Comprehensive Evaluation of the Antibacterial and Antifungal Activities of Carlina acaulis L. Essential Oil and Its Nanoemulsion
Abstract
1. Introduction
2. Results
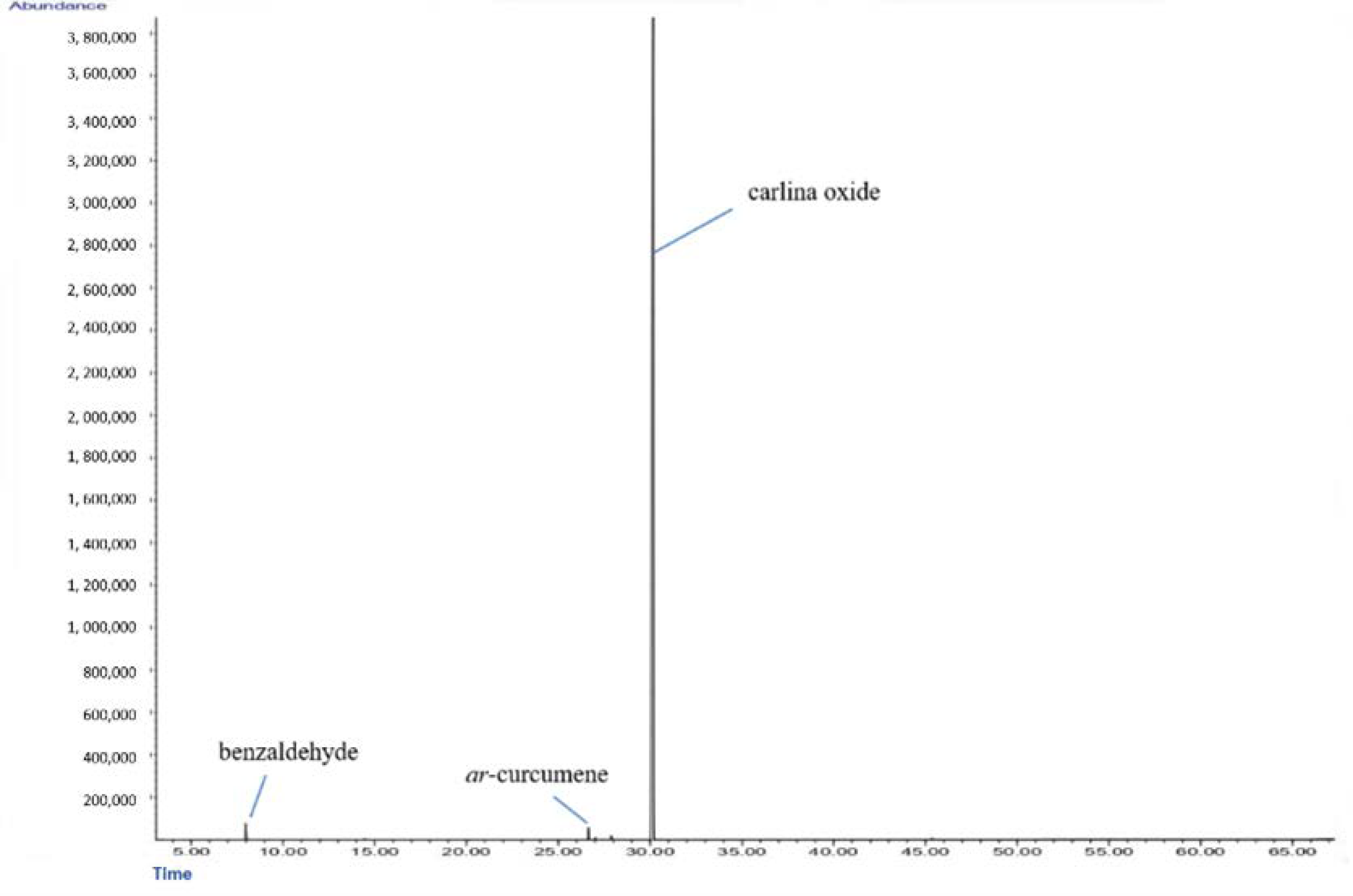
2.1. Chemical Analysis of C. acaulis Essential Oil
2.2. Nanoemulsion Development
2.3. Antimicrobial Assay
3. Discussion
4. Materials and Methods
4.1. Essential Oil Isolation and Purification of Carlina Oxide
4.2. Chemical Characterization of the Essential Oil
4.3. Nanoemulsion Preparation and Characterization
4.4. Antimicrobial Assay
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef]
- Rossiter, S.E.; Fletcher, M.H.; Wuest, W.M. Natural products as platforms to overcome antibiotic resistance. Chem. Rev. 2017, 117, 12415–12474. [Google Scholar] [CrossRef]
- Silva, L.N.; Zimmer, K.R.; Macedo, A.J.; Trentin, D.S. Plant natural products targeting bacterial virulence factors. Chem. Rev. 2016, 116, 9162–9236. [Google Scholar] [CrossRef]
- Tutin, F.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. (Eds.) Plantaginaceae to Compositae (and Rubiaceae). In Flora Europea; Cambridge University Press: Cambridge, UK, 1976; Volume 4, p. 210. [Google Scholar]
- Strzemski, M.; Wójciak-Kosior, M.; Sowa, I.; Załuski, D.; Verpoorte, R. Historical and traditional medical applications of Carlina acaulis L.—A critical ethnopharmacological review. J. Ethnopharmacol. 2019, 239, 111842. [Google Scholar] [CrossRef] [PubMed]
- Kühn, C.G. Claudii Galeni, Opera Omnia; Prostat in Officina Libraria Car. Cnoblochii: Leipzig, Germany, 1833; Volume 20, p. 143. [Google Scholar]
- Ruellio, J. Pedanii Dioscoridis Anazarbeii, de Medicinali Materia Libri Sex; Apud Balthazarem Arnolletum: Lugduni, France, 1522; Volume 3, pp. 329–331. [Google Scholar] [CrossRef]
- Parvu, C. Universul Plantelor-Mica Enciclopedie; Editura Enciclopedica: Bucuresti, Romania, 1997; p. 1154. [Google Scholar]
