Clinical Features and Molecular Characteristics of Methicillin-Susceptible Staphylococcus aureus Ocular Infection in Taiwan
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics of MSSA Ocular Infections
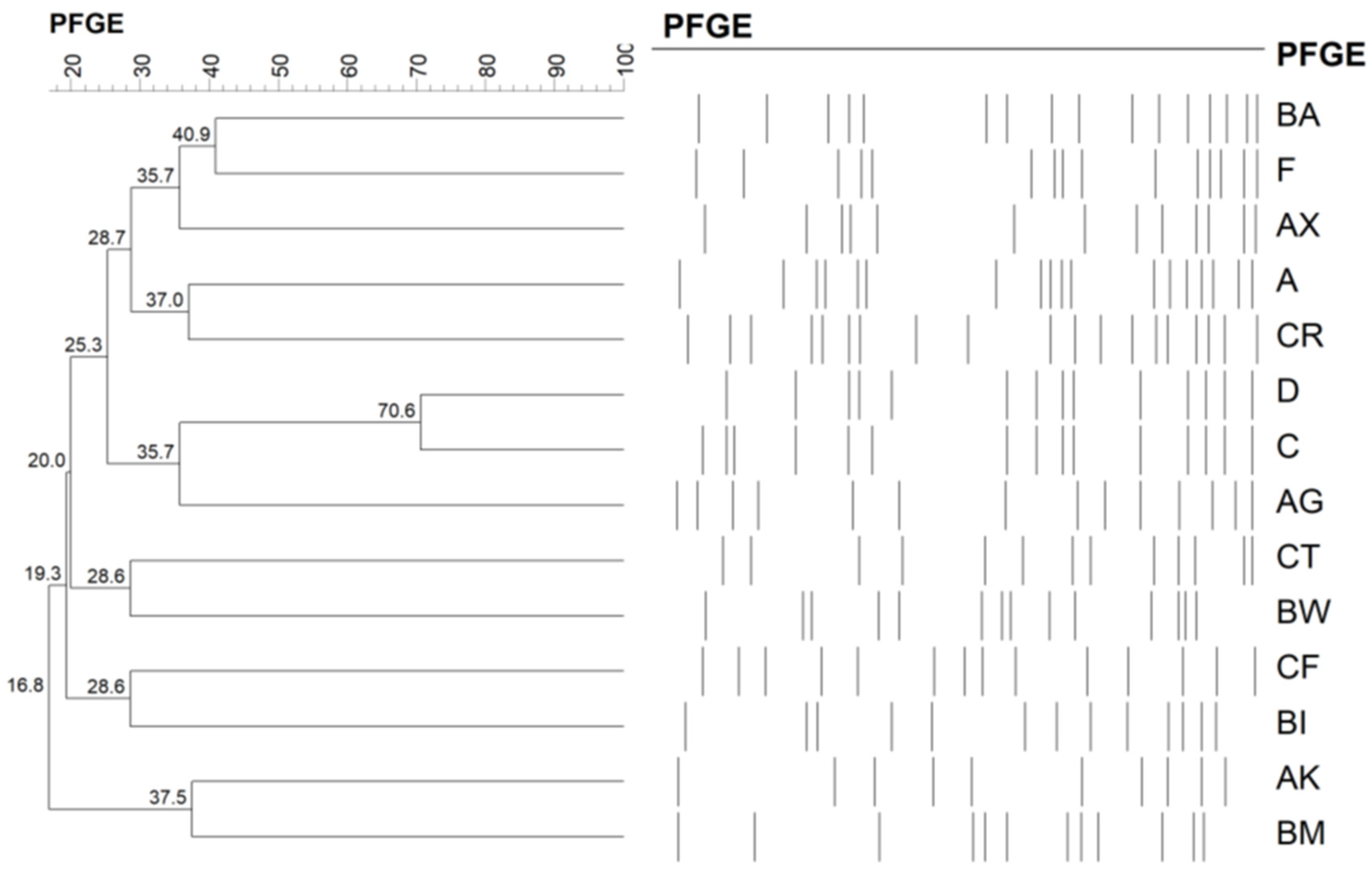
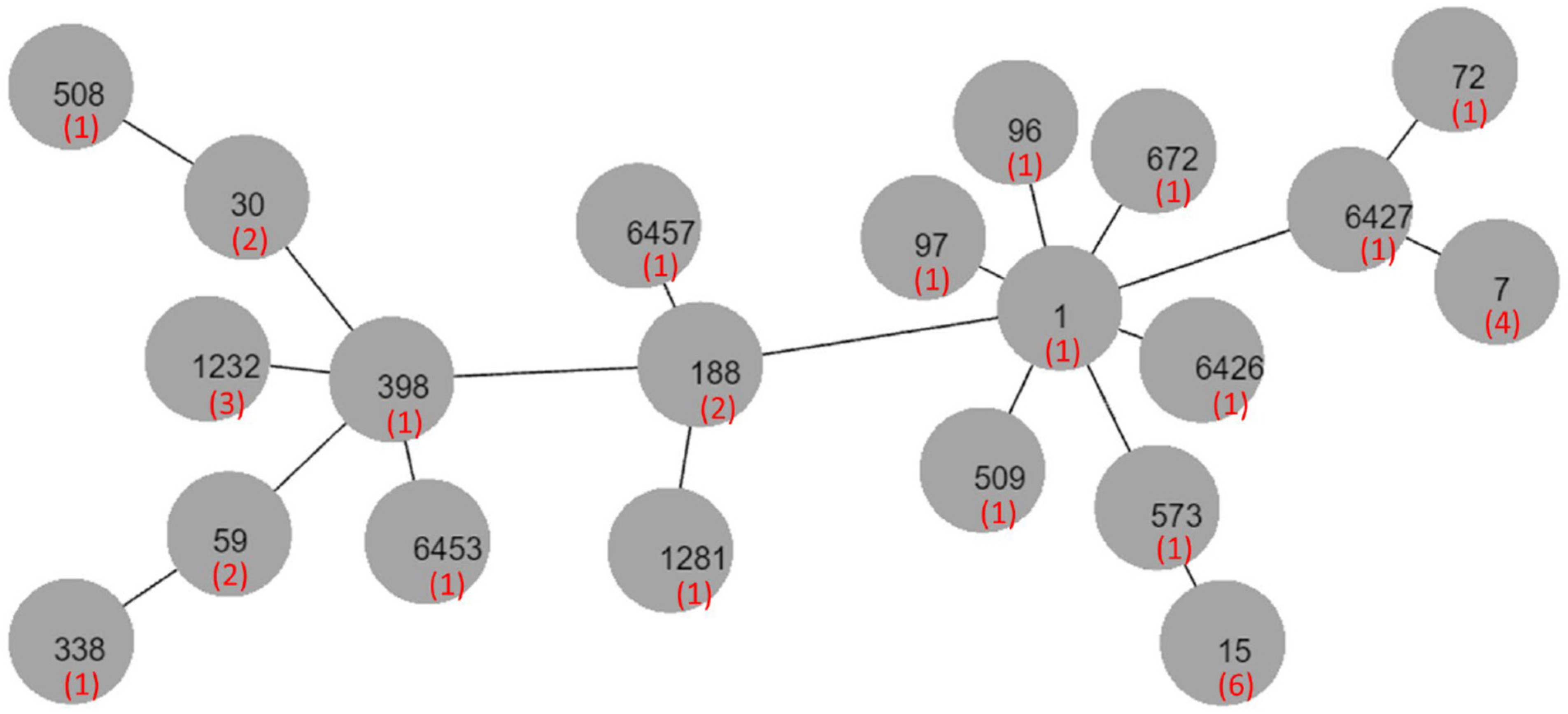
2.2. Molecular Typing
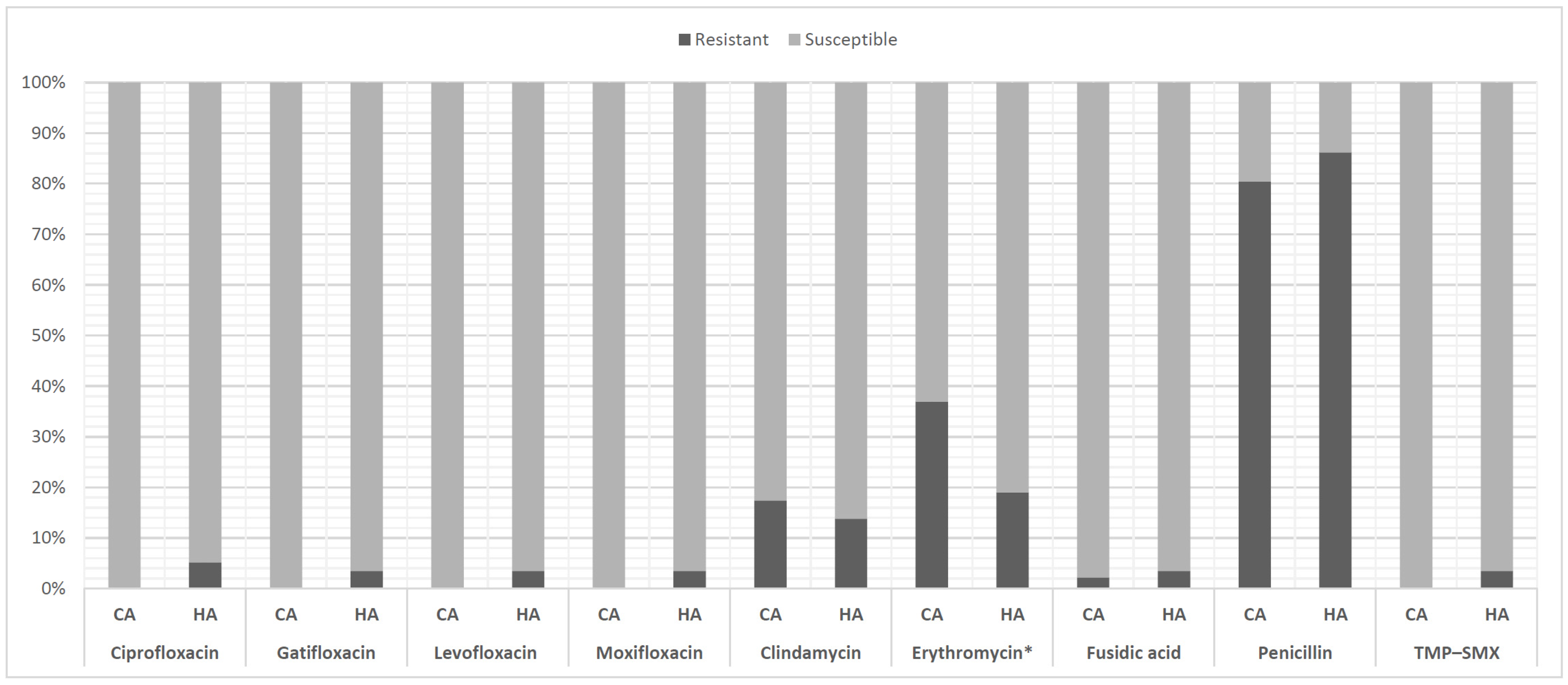
2.3. Drug Susceptibility Test
3. Discussion
4. Materials and Methods
4.1. Ethics
4.2. Study Population and Data Collection
4.3. Molecular Characteristics
4.4. Drug Susceptibility Test
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magill, S.S.; O’Leary, E.; Janelle, S.J.; Thompson, D.L.; Dumyati, G.; Nadle, J.; Wilson, L.E.; Kainer, M.A.; Lynfield, R.; Greissman, S.; et al. Changes in Prevalence of Health Care–Associated Infections in U.S. Hospitals. N. Eng. J. Med. 2018, 379, 1732–1744. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Wong, J.W.; Ip, M.; Tang, A.; Wei, V.W.; Wong, S.Y.; Riley, S.; Read, J.M.; Kwok, K.O. Prevalence and risk factors of community-associated methicillin-resistant Staphylococcus aureus carriage in Asia-Pacific region from 2000 to 2016: A systematic review and meta-analysis. Clin. Epidemiol. 2018, 10, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- King, J.M.; Kulhankova, K.; Stach, C.S.; Vu, B.G.; Salgado-Pabón, W. Phenotypes and Virulence among Staphylococcus aureus USA100, USA200, USA300, USA400, and USA600 Clonal Lineages. Msphere 2016, 1, e00071-16. [Google Scholar] [CrossRef]
- Jackson, K.A.; Gokhale, R.H.; Nadle, J.; Ray, S.M.; Dumyati, G.; Schaffner, W.; Ham, D.C.; Magill, S.S.; Lynfield, R.; See, I. Public Health Importance of Invasive Methicillin-sensitive Staphylococcus aureus Infections: Surveillance in 8 US Counties, 2016. Clin. Infect. Dis. 2020, 70, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Kourtis, A.P.; Hatfield, K.; Baggs, J.; Mu, Y.; See, I.; Epson, E.; Nadle, J.; Kainer, M.A.; Dumyati, G.; Petit, S.; et al. Vital Signs: Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections—United States. