendoprostheses in salvage revision arthroplasty after periimplant infection with extensive bone loss—A pilot study of 34 patients. BMC Musculoskelet. Disord. 2017, 18, 383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, T.L.; Maltenfort, M.G.; Chen, A.F.; Shahi, A.; Higuera, C.A.; Siqueira, M.; Parvizi, J. Development and evaluation of a preoperative risk calculator for periprosthetic joint infection following total joint arthroplasty. J. Bone Jt. Surg. 2018, 100, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj Pandian, C.; Palanivel, R.; Dhanasekaran, S. Screening antimicrobial activity of nickel nanoparticles synthesized using Ocimum sanctum leaf extract. J. Nanoparticles 2016, 2016, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Ahghari, M.R.; Soltaninejad, V.; Maleki, A. Synthesis of nickel nanoparticles by a green and convenient method as a magnetic mirror with antibacterial activities. Sci. Rep. 2020, 10, 12627. [Google Scholar] [CrossRef]
- Bellio, P.; Luzi, C.; Mancini, A.; Cracchiolo, S.; Passacantando, M.; Di Pietro, L.; Perilli, M.; Amicosante, G.; Santucci, S.; Celenza, G. Cerium oxide nanoparticles as potential antibiotic adjuvant. Effects of CeO2 nanoparticles on bacterial outer membrane permeability. Biochim. Biophys. Acta BBA—Biomembr. 2018, 1860, 2428–2435. [Google Scholar] [CrossRef]
- Vahdati, M.; Tohidi Moghadam, T. Synthesis and characterization of selenium nanoparticles-lysozyme nanohybrid system with synergistic antibacterial properties. Sci. Rep. 2020, 10, 510. [Google Scholar] [CrossRef] [Green Version]
- Geoffrion, L.D.; Hesabizadeh, T.; Medina-Cruz, D.; Kusper, M.; Taylor, P.; Vernet-Crua, A.; Chen, J.; Ajo, A.; Webster, T.J.; Guisbiers, G. Naked selenium nanoparticles for antibacterial and anticancer treatments. ACS Omega 2020, 5, 2660–2669. [Google Scholar] [CrossRef]
- Kang, S.-M.; Jang, S.-C.; Heo, N.S.; Oh, S.Y.; Cho, H.-J.; Rethinasabapathy, M.; Vilian, A.T.E.; Han, Y.-K.; Roh, C.; Huh, Y.S. Cesium-induced inhibition of bacterial growth of pseudomonas aeruginosa PAO1 and their possible potential applications for bioremediation of wastewater. J. Hazard. Mater. 2017, 338, 323–333. [Google Scholar] [CrossRef]
- Banin, E.; Friedman, A.; Gedanken, A. Lellouche antibacterial and antibiofilm properties of yttrium fluoride nanoparticles. Int. J. Nanomed. 2012, 7, 5611. [Google Scholar] [CrossRef] [Green Version]
- Adams, C.P.; Walker, K.A.; Obare, S.O.; Docherty, K.M. Size-dependent antimicrobial effects of novel palladium nanoparticles. PLoS ONE 2014, 9, e85981. [Google Scholar] [CrossRef] [Green Version]
- Mohana, S.; Sumathi, S. Multi-functional biological effects of palladium nanoparticles synthesized using agaricus bisporus. J. Clust. Sci. 2020, 31, 391–400. [Google Scholar] [CrossRef]
- Xu, C.; Akakuru, O.U.; Zheng, J.; Wu, A. Applications of iron oxide-based magnetic nanoparticles in the diagnosis and treatment of bacterial infections. Front. Bioeng. Biotechnol. 2019, 7, 141. [Google Scholar] [CrossRef]