- Menković, N.; Šavikin, K.; Tasić, S.; Zdunić, G.; Stesević, D.; Milosavljević, S.; Vincek, D. Ethnobotanical study on traditional uses of wild medicinal plants in Prokletije Mountains (Montenegro). J. Ethnopharmacol. 2011, 133, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Kernóczi, Z.; Héthelyi, E.; Dános, B.; Tétényi, P. Presence of carlina oxide in plants of hungary. Stabilization and antimicrobial effect. Acta Pharm. Hung. 1987, 57, 171–181. [Google Scholar] [PubMed]
- Belabbes, R.; Mami, I.R.; Dib, M.E.; Mejdoub, K.; Tabti, B.; Costa, J.; Muselli, A. Chemical composition and biological activities of essential oils of Echinops spinosus and Carlina vulgaris rich in polyacetylene compounds. Curr. Nutr. Food Sci. 2020, 16, 563–570. [Google Scholar] [CrossRef]
- Stojanović-Radić, Z.; Čomić, L.; Radulović, N.; Blagojević, P.; Mihajilov-Krstev, T.; Rajković, J. Commercial Carlinae radix herbal drug: Botanical identity, chemical composition and antimicrobial properties. Pharm. Biol. 2012, 50, 933–940. [Google Scholar] [CrossRef]
- Đorđević, S.; Tadić, V.; Petrović, S.; Kukić-Marković, J.; Dobrić, S.; Milenković, M.; Hadžifejzović, N. Bioactivity assays on Carlina acaulis and C. acanthifolia root and herb extracts. Digest J. Nanomater. Biostruct. 2012, 7, 213–1222. [Google Scholar]
- Herrmann, F.; Hamoud, R.; Sporer, F.; Tahrani, A.; Wink, M. Carlina oxide—A natural polyacetylene from Carlina acaulis (Asteraceae) with potent antitrypanosomal and antimicrobial properties. Planta Med. 2011, 77, 1905–1911. [Google Scholar] [CrossRef]
- Wnorowski, A.; Wnorowska, S.; Wojas-Krawczyk, K.; Grenda, A.; Staniak, M.; Michalak, A.; Woźniak, S.; Matosiuk, D.; Biała, G.; Wójciak, M.; et al. Toxicity of carlina oxide—A natural polyacetylene from the Carlina acaulis roots—In Vitro and in vivo study. Toxins 2020, 12, 239. [Google Scholar] [CrossRef]
- Benelli, G.; Pavoni, L.; Zeni, V.; Ricciardi, R.; Cosci, F.; Cacopardo, G.; Gendusa, S.; Spinozzi, E.; Petrelli, R.; Cappellacci, L.; et al. Developing a highly stable Carlina acaulis essential oil nanoemulsion for managing Lobesia botrana. Nanomaterials 2020, 10, 1867. [Google Scholar] [CrossRef]