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 214–219. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chen, P.A.; Chen, C.J.; Huang, Y.C. Molecular characteristics and clinical features of pediatric methicillin-susceptible Staphylococcus aureus infection in a medical center in northern Taiwan. BMC Infect. Dis. 2019, 19, 402. [Google Scholar] [CrossRef]
- Goering, R.V.; Shawar, R.M.; Scangarella, N.E.; O’Hara, F.P.; Amrine-Madsen, H.; West, J.M.; Dalessandro, M.; Becker, J.A.; Walsh, S.L.; Miller, L.A.; et al. Molecular epidemiology of methicillin-resistant and methicillin-susceptible Staphylococcus aureus isolates from global clinical trials. J. Clin. Microbiol. 2008, 46, 2842–2847. [Google Scholar] [CrossRef][Green Version]
- Chen, F.-J.; Siu, L.-K.K.; Lin, J.-C.; Wang, C.-H.; Lu, P.-L. Molecular typing and characterization of nasal carriage and community-onset infection methicillin-susceptible Staphylococcus aureus isolates in two Taiwan medical centers. BMC Infect. Dis. 2012, 12, 343. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Chuang, Y.-C.; Wang, J.-T.; Chang, S.-C. Impact of Prior Healthcare-Associated Exposure on Clinical and Molecular Characterization of Methicillin-Susceptible Staphylococcus aureus Bacteremia. Medicine 2015, 94, e474. [Google Scholar] [CrossRef] [PubMed]
- De Kraker, M.E.; Jarlier, V.; Monen, J.C.; Heuer, O.E.; van de Sande, N.; Grundmann, H. The changing epidemiology of bacteraemias in Europe: Trends from the European Antimicrobial Resistance Surveillance System. Clin. Microbiol. Infect. 2013, 19, 860–868. [Google Scholar] [CrossRef]