- Ren, K.; Dusad, A.; Zhang, Y.; Wang, D. Therapeutic intervention for wear debris-induced aseptic implant loosening. Acta Pharm. Sin. B 2013, 3, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, J.J.; Gilbert, J.L.; Urban, R.M. Corrosion of metal orthopaedic implants. J. Bone Jt. Surg. Am. 1998, 80, 268–282. [Google Scholar] [CrossRef]
- Jacobs, J.J.; Skipor, A.K.; Black, J.; Urban, R.M.; Galante, J.O. Release and excretion of metal in patients who have a total hip-replacement component made of titanium-base alloy. J. Bone Jt. Surg. Am. 1991, 73, 1475–1486. [Google Scholar] [CrossRef]
- Jacobs, J.J.; Silverton, C.; Hallab, N.J.; Skipor, A.K.; Patterson, L.; Black, J.; Galante, J.O. Metal release and excretion from cementless titanium alloy total knee replacements. Clin. Orthop. 1999, 358, 173–180. [Google Scholar] [CrossRef]
- Jacobs, J.J.; Skipor, A.K.; Patterson, L.M.; Hallab, N.J.; Paprosky, W.G.; Black, J.; Galante, J.O. Metal release in patients who have had a primary total hip arthroplasty. A prospective, controlled, longitudinal study. J. Bone Jt. Surg. 1998, 80, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Pauksch, L.; Hartmann, S.; Rohnke, M.; Szalay, G.; Alt, V.; Schnettler, R.; Lips, K.S. Biocompatibility of silver nanoparticles and silver ions in primary human mesenchymal stem cells and osteoblasts. Acta Biomater. 2014, 10, 439–449. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; van der Ven, L.T.M.; Sleijffers, A.; Park, M.V.D.Z.; Jansen, E.H.J.M.; van Loveren, H.; Vandebriel, R.J. Systemic and immunotoxicity of silver nanoparticles in an intravenous 28 days repeated dose toxicity study in rats. Biomaterials 2013, 34, 8333–8343. [Google Scholar] [CrossRef] [Green Version]
- Glehr, M.; Leithner, A.; Friesenbichler, J.; Goessler, W.; Avian, A.; Andreou, D.; Maurer-Ertl, W.; Windhager, R.; Tunn, P.-U. Argyria following the use of silver-coated megaprostheses: No association between the development of local argyria and elevated silver levels. Bone Jt. J. 2013, 95, 988–992. [Google Scholar] [CrossRef]
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations/the Review on Antimicrobial Resistance Chaired by Jim O’Neill. Available online: https://wellcomecollection.org/works/rdpck35v (accessed on 20 May 2021).
- Lora-Tamayo, J.; Murillo, O.; Iribarren, J.A.; Soriano, A.; Sánchez-Somolinos, M.; Baraia-Etxaburu, J.M.; Rico, A.; Palomino, J.; Rodríguez-Pardo, D.; Horcajada, J.P.; et al. A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2013, 56, 182–194. [Google Scholar] [CrossRef] [Green Version]