- Benelli, G.; Rizzo, R.; Zeni, V.; Govigli, A.; Samková, A.; Sinacori, M.; Lo Verde, G.; Pavela, R.; Cappellacci, L.; Petrelli, R.; et al. Carlina acaulis and Trachyspermum ammi essential oils formulated in protein baits are highly toxic and reduce aggressiveness in the medfly, Ceratitis capitata. Ind. Crops Prod. 2021, 161, 113191. [Google Scholar] [CrossRef]
- Pavela, R.; Maggi, F.; Petrelli, R.; Cappellacci, L.; Buccioni, M.; Palmieri, A.; Canale, A.; Benelli, G. Outstanding insecticidal activity and sublethal effects of Carlina acaulis root essential oil on the housefly, Musca domestica, with insights on its toxicity on human cells. Food Chem. Toxicol. 2020, 136, 111037. [Google Scholar] [CrossRef]
- Konovalov, D.A. Polyacetylene compounds of plants of the Asteraceae family. Pharm. Chem. J. 2014, 48, 613–631. [Google Scholar] [CrossRef]
- Christensen, L.P. Biological activities of naturally occurring acetylenes and related compounds from higher plants. In Recent Research Developments in Phytochemistry; Pandalai, S.G., Ed.; Research Signpost: Trivandrum, India, 1998; pp. 227–257. [Google Scholar]
- Benelli, G.; Pavela, R.; Petrelli, R.; Nzekoue, F.K.; Cappellacci, L.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Sut, S.; Dall’Acqua, S. Carlina oxide from Carlina acaulis root essential oil acts as a potent mosquito larvicide. Ind. Crops Prod. 2019, 137, 356–366. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). M27-A3 Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard—Third Edition. CLSI document M27-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; ISBN 1-56238-666-2. [Google Scholar]
- Pharmacopoea Bavarica; Sumptibus Josephi Lindauer: Munich, Germany, 1822; p. 57.
- Jourdan, A.J.L. Pharmacopoea Universalis; Verlag des Grossh. Sächs. pr. Landes-Industrie-Comptoirs: Weimar, Germany, 1832; p. 446. [Google Scholar]
- Gazzetta Ufficiale. Available online: https://www.gazzettaufficiale.it/eli/gu/2018/09/26/224/sg/pdf (accessed on 22 September 2021).