- Gudmundsson, O.G.; Ormerod, L.D.; Kenyon, K.R.; Glynn, R.J.; Baker, A.S.; Haaf, J.; Lubars, S.; Abelson, M.B.; Boruchoff, S.A.; Foster, C.S.; et al. Factors influencing predilection and outcome in bacterial keratitis. Cornea 1989, 8, 115–121. [Google Scholar] [CrossRef]
- Knauf, H.P.; Silvany, R.; Southern, P.M., Jr.; Risser, R.C.; Wilson, S.E. Susceptibility of corneal and conjunctival pathogens to ciprofloxacin. Cornea 1996, 15, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Asbell, P.A.; Sanfilippo, C.M.; Sahm, D.F.; DeCory, H.H. Trends in Antibiotic Resistance Among Ocular Microorganisms in the United States From 2009 to 2018. JAMA Ophtalmol. 2020, 138, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.C.; Hsiao, C.H.; Tan, H.Y.; Ma, D.H.; Lin, K.K.; Chang, C.J.; Huang, Y.C. Staphylococcus aureus ocular infection: Methicillin-resistance, clinical features, and antibiotic susceptibilities. PLoS ONE 2012, 8, e42437. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.H.; Chuang, C.C.; Tan, H.Y.; Ma, D.H.; Lin, K.K.; Chang, C.J.; Huang, Y.C. Methicillin-resistant Staphylococcus aureus ocular infection: A 10-year hospital-based study. Ophtalmology 2012, 119, 522–527. [Google Scholar] [CrossRef]
- Kang, Y.C.; Hsiao, C.H.; Yeh, L.K.; Ma, D.H.K.; Chen, P.Y.F.; Lin, H.C.; Tan, H.Y.; Chen, H.C.; Chen, S.Y.; Huang, Y.C. Methicillin-Resistant Staphylococcus aureus Ocular Infection in Taiwan: Clinical Features, Genotyping, and Antibiotic Susceptibility. Medicine 2015, 94, e1620. [Google Scholar] [CrossRef]
- Marangon, F.B.; Miller, D.; Muallem, M.S.; Romano, A.C.; Alfonso, E.C. Ciprofloxacin and levofloxacin resistance among methicillin-sensitive Staphylococcus aureus isolates from keratitis and conjunctivitis. Am. J. Ophthalmol. 2004, 137, 453–458. [Google Scholar] [CrossRef]