- Pfang, B.G.; García-Cañete, J.; García-Lasheras, J.; Blanco, A.; Auñón, Á.; Parron-Cambero, R.; Macías-Valcayo, A.; Esteban, J. Orthopedic implant-associated infection by multidrug resistant enterobacteriaceae. J. Clin. Med. 2019, 8, 220. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, A.; Ribera, A.; Mavrogenis, A.F.; Rodriguez-Pardo, D.; Bonnet, E.; Salles, M.J.; del Toro, D.M.; Nguyen, S.; Blanco-García, A.; Skaliczki, G.; et al. Multidrug-resistant and extensively drug-resistant gram-negative prosthetic joint infections: Role of surgery and impact of colistin administration. Int. J. Antimicrob. Agents 2019, 53, 294–301. [Google Scholar] [CrossRef]
- Randall, C.P.; Gupta, A.; Jackson, N.; Busse, D.; O’Neill, A.J. Silver resistance in gram-negative bacteria: A dissection of endogenous and exogenous mechanisms. J. Antimicrob. Chemother. 2015, 70, 1037–1046. [Google Scholar] [CrossRef] [Green Version]
- Bondarczuk, K.; Piotrowska-Seget, Z. Molecular basis of active copper resistance mechanisms in gram-negative bacteria. Cell Biol. Toxicol. 2013, 29, 397–405. [Google Scholar] [CrossRef] [Green Version]
- Runner, R.P.; Mener, A.; Roberson, J.R.; Bradbury, T.L.; Guild, G.N.; Boden, S.D.; Erens, G.A. Prosthetic joint infection trends at a dedicated orthopaedics specialty hospital. Adv. Orthop. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Perez-Jorge, C.; Gomez-Barrena, E.; Horcajada, J.-P.; Puig-Verdie, L.; Esteban, J. Drug treatments for prosthetic joint infections in the era of multidrug resistance. Expert Opin. Pharmacother. 2016, 17, 1233–1246. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Shirai, T.; Nishida, H.; Murakami, H.; Kabata, T.; Yamamoto, N.; Watanabe, K.; Nakase, J. Innovative antimicrobial coating of titanium implants with iodine. J. Orthop. Sci. 2012, 17, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Browne, K.; Chakraborty, S.; Chen, R.; Willcox, M.D.; Black, D.S.; Walsh, W.R.; Kumar, N. A new era of antibiotics: The clinical potential of antimicrobial peptides. Int. J. Mol. Sci. 2020, 21, E7047. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.L.; Letson, H.L.; Elliott, L.; Grant, A.L.; Wilkinson, M.; Hazratwala, K.; McEwen, P. Evaluation of bacteriophage as an adjunct therapy for treatment of peri-prosthetic joint infection caused by Staphylococcus aureus. PLoS ONE 2019, 14, e0226574. [Google Scholar] [CrossRef] [Green Version]
- Van Belleghem, J.D.; Manasherob, R.; Miȩdzybrodzki, R.; Rogóż, P.; Górski, A.; Suh, G.A.; Bollyky, P.L.; Amanatullah, D.F. The rationale for using bacteriophage to treat and prevent periprosthetic joint infections. Front. Microbiol. 2020, 11, 591021. [Google Scholar] [CrossRef]
- Doub, J.B.; Ng, V.Y.; Wilson, E.; Corsini, L.; Chan, B.K. Successful treatment of a recalcitrant Staphylococcus epidermidis prosthetic knee infection with intraoperative bacteriophage therapy. Pharm. Basel Switz. 2021, 14, 231. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef]
- Romanò, C.L.; Scarponi, S.; Gallazzi, E.; Romanò, D.; Drago, L. Antibacterial coating of implants in orthopaedics and trauma: A classification proposal in an evolving panorama. J. Orthop. Surg. 2015, 10, 157. [Google Scholar] [CrossRef] [Green Version]