- Cousyn, G.; Dalfrà, S.; Scarpa, B.; Geelen, J.; Anton, R.; Serafini, M.; Delmulle, L. Project belfrit: Harmonizing the use of plants in food supplements in the European Union: Belgium, France and Italy—A first step. Eur. Food Feed Law Rev. 2013, 8, 187–196. [Google Scholar]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corp.: Carol Stream, IL, USA, 2007. [Google Scholar]
- NIST. Mass Spectral Library (NIST/EPA/NIH). 17; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2017. [Google Scholar]
- FFNSC. Flavors and Fragrances of Natural and Synthetic Compounds. Mass Spectral Database. 2; Shimadzu Corps: Kyoto, Japan, 2012. [Google Scholar]
- Van den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). M07–A10 Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Ninety Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015; Volume 32. [Google Scholar]
- Rosato, A.; Maggi, F.; Cianfaglione, K.; Conti, F.; Ciaschetti, G.; Rakotosaona, R.; Fracchiolla, G.; Clodoveo, M.L.; Franchini, C.; Corbo, F. Chemical composition and antibacterial activity of seven uncommon essential oils. J. Essent. Oil Res. 2018, 30, 233–243. [Google Scholar] [CrossRef]
- Van den Bogaard, A.E.; Stobberingh, E.E. Epidemiology of resistance to antibiotics: Links between animals and humans. Int. J. Antimicrob. Agents 2000, 14, 327–335. [Google Scholar] [CrossRef]
- Kon, K.V.; Rai, M.K. Plant essential oils and their constituents in coping with multidrug-resistant bacteria. Expert Rev. Anti-Infect. Ther. 2012, 10, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Rosato, A.; Carocci, A.; Catalano, A.; Clodoveo, M.L.; Franchini, C.; Corbo, F.; Carbonara, G.G.; Carrieri, A.; Fracchiolla, G. Elucidation of the synergistic action of Mentha piperita essential oil with common antimicrobials. PLoS ONE 2018, 13, e0200902. [Google Scholar] [CrossRef] [PubMed]
| Time (Days) | Z-Average (nm) | PDI |
|---|---|---|
| T0 | 134.62 ± 2.22 | 0.268 ± 0.009 |
| T30 | 129.63 ± 1.11 | 0.265 ± 0.005 |
| T90 | 121.5 ± 0.78 | 0.260 ± 0.008 |
| T180 | 117.63 ± 2.23 | 0.256 ± 0.009 |
| T270 | 114.7 ± 0.69 | 0.262 ± 0.002 |
| EO MIC 1 (mg/mL) | EO ADIFF 2 (mm) | NE MIC 3 (mg/mL) | Carlina Oxide MIC 4 (mg/mL) | Rifaximin MIC 5 (μg/mL) | |
|---|---|---|---|---|---|
| Bacillus cereus 10876 | 5.4 | 0.9 | 15 | na 6 | 0.1 |
| Corynebacterium striatum RM | 5.4 | 1.3 | 15 | 1.33 | 0.4 |
| Enterococcus faecalis 1011 | 10.9 | 1.0 | 60 | 5.35 | 0.4 |
| Enterococcus faecalis 29212 | 5.4 | 0.8 | 60 | 10.7 | 0.1 |
| Staphylococcus aureus 23 | 10.9 | 0.8 | 60 | 5.35 | 0.1 |
| Staphylococcus aureus 24 | 5.4 | 1.0 | 15 | 0.33 | 0.4 |
| Staphylococcus aureus 25923 | 2.7 | 0.7 | 15 | 0.33 | 0.1 |
| Staphylococcus aureus 29213 | 5.4 | 1.1 | 15 | 0.33 | 0.1 |
| Staphylococcus aureus 43300 | 2.7 | 0.9 | 7.5 | 1.33 | 3.1 |
| Staphylococcus aureus 6538 | 2.7 | 1.0 | 7.5 | 1.33 | 0.1 |
| Staphylococcus aureus 6538P | 5.4 | 1.0 | 15 | 0.67 | 0.1 |
| Staphylococcus aureus IAC | 5.4 | 0.9 | 7.5 | 0.67 | 0.1 |
| Staphylococcus aureus TER | 10.9 | 0.7 | 15 | 1.32 | 0.1 |