- Ho, C.-M.; Lin, C.-Y.; Ho, M.-W.; Lin, H.-C.; Peng, C.-T.; Lu, J.-J. Concomitant genotyping revealed diverse spreading between methicillin-resistant Staphylococcus aureus and methicillin-susceptible Staphylococcus aureus in central Taiwan. J. Microbiol. Immunol. Infect. 2016, 49, 363–370. [Google Scholar] [CrossRef]
- Yu, F.; Li, T.; Huang, X.; Xie, J.; Xu, Y.; Tu, J.; Qin, Z.; Parsons, C.; Wang, J.; Hu, L.; et al. Virulence gene profiling and molecular characterization of hospital-acquired Staphylococcus aureus isolates associated with bloodstream infection. Diagn. Microbiol. Infect. Dis. 2012, 74, 363–368. [Google Scholar] [CrossRef]
- Hesje, C.K.; Sanfilippo, C.M.; Haas, W.; Morris, T.W. Molecular epidemiology of methicillin-resistant and methicillin-susceptible Staphylococcus aureus isolated from the eye. Curr. Eye Res. 2011, 36, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.C.; Durkee, H.; Miller, D.; Maestre-Mesa, J.; Arboleda, A.; Aguilar, M.C.; Relhan, N.; Flynn, H.W., Jr.; Amescua, G.; Parel, J.M.; et al. Molecular epidemiology and resistance profiles among healthcare- and community-associated Staphylococcus aureus keratitis isolates. Infect. Drug Resist 2019, 12, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-J.; Hiramatsu, K.; Huang, I.W.; Wang, C.-H.; Lauderdale, T.-L.Y. Panton-Valentine leukocidin (PVL)-positive methicillin-susceptible and resistant Staphylococcus aureus in Taiwan: Identification of oxacillin-susceptible mecA-positive methicillin-resistant. S. Aureus. Diagn. Microbiol. Infect. Dis. 2009, 65, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Asbell, P.A.; Colby, K.A.; Deng, S.; McDonnell, P.; Meisler, D.M.; Raizman, M.B.; Sheppard, J.D., Jr.; Sahm, D.F. Ocular TRUST: Nationwide antimicrobial susceptibility patterns in ocular isolates. Am. J. Ophthalmol. 