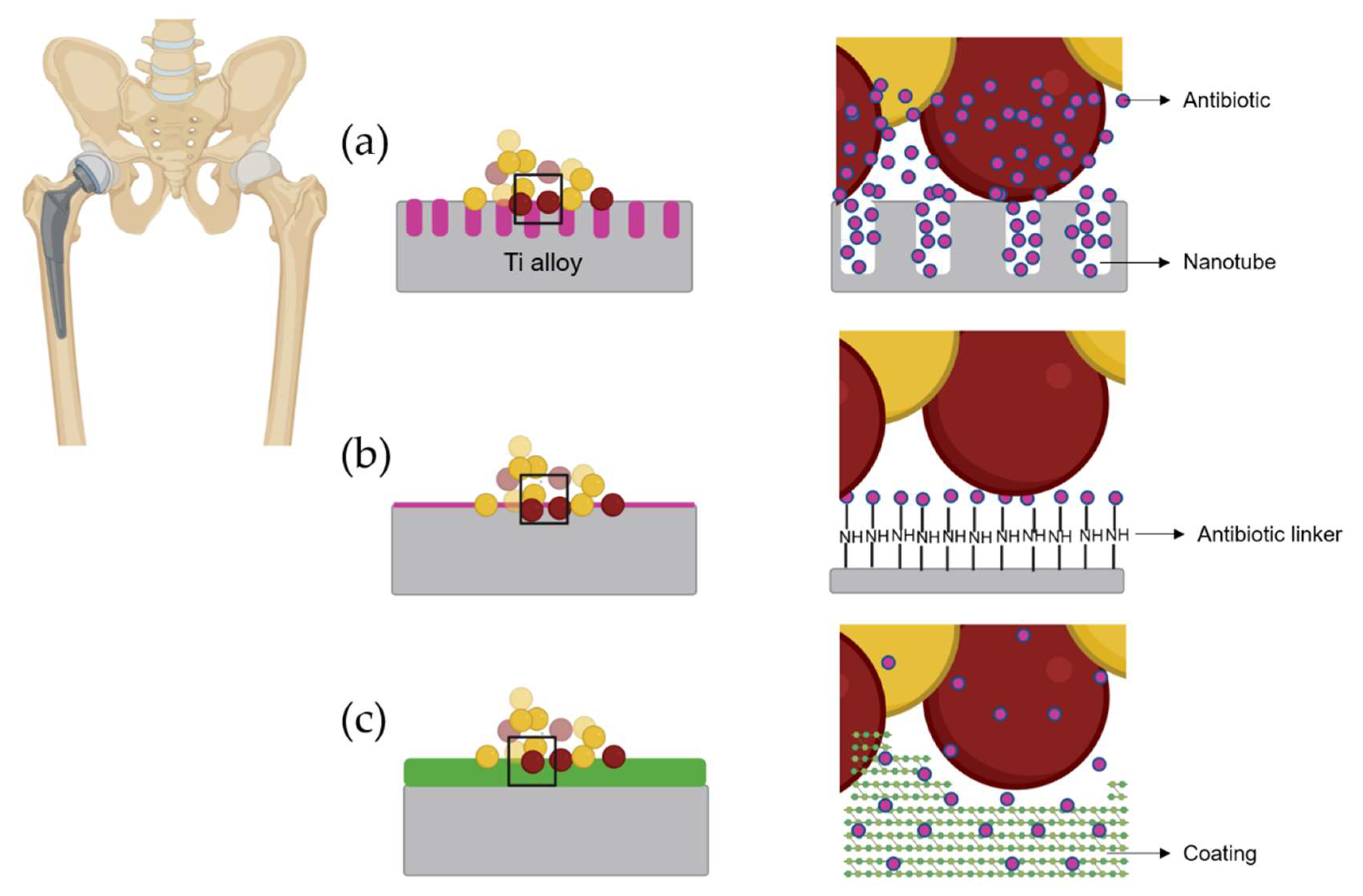
| Year | Type of Surface Modification | Bacteria Evaluated | Bacterial State | Cytotoxicity (%) | Level Study | Cell Lines/Animal Used In Vivo | Reference |
|---|---|---|---|---|---|---|---|
| 2014 | Gentamicin-loaded nanotubes with different diameters | SA, SE | Biofilm | ND | In vitro | hBMMS cells | [71] |
| 2016 | Chitosan-coated gentamicin-loaded nanotubes | SA | Planktonic | 20 | In vitro | MG-63 osteoblasts | [72] |
| 2017 | Gentamicin-loaded nanotubes made with anodization | SA | Biofilm | ND | In vivo | ‒/rabbit | [73] |
| 2018 | Chitosan-hyaluronic acid-coated vancomycin-loaded nanotubes | SA | Planktonic/Biofilm | 0 | In vitro/in vivo | Primary osteoblasts/rat | [74] |
| Vancomycin-loaded micro-patterning | MRSA | Biofilm | ND | In vivo | ‒/rabbit | [75] | |
| Gentamicin and/or vancomycin F-dopped nanotubes | SA, SE, EC | Planktonic | ND | In vitro | ‒/‒ | [66] | |
| 2019 | Gentamicin plus vancomycin F- and P-dopped bottle-shaped nanotubes | SA | Biofilm | 0 | In vitro/in vivo | MC3T3-E1 osteoblasts/rabbit | [76] |
| Year | Antibiotic Covalently Bound | Bacteria Evaluated | Bacterial State | Cytotoxicity (%) | Level Study | Cell Lines/Animal Used In Vivo | Reference |
|---|---|---|---|---|---|---|---|
| 2010 | Daptomycin | SA | Biofilm | ND | In vitro | ‒/‒ | [90] |
| 2014 | Doxycycline | ‒ | ‒ | 0− <40 | In vitro/in vivo | MC3T3-E1 osteoblasts/rabbit | [85] |
| 2015 | Ciprofloxacin | PA | Biofilm | 0 | In vitro/in vivo | NIH3T3 fibroblasts/mouse | [84] |
| 2016 | Vancomycin/caspofungin | SA, CA | Biofilm | 0 | In vitro/in vivo | hME cells/rat | [86] |
| SPI031 | SA, PA | Biofilm | 0 | In vitro/in vivo | hBMMS cells, hME cells/mouse | [89] | |
| Enoxacin | MRSA, SE, EC | Planktonic, Biofilm | 0 | In vitro/in vivo | hBMMS cells/rat | [87] | |
| 2017 | Bacitracin | SA | Biofilm | ND | In vivo | ‒/rat | [88] |
| Year | Type of Coating | Evaluated Bacteria | Bacterial State | Cytotoxicity (%) | Level Study | Cell Lines/Animal Used In Vivo | Reference |
|---|---|---|---|---|---|---|---|
| 2010 | Vancomycin-loaded PMMA | SE | Biofilm | ND | In vitro | ‒/‒ | [91] |
| Inorganic sol–gel with Polymyxin B covalently bound | EC | Planktonic | ND | In vitro | ‒/‒ | [92] | |
| Gentamicin-loaded polyelectrolyte multilayer | SA | Planktonic, Biofilm | 0–80 | In vitro/in vivo | MC3T3-E1 osteoblasts/rabbit | [93] | |
| 2014 | Rifampicin and fosfomycin-loaded Hydroxyapatite coating | MSSA, MRSA | Biofilm | ND | In vivo | ‒/rabbit | [94] |
| Ciprofloxacin-loaded chitosan-nanoparticles coating | SA | Planktonic | <30 | In vitro | MG63 osteoblast-like cells | [95] | |
| Chitosan–vancomycin composite coatings | SA | Planktonic | 0 | In vitro | MG63 osteoblast-like cells | [96] | |
| Vancomycin-loaded PLGA-coating | SA | Planktonic/Biofilm | 0 | In vitro | MC3T3-E1 osteoblasts/rabbit | [97] | |