| Staphylococcus lugdunensis | 10.9 | 1.0 | 15 | 1.33 | 0.1 |
| Staphylococcus sciuri | 10.9 | 1.0 | 15 | 2.77 | 0.1 |
| Staphylococcus warneri | 5.4 | 1.0 | 15 | 1.33 | 0.1 |
| Acinetobacter baumannii BS | na | na | 15 | na | 25.0 |
| Citrobacter freundii IG | na | na | 500 | na | 12.5 |
| Escherichia coli 25922 | na | na | 125 | na | 6.3 |
| Escherichia coli 35218 | na | na | 125 | na | 6.3 |
| Escherichia coli ESBL | na | na | 250 | na | 6.3 |
| Klebsiella pneumoniae 13883 | na | na | 500 | na | 12.5 |
| Klebsiella pneumoniae BS | na | na | 125 | na | 25.0 |
| Proteus mirabilis | na | na | 500 | na | 6.3 |
| Pseudomonas aeruginosa 27853 | na | na | 250 | na | 6.3 |
| Serratia marcescens IG | na | na | 500 | na | 6.3 |
| EO MIC 1 (mg/mL) | EO ADIFF 2 (mm) | NE MIC 3 (mg/mL) | Carlina Oxide MIC 4 (mg/mL) | Amphotericin B MIC 5 (μg/mL) | |
|---|---|---|---|---|---|
| Candida albicans ATCC 10231 | 0.68 | 1.30 | 1.9 | 0.04 | 1.0 |
| Candida albicans ATCC 90028 | 1.35 | 1.00 | 1.9 | 0.04 | 1.0 |
| Candida glabrata ATCC 15126 | 2.70 | 1.22 | 0.9 | 0.04 | 0.5 |
| Candida kefyr ATCC 200,0493 | 2.70 | 0.91 | 0.9 | 0.02 | 0.5 |
| Candida krusei ATCC 6258 | 2.70 | 1.10 | 0.9 | 0.02 | 1.0 |
| Candida albicans 10A12 | 2.70 | 1.20 | 0.9 | 0.04 | 1.0 |
| Candida krusei 31A29 | 0.67 | 1.52 | 1.9 | 0.04 | 1.0 |
| Candida parapsilosis 11A13 | 0.67 | 1.41 | 1.9 | 0.08 | 0.5 |
| Candida parapislosis 1A1 | 0.34 | 1.41 | 1.9 | 0.04 | 1.0 |
| Candida parapsilosis 910 | 1.35 | 1.22 | 1.9 | 0.04 | 0.5 |
| Candida parapsilosis 911 | 1.35 | 1.21 | 1.9 | 0.04 | 0.5 |
| Candida tropicalis 810 | 0.36 | 0.90 | 1.9 | 0.08 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosato, A.; Barbarossa, A.; Mustafa, A.M.; Bonacucina, G.; Perinelli, D.R.; Petrelli, R.; Maggi, F.; Spinozzi, E. Comprehensive Evaluation of the Antibacterial and Antifungal Activities of Carlina acaulis L. Essential Oil and Its Nanoemulsion. Antibiotics 2021, 10, 1451. https://doi.org/10.3390/antibiotics10121451
Rosato A, Barbarossa A, Mustafa AM, Bonacucina G, Perinelli DR, Petrelli R, Maggi F, Spinozzi E. Comprehensive Evaluation of the Antibacterial and Antifungal Activities of Carlina acaulis L. Essential Oil and Its Nanoemulsion. Antibiotics. 2021; 10(12):1451. https://doi.org/10.3390/antibiotics10121451
Chicago/Turabian StyleRosato, Antonio, Alexia Barbarossa, Ahmed M. Mustafa, Giulia Bonacucina, Diego Romano Perinelli, Riccardo Petrelli, Filippo Maggi, and Eleonora Spinozzi. 2021. "Comprehensive Evaluation of the Antibacterial and Antifungal Activities of Carlina acaulis L. Essential Oil and Its Nanoemulsion" Antibiotics 10, no. 12: 1451. https://doi.org/10.3390/antibiotics10121451
APA StyleRosato, A., Barbarossa, A., Mustafa, A. M., Bonacucina, G., Perinelli, D. R., Petrelli, R., Maggi, F., & Spinozzi, E. (2021). Comprehensive Evaluation of the Antibacterial and Antifungal Activities of Carlina acaulis L. Essential Oil and Its Nanoemulsion. Antibiotics, 10(12), 1451. https://doi.org/10.3390/antibiotics10121451