2008, 145, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, R.; Bandello, F.; Martinelli, M.; Calaresu, E.; Cocuzza, C.E. In vitro bactericidal activity of 0.6% povidone-iodine eye drops formulation. Eur. J. Ophthalmol. 2019, 29, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Pinna, A.; Donadu, M.G.; Usai, D.; Dore, S.; D’Amico-Ricci, G.; Boscia, F.; Zanetti, S. In vitro antimicrobial activity of a new ophthalmic solution containing povidone-iodine 0.6% (IODIM(®)). Acta Ophthalmol. 2020, 98, e178–e180. [Google Scholar] [CrossRef]
- Pinna, A.; Donadu, M.G.; Usai, D.; Dore, S.; Boscia, F.; Zanetti, S. In Vitro Antimicrobial Activity of a New Ophthalmic Solution Containing Hexamidine Diisethionate 0.05% (Keratosept). Cornea 2020, 39, 1415–1418. [Google Scholar] [CrossRef]
- Nasir, S.; Vohra, M.S.; Gul, D.; Swaiba, U.E.; Aleem, M.; Mehmood, K.; Andleeb, S. Novel Antibiotic Combinations of Diverse Subclasses for Effective Suppression of Extensively Drug-Resistant Methicillin-Resistant Staphylococcus aureus (MRSA). Int. J. Microbiol. 2020, 2020, 8831322. [Google Scholar] [CrossRef]
- Morrison, M.A.; Hageman, J.C.; Klevens, R.M. Case definition for community-associated methicillin-resistant Staphylococcus aureus. J. Hosp. Infect. 2006, 62, 241. [Google Scholar] [CrossRef]
- Lina, G.; Piémont, Y.; Godail-Gamot, F.; Bes, M.; Peter, M.O.; Gauduchon, V.; Vandenesch, F.; Etienne, J. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 1999, 29, 1128–1132. [Google Scholar] [CrossRef]
- Enright, M.C.; Day, N.P.; Davies, C.E.; Peacock, S.J.; Spratt, B.G. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 2000, 38, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informnational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2013. [Google Scholar]