| 2015 | Doxycycline-loaded polymer-lipid encapsulation matrix coating | MSSA, MRSA | Planktonic, Biofilm | ND | In vitro/in vivo | ‒/mouse | [98] |
| 2015 | PLGA-gentamicin-hydroxyapatite-coating | SA, SE | Planktonic, Biofilm | ND | In vitro/in vivo | ‒/rabbit | [99] |
| 2016 | Gentamicin-derivates coating | SA | Biofilm | ND | In vivo | ‒/rats | [100] |
| 2016 | Vancomycin-loaded phosphatidyl-choline | SA | Biofilm | ND | In vivo | ‒/rabbit | [101] |
| 2016 | Tetracycline loaded chitosan-gelatin nanosphere coating | SA | Biofilm | >90 | In vitro/in vivo | MC3T3-E1 osteoblasts/rabbit | [102] |
| 2017 | Doxycycline-loaded coaxial PCL-PVA nanofiber coating | SA | Biofilm | ND | In vivo | ‒/rat | [103] |
| Tobramycin-loaded PDLLA coating | SA | Biofilm | ND | In vivo | ‒/rabbit | [1] | |
| 2018 | Vancomycin-loaded mesoporous bioglass-PLGA coating | SA | Planktonic, Biofilm | 0 | In vitro | hBMMS cells | [104] |
| Vancomycin-loaded mesoporous silica nanoparticles-containing gelatin coating | SA | Biofilm | 0 | In vitro | hBMMS cells | [105] | |
| 2019 | Gentamicin-loaded polyelectrolyte multilayer | SA, SE | Planktonic, Biofilm | <5 | In vitro/in vivo | MC3T3-E1 osteoblast/rats | [106] |
| Tobramycin-loaded hydroxyapatite coating | SA | Planktonic, Biofilm | ND | In vitro/in vivo | Endothelial cells, primary osteoblasts/rabbit | [107] | |
| Vancomycin plus tigecycline-loaded PEG-PPS coating | SA | Biofilm | ND | In vivo | ‒/mouse | [108] | |
| Gentamicin-loaded calcium phosphate-based coating | SA | Biofilm | ND | In vivo | ‒/rat | [109] | |
| Vancomycin-loaded polymethacrylate coating | SA | Planktonic/Biofilm | ND | In vitro/in vivo | ‒/mouse | [110] | |
| 2020 | Cephalexin- and VEGF-loaded agarose-nanocrystalline apatite coating | SA | Planktonic | 0 | In vitro | MC3T3-E1 osteoblast | [111] |
| Moxifloxacin-loaded organic-inorganic sol–gel | SA, SE, EC | Planktonic, Biofilm | 0 | In vitro/in vivo | MC3T3-E1 osteoblasts/mouse | [112] | |
| Gentamicin loaded autologous blood glue | PA | Planktonic, Biofilm | 0 | In vitro | hBMMS cells | [113] | |
| Fluconazole/anidulafungin-loaded organic-inorganic sol–gel | CA, CP | Planktonic, Biofilm | 0 | In vitro | MC3T3-E1 osteoblasts | [114] | |
| Anidulafungin-loaded organic-inorganic sol–gel | CA | Biofilm | - | In vivo | ‒/mouse | [115] | |
| Vancomycin-loaded starch coating | SA | Planktonic | ND | In vitro | ‒/‒ | [116] |
| Year | Type of Surface Modification | Incorporated Metal | Metal Incorporation | Bacteria Evaluated | Bacterial State | Cytotoxicity (%) | Level Study | Cell Lines/Animal Used In Vivo | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 2009 | Metallurgical addition | Cu | Forge | SA, EC | Planktonic/biofilm | Ctyocompatible | In vitro/ in vivo | V79 cell line/rabbits | [148] |
| 2010 | Co-sputtering | Cu-Mn-O, Ag-Mn-O | ternary and quaternary oxides | SA, SE | Planktonic | - | In vitro | - | [149] |
| Single step silver plasma immersion ion implantation | Ag | Nanoparticles | SA, EC | Planktonic | Cytocompatible | In vitro | MG63 human osteoblast-like cells | [150] | |
| 2011 | TiO2-chitosan/heparin coating | Ag | Nanoparticles | SA | Biofilm | - | In vivo | - | [151] |
| Hydroxyapatite coating | Ag | Nanoparticles | EC | Planktonic | - | In vitro | - | [152] | |
| 2013 | Metallurgical addition | Cu | Powder metallurgy | SA, EC | Planktonic | - | In vitro | - | [153] |
| Titanium nanotubular | Ag | Nanoparticle loading | SA, EC | Planktonic | - | In vitro | - | [154] | |
| Polydopamine-modified alloy surface | Ag | Silver inonic inmobilization | EC | Planktonic | - | In vitro | - | [155] | |
| Poly(ethylene glycol diacrylate)-co-acrylic acid coating | Ag | Nanoparticles | SA, EC, PA | Planktonic | Cytocompatible | In vitro | MG63 human osteoblast-like cells | [156] | |
| 2014 | Metallurgical addition | Cu | Powder metallurgy | SA, EC | Planktonic | - | In vitro | - | [157] |
| Metallurgical addition | Cu | Casting with post-treatment | SA, EC | Planktonic | Cytocompatible | In vitro | L929 cell line | [158] | |
| BMP-2/heparinchitosan-hydroxyapatite coating | Ag | Nanoparticles | SE, EC | Planktonic | Cytocompatible | In vitro | MC3T3-E1 cells, BMS cells | [159] | |
| Aminosilanized titanium alloy | Ag | Nanoparticles | SA | Planktonic | - | In vitro | - | [160] | |
| 2016 | Metallurgical addition | Ag | Sintering | SA | Planktonic | - | In vitro | - | [161] |
| 2017 | Metallurgical addition | Ag | Sintering, casting, casting with appropiate post-treatment w/o surface tretament | SA | Planktonic | Cytocompatible | In vitro | MC3T3-E1 cells | [162] |
| 2018 | Metallurgical addition | Cu | Powder metallurgy | SA, EC | Planktonic | Cytocompatible | In vitro | HeLa cells | [163] |
| Metallurgical addition | Ag | Spark plasma sintering and acid etching | SA | Planktonic | Cytocompatible | In vitro | MC3T3-E1 cells | [164] | |
| Metallurgical addition | Cu | Casting with post-treatment | SA | Planktonic | - | In vitro | - | [165] | |
| 2019 | Metallurgical addition | Cu | Sintering | SA | Biofilm | - | In vivo | - | [166] |
| Metallurgical addition | Ga | Powder metallurgy | MRSA | Planktonic/biofilm | Cytocompatible | In vitro | ATCC CRL-11372 and ATCC HTB-96 | [167] | |
| 2020 | Metallurgical addition | Cu | Microwave sintering | SA, EC | Planktonic | - | In vitro | - | [168] |
| Metallurgical addition | Cu | Powder metallurgy | EC | Planktonic | - | In vitro | - | [169] | |
| Metallurgical addition | Ag | Casting with appropiate post-treatment w/o surface tretament | SA | Planktonic | Cytocompatible | In vitro | MC3T3-E1 cells | [170] | |
| 2021 | Metallurgical addition | Cu | As-cast | SA | Biofilm | - | In vitro/in vivo | Mouse | [171] |
| Metallurgical addition | Cu | As-cast | MRSA | Planktonic/biofilm | Cytocompatible | In vitro/in vivo | MC3T3-E1 cells/rat | [172] |
| Preventive Approach of PJI | Advantages | Disadvantages |
|---|---|---|