| CA (n = 46) No. (%) | HA (n = 58) No. (%) | p Value | |
|---|---|---|---|
| Demographics | |||
| Age in years, mean ± SD (range) | 44.6 ± 24.0 (0.1–84) | 52.6 ± 25.0 (0.1–95) | 0.102 |
| Sex (male/female) | 22/24 (47.8/52.2) | 25/33 (43.1/56.9) | 0.694 |
| Underlying Condition | |||
| Diabetes mellitus | 9 (19.6) | 15 (25.9) | 0.491 |
| Hypertension | 13 (28.3) | 20 (34.5) | 0.532 |
| Pulmonary disease | 3 (6.5) | 6 (10.3) | 0.728 |
| Renal disease | 4 (8.7) | 6 (10.3) | 1 |
| Liver disease | 2 (4.3) | 5 (8.6) | 0.46 |
| Malignancy | 1 (2.2) | 12 (20.7) | 0.006 |
| Immunodeficiency | 1 (2.2) | 5 (8.6) | 0.224 |
| Current infection a | 0 (0) | 19 (32.9) | <0.001 |
| Recent antibiotic use | 1 (2.2) | 15 (25.9) | 0.001 |
| Alcoholic | 2 (4.3) | 3 (5.2) | 1 |
| Ocular history | |||
| Contact lens use | 8 (17.4) | 1 (1.7) | 0.010 |
| Ocular surface disease | 11 (23.9) | 23 (39.7) | 0.098 |
| Surgery | 9 (19.6) | 37 (63.8) | <0.001 |
| Trauma | 8 (17.4) | 9 (15.5) | 0.797 |
| CA (n = 46) No. (%) | HA (n = 58) No. (%) | p Value | |
|---|---|---|---|
| Diagnosis | |||
| Lid disorder | 4 (8.7) | 4 (6.9) | 0.730 |
| Conjunctivitis | 5 (10.9) | 23 (39.7) | 0.001 |
| Keratitis | 29 (63.0) | 17 (29.3) | <0.001 |
| Endophthalmitis | 3 (6.5) | 2 (3.4) | 0.653 |
| Wound infection) | 1 (2.2) | 5 (8.6) | 0.224 |
| Lacrimal system disorder | 4 (8.7) | 4 (6.9) | 0.730 |
| Others (%) | 0 (0) | 3 (5.2) | 0.253 |
| Treatment | |||
| Surgical intervention | 5 (10.9) | 11 (19) | 0.288 |
| Inpatient | 14 (30.4) | 33 (56.9) | 0.009 |
| Outpatient/ED | 32 (69.6) | 25 (43.1) | 0.010 |
| Pulsotypes | BA | F | AX | BW | D | Others |
|---|---|---|---|---|---|---|
| No. isolates (n = 104) | 35 (33.7%) | 20 (19.2%) | 8 (7.7%) | 8 (7.7%) | 7 (6.7%) | 26 (25%) |
| CA (n = 46) | 13 (28.3%) | 10 (21.7%) | 4 (8.7%) | 3 (6.5%) | 5 (10.9%) | 11 (23.9%) |
| HA (n = 58) | 22 (37.9%) | 10 (17.2%) | 4 (6.9%) | 5 (8.6%) | 2 (3.4%) | 15 (25.9%) |
| p-value | 0.404 | 0.621 | 0.730 | 1 | 0.237 | 1 |
| PVL-positive (n = 7) | 0 | 0 | 1 | 1 | 2 | 3 |
| Sequence type | 1 (1/7), 7 (4/7), 6427 (1/7), 6457 (1/7) | 15 (6/6) | 188 (2/3), 6426 a (1/3) | Untypeable a (4/4) | 59 a (2/4), 97 (1/3), 338 a (1/4) | 1232 aa (3), 59, 1281, 72, 30 (2), 96, 398, 508, 509, 573, 672 a, 6453, untypeable |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-L.; Kang, E.Y.-C.; Yeh, L.-K.; Ma, D.H.K.; Tan, H.-Y.; Chen, H.-C.; Hung, K.-H.; Huang, Y.-C.; Hsiao, C.-H. Clinical Features and Molecular Characteristics of Methicillin-Susceptible Staphylococcus aureus Ocular Infection in Taiwan. Antibiotics 2021, 10, 1445. https://doi.org/10.3390/antibiotics10121445
Chen Y-L, Kang EY-C, Yeh L-K, Ma DHK, Tan H-Y, Chen H-C, Hung K-H, Huang Y-C, Hsiao C-H. Clinical Features and Molecular Characteristics of Methicillin-Susceptible Staphylococcus aureus Ocular Infection in Taiwan. Antibiotics. 2021; 10(12):1445. https://doi.org/10.3390/antibiotics10121445
Chicago/Turabian StyleChen, Yueh-Ling, Eugene Yu-Chuan Kang, Lung-Kun Yeh, David H. K. Ma, Hsin-Yuan Tan, Hung-Chi Chen, Kuo-Hsuan Hung, Yhu-Chering Huang, and Ching-Hsi Hsiao. 2021. "Clinical Features and Molecular Characteristics of Methicillin-Susceptible Staphylococcus aureus Ocular Infection in Taiwan" Antibiotics 10, no. 12: 1445. https://doi.org/10.3390/antibiotics10121445
APA StyleChen, Y.-L., Kang, E. Y.-C., Yeh, L.-K., Ma, D. H. K., Tan, H.-Y., Chen, H.-C., Hung, K.-H., Huang, Y.-C., & Hsiao, C.-H. (2021). Clinical Features and Molecular Characteristics of Methicillin-Susceptible Staphylococcus aureus Ocular Infection in Taiwan. Antibiotics, 10(12), 1445. https://doi.org/10.3390/antibiotics10121445