| Antibiotic-based strategies | ||
| Nanostructured titanium surfaces | Possibility of increasing the osteointegration of the titanium surfaces | Reduced durability of antibiotic protection |
| Unknown biomechanical stability | ||
| Loaded antibiotic can act against both bacteria directly adhered on the titanium surface and bacteria near but not in contact with it | Unknown effects on the useful life of the implant, osteointegration, and coagulation profile | |
| Impossibility of intra-operative antibiotic load No clinical trials to support their use | ||
| Antibiotics covalently bound to titanium surfaces | Long durability of antibiotic protection, up to months or years | Loaded antibiotic can only act against bacteria directly adhered on the titanium surface |
| Unknown durability of antibiotic protection | ||
| Impossibility of intra-operative antibiotic load | ||
| No clinical trials to support their use | ||
| Coatings loaded with antibiotic for titanium alloys | Possibility of increasing the osteointegration of the titanium surfaces | Incomplete surface protection |
| Loaded antibiotic can act against both bacteria directly adhered on the titanium surface and bacteria near but not in contact with it | Unknown effects on the useful life of the implant, osteointegration, and coagulation profile | |
| Possibility of intra-operative antibiotic load | ||
| Clinical trials to support their use | Clinical trials that support their use has been carried out with few antibiotics | |
| Heavy metals-based strategies | Broad spectrum antimicrobial effect (beyond antibacterial effect) | Local and systemic toxicity supported by clinical trials |
| Loaded metals can act against both microorganisms directly adhered on the titanium surface and those near but not in contact with it | ||
| Long durability | ||
| Clinical trials to support their use | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteban, J.; Vallet-Regí, M.; Aguilera-Correa, J.J. Antibiotics- and Heavy Metals-Based Titanium Alloy Surface Modifications for Local Prosthetic Joint Infections. Antibiotics 2021, 10, 1270. https://doi.org/10.3390/antibiotics10101270
Esteban J, Vallet-Regí M, Aguilera-Correa JJ. Antibiotics- and Heavy Metals-Based Titanium Alloy Surface Modifications for Local Prosthetic Joint Infections. Antibiotics. 2021; 10(10):1270. https://doi.org/10.3390/antibiotics10101270
Chicago/Turabian StyleEsteban, Jaime, María Vallet-Regí, and John J. Aguilera-Correa. 2021. "Antibiotics- and Heavy Metals-Based Titanium Alloy Surface Modifications for Local Prosthetic Joint Infections" Antibiotics 10, no. 10: 1270. https://doi.org/10.3390/antibiotics10101270
APA StyleEsteban, J., Vallet-Regí, M., & Aguilera-Correa, J. J. (2021). Antibiotics- and Heavy Metals-Based Titanium Alloy Surface Modifications for Local Prosthetic Joint Infections. Antibiotics, 10(10), 1270. https://doi.org/10.3390/antibiotics10101270








